Curative Potential of Substances with Bioactive Properties to Alleviate Cd Toxicity: A Review
Abstract
1. Introduction
2. Cadmium Toxicity
3. Mitigation of Cd Toxicity via Food Supplementation
3.1. Vitamins
3.1.1. Vitamin C (Ascorbic Acid)
3.1.2. Vitamin E
3.2. Mineral Elements
3.2.1. Selenium
3.2.2. Zinc
3.2.3. Calcium
3.2.4. Silicon, Magnesium, Manganese
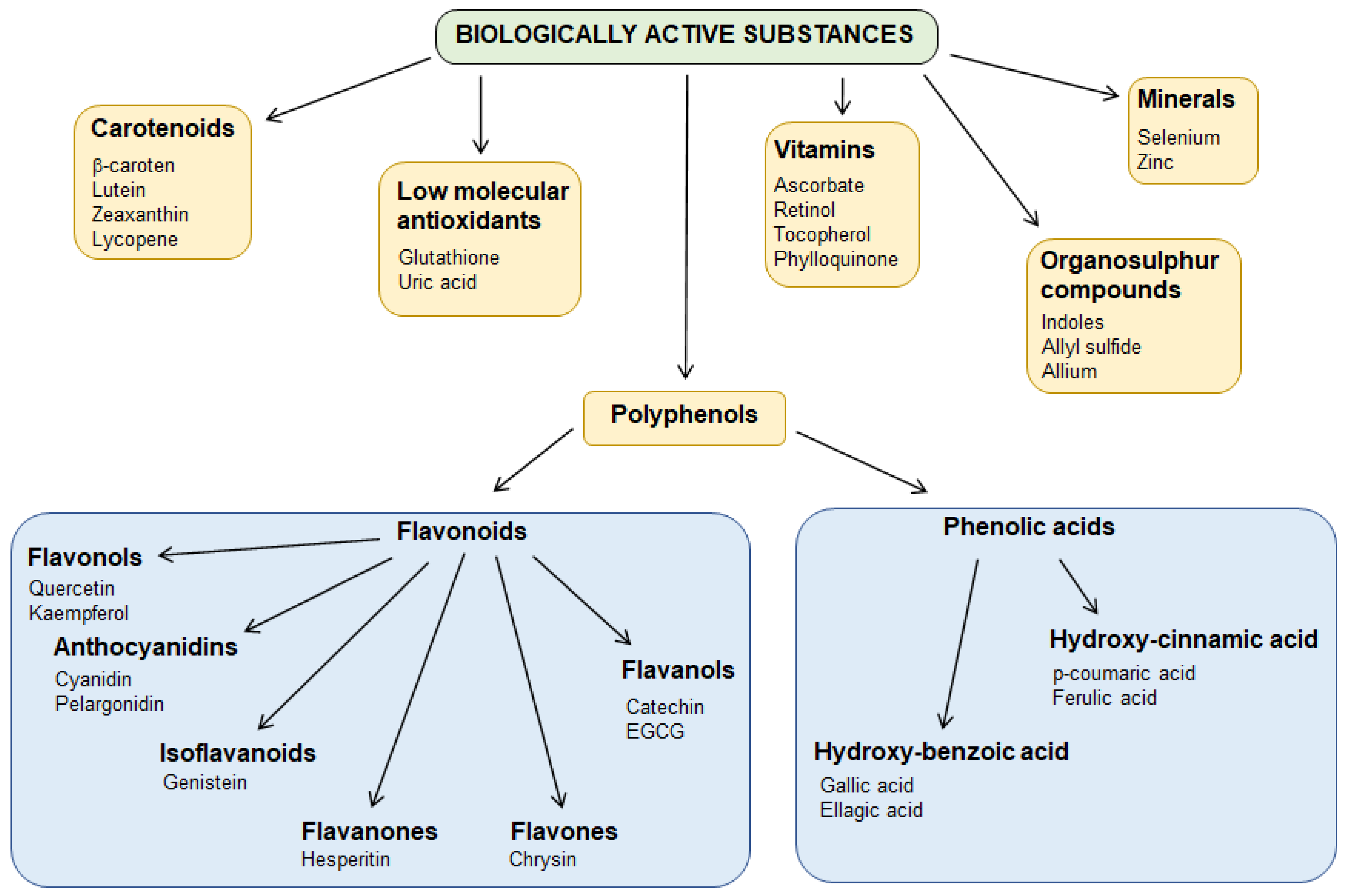
3.3. Bioactive Substances
3.3.1. Polyphenols, Phenols, and Phenolic Acids
Flavonoids
- Rutin hydrate
- Chrysin
- Diosmin
- Quercetin
- Hesperetin
- Anthocyanins
- Naringin
- Curcumin
- Carvacrol
Phenolic Acids
- Ferulic acid
- Vanillic acid
3.3.2. Hormones, Phytohormones, Metabolites
Salicylic Acid
Abscisic Acid
Melatonin
Fulvic Acid
3.4. Whole Plant Extracts
3.4.1. Senna alexandrina Extract
3.4.2. Portulaca oleracea (Purslane) Extract
3.4.3. Aronia melanocarpa L. Extract
3.4.4. Physalis peruviana L. Extract
3.4.5. Mimosa caesalpiniifolia Extract
3.4.6. Spirulina platensis Extract
3.4.7. Purple Carrot Extract
3.4.8. Grape Seed Extract and Grape Seed Proanthocyanidins
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nogawa, K.; Kido, T. Biological Monitoring of Cadmium Exposure in Itai-Itai Disease Epidemiology. Int. Arch. Occup. Env. Heath 1993, 65, S43–S46. [Google Scholar] [CrossRef] [PubMed]
- Nogawa, K.; Sakurai, M.; Ishizaki, M.; Kido, T.; Nakagawa, H.; Suwazono, Y. Threshold Limit Values of the Cadmium Concentration in Rice in the Development of Itai-Itai Disease Using Benchmark Dose Analysis. J. Appl. Toxicol. 2017, 37, 962–966. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Plant, J.A.; Voulvoulis, N.; Oates, C.J.; Ihlenfeld, C. Cadmium Levels in Europe: Implications for Human Health. Environ. Geochem. Health 2010, 32, 1–12. [Google Scholar] [CrossRef]
- Schaefer, H.R.; Dennis, S.; Fitzpatrick, S. Cadmium: Mitigation Strategies to Reduce Dietary Exposure. J. Food Sci. 2020, 85, 260–267. [Google Scholar] [CrossRef]
- Rani, A.; Kumar, A.; Lal, A.; Pant, M. Cellular Mechanisms of Cadmium-Induced Toxicity: A Review. Int. J. Environ. Health Res. 2014, 24, 378–399. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; Garrett, S.H.; Sens, M.A.; Sens, D.A. Cadmium, Environmental Exposure, and Health Outcomes. Environ. Health Perspect. 2010, 118, 182–190. [Google Scholar] [CrossRef]
- Satarug, S. Dietary Cadmium Intake and Its Effects on Kidneys. Toxics 2018, 6, 15. [Google Scholar] [CrossRef]
- FAO; World Health Organization; WHO Expert Committee on Food Additives. Evaluation of Certain Food Additives and Contaminants: Seventy-Third [73rd] Report of the Joint FAO/WHO Expert Committee on Food Additives; World Health Organization: Geneva, Switzerland, 2011; Available online: https://apps.who.int/iris/handle/10665/44515 (accessed on 17 June 2022).
- Bernhoft, R.A. Cadmium Toxicity and Treatment. Sci. World J. 2013, 2013, e394652. [Google Scholar] [CrossRef]
- Marettová, E.; Maretta, M.; Legáth, J. Toxic Effects of Cadmium on Testis of Birds and Mammals: A Review. Anim. Reprod. Sci. 2015, 155, 1–10. [Google Scholar] [CrossRef]
- Matović, V.; Buha, A.; Bulat, Z.; Đukić-Ćosić, D. Cadmium Toxicity Revisited: Focus on Oxidative Stress Induction and Interactions with Zinc and Magnesium. Arch. Ind. Hyg. Toxicol. 2011, 62, 65–76. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, J.; Wu, T.; Jiang, X.; Jia, H.; Qing, S.; An, Q.; Zhang, Y.; Su, J. Reproductive Toxicity of Acute Cd Exposure in Mouse: Resulting in Oocyte Defects and Decreased Female Fertility. Toxicol. Appl. Pharmacol. 2019, 379, 114684. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, T.; Lei, W.; Liu, D.; Li, Y.; Xuan, R.; Ma, J. Cadmium-Induced Oxidative Stress and Apoptotic Changes in the Testis of Freshwater Crab, Sinopotamon henanense. PLoS ONE 2011, 6, e27853. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-M.; Arnush, M.; Chen, Q.-Y.; Wu, X.-D.; Pang, B.; Jiang, X.-Z. Cadmium-Induced Damage to Primary Cultures of Rat Leydig Cells. Reprod. Toxicol. 2003, 17, 553–560. [Google Scholar] [CrossRef]
- Zhu, Q.; Li, X.; Ge, R.-S. Toxicological Effects of Cadmium on Mammalian Testis. Front. Genet. 2020, 11, 527. [Google Scholar] [CrossRef]
- Zhou, T.; Jia, X.; Chapin, R.E.; Maronpot, R.R.; Harris, M.W.; Liu, J.; Waalkes, M.P.; Eddy, E.M. Cadmium at a Non-Toxic Dose Alters Gene Expression in Mouse Testes. Toxicol. Lett. 2004, 154, 191–200. [Google Scholar] [CrossRef]
- Liu, W.; Sun, L.; Zhong, M.; Zhou, Q.; Gong, Z.; Li, P.; Tai, P.; Li, X. Cadmium-Induced DNA Damage and Mutations in Arabidopsis Plantlet Shoots Identified by DNA Fingerprinting. Chemosphere 2012, 89, 1048–1055. [Google Scholar] [CrossRef]
- Fernández, E.L.; Gustafson, A.-L.; Andersson, M.; Hellman, B.; Dencker, L. Cadmium-Induced Changes in Apoptotic Gene Expression Levels and DNA Damage in Mouse Embryos Are Blocked by Zinc. Toxicol. Sci. 2003, 76, 162–170. [Google Scholar] [CrossRef]
- Xia, L.; Chen, S.; Dahms, H.-U.; Ying, X.; Peng, X. Cadmium Induced Oxidative Damage and Apoptosis in the Hepatopancreas of Meretrix Meretrix. Ecotoxicology 2016, 25, 959–969. [Google Scholar] [CrossRef]
- Zhang, H.; Cai, C.; Shi, C.; Cao, H.; Han, Z.; Jia, X. Cadmium-Induced Oxidative Stress and Apoptosis in the Testes of Frog Rana limnocharis. Aquat. Toxicol. 2012, 122–123, 67–74. [Google Scholar] [CrossRef]
- Oliveira, H.; Monteiro, C.; Pinho, F.; Pinho, S.; Ferreira de Oliveira, J.M.P.; Santos, C. Cadmium-Induced Genotoxicity in Human Osteoblast-like Cells. Mutat. Res. Genet. Toxicol. Environ. Mutagenesis 2014, 775–776, 38–47. [Google Scholar] [CrossRef]
- Ou, L.; Wang, H.; Wu, Z.; Wang, P.; Yang, L.; Li, X.; Sun, K.; Zhu, X.; Zhang, R. Effects of Cadmium on Osteoblast Cell Line: Exportin 1 Accumulation, p-JNK Activation, DNA Damage and Cell Apoptosis. Ecotoxicol. Environ. Saf. 2021, 208, 111668. [Google Scholar] [CrossRef] [PubMed]
- Skipper, A.; Sims, J.N.; Yedjou, C.G.; Tchounwou, P.B. Cadmium Chloride Induces DNA Damage and Apoptosis of Human Liver Carcinoma Cells via Oxidative Stress. Int. J. Environ. Res. Public Health 2016, 13, 88. [Google Scholar] [CrossRef] [PubMed]
- Filipič, M.; Fatur, T.; Vudrag, M. Molecular Mechanisms of Cadmium Induced Mutagenicity. Hum. Exp. Toxicol. 2006, 25, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Filipič, M. Mechanisms of Cadmium Induced Genomic Instability. Mutat. Res. Fundam. Mol. Mech. Mutagenesis 2012, 733, 69–77. [Google Scholar] [CrossRef]
- Lützen, A.; Liberti, S.E.; Rasmussen, L.J. Cadmium Inhibits Human DNA Mismatch Repair in vivo. Biochem. Biophys. Res. Commun. 2004, 321, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.H.; Clark, A.B.; Slebos, R.J.C.; Al-Refai, H.; Taylor, J.A.; Kunkel, T.A.; Resnick, M.A.; Gordenin, D.A. Cadmium Is a Mutagen That Acts by Inhibiting Mismatch Repair. Nat. Genet. 2003, 34, 326–329. [Google Scholar] [CrossRef]
- Bork, U.; Lee, W.-K.; Kuchler, A.; Dittmar, T.; Thévenod, F. Cadmium-Induced DNA Damage Triggers G2/M Arrest via Chk1/2 and Cdc2 in P53-Deficient Kidney Proximal Tubule Cells. Am. J. Physiol. Ren. Physiol. 2010, 298, F255–F265. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cheng, C.-H.; Ma, H.-L.; Deng, Y.-Q.; Feng, J.; Jie, Y.-K.; Guo, Z.-X. Oxidative Stress, Cell Cycle Arrest, DNA Damage and Apoptosis in the Mud Crab (Scylla paramamosain) Induced by Cadmium Exposure. Chemosphere 2021, 263, 128277. [Google Scholar] [CrossRef]
- Nzengue, Y.; Steiman, R.; Garrel, C.; Lefèbvre, E.; Guiraud, P. Oxidative Stress and DNA Damage Induced by Cadmium in the Human Keratinocyte HaCaT Cell Line: Role of Glutathione in the Resistance to Cadmium. Toxicology 2008, 243, 193–206. [Google Scholar] [CrossRef]
- Chater, S.; Douki, T.; Garrel, C.; Favier, A.; Sakly, M.; Abdelmelek, H. Cadmium-Induced Oxidative Stress and DNA Damage in Kidney of Pregnant Female Rats. C. R. Biol. 2008, 331, 426–432. [Google Scholar] [CrossRef]
- Nemmiche, S.; Chabane-Sari, D.; Kadri, M.; Guiraud, P. Cadmium Chloride-Induced Oxidative Stress and DNA Damage in the Human Jurkat T Cell Line Is Not Linked to Intracellular Trace Elements Depletion. Toxicol. Vitr. 2011, 25, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, A. Cadmium and Cancer. In Cadmium: From Toxicity to Essentiality; Sigel, A., Sigel, H., Sigel, R.K., Eds.; Metal Ions in Life Sciences; Springer: Dordrecht, The Netherlands, 2013; pp. 491–507. [Google Scholar] [CrossRef]
- Pozgajova, M.; Navratilova, A.; Arvay, J.; Duranova, H.; Trakovicka, A. Impact of Cadmium and Nickel on Ion Homeostasis in the Yeast Schizosaccharomyces pombe. J. Environ. Sci. Health Part B 2020, 55, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wang, Y.; Wang, C.; Zhao, P.; Liu, H.; Li, J.; Bao, J. Ameliorative Effects of Dietary Selenium Against Cadmium Toxicity Is Related to Changes in Trace Elements in Chicken Kidneys. Biol. Trace Elem. Res. 2017, 176, 391–400. [Google Scholar] [CrossRef]
- Zhang, X.; Shao, J.; Chen, A.; Shang, C.; Hu, X.; Luo, S.; Lei, M.; Peng, L.; Zeng, Q. Effects of Cadmium on Calcium Homeostasis in the White-Rot Fungus Phanerochaete Chrysosporium. Ecotoxicol. Environ. Saf. 2018, 157, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Kısa, D.; Öztürk, L.; Tekin, Ş. Gene Expression Analysis of Metallothionein and Mineral Elements Uptake in Tomato (Solanum lycopersicum) Exposed to Cadmium. J. Plant Res. 2016, 129, 989–995. [Google Scholar] [CrossRef]
- Oishi, S.; Nakagawa, J.; Ando, M. Effects of Ingestion of Cadmium-Polluted Rice or Low-Dose Cadmium-Supplemented Diet on the Endogenous Metal Balance in Female Rats. Biol. Trace Elem. Res. 2001, 84, 155. [Google Scholar] [CrossRef]
- Guan, D.-L.; Ding, R.-R.; Hu, X.-Y.; Yang, X.-R.; Xu, S.-Q.; Gu, W.; Zhang, M. Cadmium-Induced Genome-Wide DNA Methylation Changes in Growth and Oxidative Metabolism in Drosophila melanogaster. BMC Genom. 2019, 20, 356. [Google Scholar] [CrossRef]
- Ren, C.; Ren, L.; Yan, J.; Bai, Z.; Zhang, L.; Zhang, H.; Xie, Y.; Li, X. Cadmium Causes Hepatopathy by Changing the Status of DNA Methylation in the Metabolic Pathway. Toxicol. Lett. 2021, 340, 101–113. [Google Scholar] [CrossRef]
- Takiguchi, M.; Achanzar, W.E.; Qu, W.; Li, G.; Waalkes, M.P. Effects of Cadmium on DNA-(Cytosine-5) Methyltransferase Activity and DNA Methylation Status during Cadmium-Induced Cellular Transformation. Exp. Cell Res. 2003, 286, 355–365. [Google Scholar] [CrossRef]
- Feng, S.J.; Liu, X.S.; Tao, H.; Tan, S.K.; Chu, S.S.; Oono, Y.; Zhang, X.D.; Chen, J.; Yang, Z.M. Variation of DNA Methylation Patterns Associated with Gene Expression in Rice (Oryza sativa) Exposed to Cadmium. Plant Cell Environ. 2016, 39, 2629–2649. [Google Scholar] [CrossRef]
- Carne, G.; Makowski, D.; Carrillo, S.; Guérin, T.; Jitaru, P.; Reninger, J.; Rivière, G.; Bemrah, N. Probabilistic Determination of a Maximum Acceptable Level of Contaminant to Reduce the Risk of Overexposure for a Novel or Emerging Food: The Case of Cadmium in Edible Seaweed in the French Population. Food Addit. Contam. Part A 2022, 39, 1439–1452. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Qu, J.; Sun, S.; Shi, Q.; Feng, H.; Zhang, Y.; Cao, S. Health Risk Assessment of Total Exposure from Cadmium in South China. Chemosphere 2021, 269, 128673. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, J.; Wong, L.P.; Chua, Y.P.; Channa, N.; Memon, U.-R.; Garn, J.V.; Yasmin, A.; VanDerslice, J.A. Heavy Metals Drinking Water Contamination and Health Risk Assessment among Primary School Children of Pakistan. J. Environ. Sci. Health Part A 2021, 56, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Sabolić, I.; Breljak, D.; Škarica, M.; Herak-Kramberger, C.M. Role of Metallothionein in Cadmium Traffic and Toxicity in Kidneys and Other Mammalian Organs. Biometals 2010, 23, 897–926. [Google Scholar] [CrossRef] [PubMed]
- Ruslee, S.S.; Zaid, S.S.M.; Bakrin, I.H.; Goh, Y.M.; Mustapha, N.M. Protective Effect of Tualang Honey against Cadmium-Induced Morphological Abnormalities and Oxidative Stress in the Ovary of Rats. BMC Complement. Med. Ther. 2020, 20, 160. [Google Scholar] [CrossRef]
- Kim, H.; Lee, H.J.; Hwang, J.-Y.; Ha, E.-H.; Park, H.; Ha, M.; Kim, J.H.; Hong, Y.-C.; Chang, N. Blood Cadmium Concentrations of Male Cigarette Smokers Are Inversely Associated with Fruit Consumption. J. Nutr. 2010, 140, 1133–1138. [Google Scholar] [CrossRef]
- Amamou, F.; Nemmiche, S.; Meziane, R.K.; Didi, A.; Yazit, S.M.; Chabane-Sari, D. Protective Effect of Olive Oil and Colocynth Oil against Cadmium-Induced Oxidative Stress in the Liver of Wistar Rats. Food Chem. Toxicol. 2015, 78, 177–184. [Google Scholar] [CrossRef]
- Venkatesh, J.; Park, S.W. Role of L-Ascorbate in Alleviating Abiotic Stresses in Crop Plants. Bot. Stud. 2014, 55, 38. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, G.; Bao, M.; Wang, L.; Xie, X. Exogenous Application of Ascorbic Acid Mitigates Cadmium Toxicity and Uptake in Maize (Zea mays L.). Environ. Sci. Pollut. Res. 2019, 26, 19261–19271. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Q.; Wu, W.; Guo, J.; Yang, Y. Cadmium Stress Tolerance in Wheat Seedlings Induced by Ascorbic Acid Was Mediated by NO Signaling Pathways. Ecotoxicol. Environ. Saf. 2017, 135, 75–81. [Google Scholar] [CrossRef]
- Jung, H.; Lee, B.-R.; Chae, M.-J.; Lee, E.-J.; Lee, T.-G.; Jung, G.-B.; Kim, M.-S.; Lee, J. Ascorbate-Mediated Modulation of Cadmium Stress Responses: Reactive Oxygen Species and Redox Status in Brassica napus. Front. Plant Sci. 2020, 11, 6547. [Google Scholar] [CrossRef] [PubMed]
- Navrátilová, A.; Kovár, M.; Požgajová, M. Ascorbic Acid Mitigates Cadmium-Induced Stress, and Contributes to Ionome Stabilization in Fission Yeast. Environ. Sci. Pollut. Res. 2021, 28, 15380–15393. [Google Scholar] [CrossRef] [PubMed]
- Mumtaz, S.; Ali, S.; Khan, R.; Andleeb, S.; Ulhaq, M.; Khan, M.A.; Shakir, H.A. The Protective Role of Ascorbic Acid in the Hepatotoxicity of Cadmium and Mercury in Rabbits. Environ. Sci. Pollut. Res. 2019, 26, 14087–14096. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Awan, Z.; Mumtaz, S.; Shakir, H.A.; Ahmad, F.; Ulhaq, M.; Tahir, H.M.; Awan, M.S.; Sharif, S.; Irfan, M.; et al. Cardiac Toxicity of Heavy Metals (Cadmium and Mercury) and Pharmacological Intervention by Vitamin C in Rabbits. Environ. Sci. Pollut. Res. 2020, 27, 29266–29279. [Google Scholar] [CrossRef]
- Donpunha, W.; Kukongviriyapan, U.; Sompamit, K.; Pakdeechote, P.; Kukongviriyapan, V.; Pannangpetch, P. Protective Effect of Ascorbic Acid on Cadmium-Induced Hypertension and Vascular Dysfunction in Mice. Biometals 2011, 24, 105–115. [Google Scholar] [CrossRef]
- Lee, G.Y.; Han, S.N. The Role of Vitamin E in Immunity. Nutrients 2018, 10, 1614. [Google Scholar] [CrossRef] [PubMed]
- Khadangi, F.; Azzi, A. Vitamin E—The Next 100 Years. IUBMB Life 2019, 71, 411–415. [Google Scholar] [CrossRef]
- Pekmezci, D. Chapter Eight—Vitamin E and Immunity. In Vitamins & Hormones; Litwack, G., Ed.; Vitamins and the Immune System; Academic Press: New York, NY, USA, 2011; Volume 86, pp. 179–215. [Google Scholar] [CrossRef]
- Ayyat, M.S.; Mahmoud, H.K.; El-Hais, A.E.-A.M.; Abd El-Latif, K.M. The Role of Some Feed Additives in Fish Fed on Diets Contaminated with Cadmium. Environ. Sci. Pollut. Res. 2017, 24, 23636–23645. [Google Scholar] [CrossRef]
- Duan, Y.; Duan, J.; Feng, Y.; Huang, X.; Fan, W.; Wang, K.; Ouyang, P.; Deng, Y.; Du, Z.; Chen, D.; et al. Hepatoprotective Activity of Vitamin E and Metallothionein in Cadmium-Induced Liver Injury in Ctenopharyngodon idellus. Oxidative Med. Cell. Longev. 2018, 2018, e9506543. [Google Scholar] [CrossRef]
- Huang, X.; Xiong, G.; Feng, Y.; Fan, W.; Yang, S.; Duan, J.; Duan, Y.; Wang, K.; Ou, Y.; Rehman, T.; et al. Protective Effects of Metallothionein and Vitamin E in the Trunk Kidney and Blood of Cadmium Poisoned Ctenopharyngodon idellus. Fish Physiol. Biochem. 2020, 46, 1053–1061. [Google Scholar] [CrossRef]
- Fang, J.; Xie, S.; Chen, Z.; Wang, F.; Chen, K.; Zuo, Z.; Cui, H.; Guo, H.; Ouyang, P.; Chen, Z.; et al. Protective Effect of Vitamin E on Cadmium-Induced Renal Oxidative Damage and Apoptosis in Rats. Biol. Trace Elem. Res. 2021, 199, 4675–4687. [Google Scholar] [CrossRef] [PubMed]
- Al-Attar, A.M. Vitamin E Attenuates Liver Injury Induced by Exposure to Lead, Mercury, Cadmium and Copper in Albino Mice. Saudi J. Biol. Sci. 2011, 18, 395–401. [Google Scholar] [CrossRef]
- Adi, P.J.; Burra, S.P.; Vataparti, A.R.; Matcha, B. Calcium, Zinc and Vitamin E Ameliorate Cadmium-Induced Renal Oxidative Damage in Albino Wistar Rats. Toxicol. Rep. 2016, 3, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Yin, H.; Yang, Z.; Tan, M.; Wang, F.; Chen, K.; Zuo, Z.; Shu, G.; Cui, H.; Ouyang, P.; et al. Vitamin E Protects against Cadmium-Induced Sub-Chronic Liver Injury Associated with the Inhibition of Oxidative Stress and Activation of Nrf2 Pathway. Ecotoxicol. Environ. Saf. 2021, 208, 111610. [Google Scholar] [CrossRef] [PubMed]
- Karabulut-Bulan, O.; Bolkent, S.; Yanardag, R.; Bilgin-Sokmen, B. The Role of Vitamin C, Vitamin E, and Selenium on Cadmium-Induced Renal Toxicity of Rats. Drug Chem. Toxicol. 2008, 31, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-H.; Rhee, S.-J. Effects of Vitamin E on Renal Dysfunction in Chronic Cadmium-Poisoned Rats. J. Med. Food 2003, 6, 209–215. [Google Scholar] [CrossRef]
- Amanpour, P.; Khodarahmi, P.; Salehipour, M. Protective Effects of Vitamin E on Cadmium-Induced Apoptosis in Rat Testes. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020, 393, 349–358. [Google Scholar] [CrossRef]
- Olaniyan, O.T.; Ojewale, A.O.; Eweoya, O.O.; Adedoyin, A.A.; Adesanya, O.A.; Adeoye, A.O.; Okeniran, O.S. Modulatory Role of Vitamin E on Proton Pump (ATPase) Activity of Cadmium Chloride-Induced Testicular Damage in Wistar Rats. BioMed Res. Int. 2021, 2021, e4615384. [Google Scholar] [CrossRef]
- Ognjanović, B.I.; Pavlović, S.Z.; Maletić, S.D.; Zikić, R.V.; Stajn, A.S.; Radojicić, R.M.; Saicić, Z.S.; Petrović, V.M. Protective Influence of Vitamin E on Antioxidant Defense System in the Blood of Rats Treated with Cadmium. Physiol. Res. 2003, 52, 563–570. [Google Scholar]
- Paunović, M.G.; Matić, M.M.; Ognjanović, B.I.; Saičić, Z.S. Antioxidative and Haematoprotective Activity of Coenzyme Q10 and Vitamin E against Cadmium-Induced Oxidative Stress in Wistar Rats. Toxicol. Ind. Health 2017, 33, 746–756. [Google Scholar] [CrossRef]
- Konar, V.; Kara, H.; Yilmaz, M.; Dayangac, A.; Karatas, F. Effects of Selenium and Vitamin E, in Addıtıon to Melatonin, Against Oxidative Stress Caused by Cadmium in Rats. Biol. Trace Elem. Res. 2007, 118, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Scientific Opinion on Dietary Reference Values for selenium|EFSA. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/3846 (accessed on 17 May 2022).
- Wang, N.; Tan, H.-Y.; Li, S.; Xu, Y.; Guo, W.; Feng, Y. Supplementation of Micronutrient Selenium in Metabolic Diseases: Its Role as an Antioxidant. Oxidative Med. Cell. Longev. 2017, 2017, e7478523. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Hossain, K.F.B.; Banik, S.; Sikder, M.T.; Akter, M.; Bondad, S.E.C.; Rahaman, M.S.; Hosokawa, T.; Saito, T.; Kurasaki, M. Selenium and Zinc Protections against Metal-(Loids)-Induced Toxicity and Disease Manifestations: A Review. Ecotoxicol. Environ. Saf. 2019, 168, 146–163. [Google Scholar] [CrossRef] [PubMed]
- Zwolak, I. The Role of Selenium in Arsenic and Cadmium Toxicity: An Updated Review of Scientific Literature. Biol. Trace Elem. Res. 2020, 193, 44–63. [Google Scholar] [CrossRef]
- Qu, K.-C.; Li, H.-Q.; Tang, K.-K.; Wang, Z.-Y.; Fan, R.-F. Selenium Mitigates Cadmium-Induced Adverse Effects on Trace Elements and Amino Acids Profiles in Chicken Pectoral Muscles. Biol. Trace Elem. Res. 2020, 193, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, L.-L.; Cao, C.-Y.; Li, N.; Talukder, M.; Li, J.-L. Selenium Mitigates Cadmium-Induced Crosstalk between Autophagy and Endoplasmic Reticulum Stress via Regulating Calcium Homeostasis in Avian Leghorn Male Hepatoma (LMH) Cells. Environ. Pollut. 2020, 265, 114613. [Google Scholar] [CrossRef] [PubMed]
- Cong, Y.; Chi, Q.; Teng, X.; Li, S. The Protection of Selenium Against Cadmium-Induced Mitochondrial Damage via the Cytochrome P450 in the Livers of Chicken. Biol. Trace Elem. Res. 2019, 190, 484–492. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, H.; Chang, W.; Chen, R.; Xu, S.; Tao, D. Protective Effects of Selenium Yeast against Cadmium-Induced Necroptosis via Inhibition of Oxidative Stress and MAPK Pathway in Chicken Liver. Ecotoxicol. Environ. Saf. 2020, 206, 111329. [Google Scholar] [CrossRef]
- Lynch, S.J.; Horgan, K.A.; White, B.; Walls, D. Selenium Source Impacts Protection of Porcine Jejunal Epithelial Cells from Cadmium-Induced DNA Damage, with Maximum Protection Exhibited with Yeast-Derived Selenium Compounds. Biol. Trace Elem. Res. 2017, 176, 311–320. [Google Scholar] [CrossRef]
- Li, H.; Liu, X.; Wassie, M.; Chen, L. Selenium Supplementation Alleviates Cadmium-Induced Damages in Tall Fescue through Modulating Antioxidant System, Photosynthesis Efficiency, and Gene Expression. Environ. Sci. Pollut. Res. 2020, 27, 9490–9502. [Google Scholar] [CrossRef]
- Auobi Amirabad, S.; Behtash, F.; Vafaee, Y. Selenium Mitigates Cadmium Toxicity by Preventing Oxidative Stress and Enhancing Photosynthesis and Micronutrient Availability on Radish (Raphanus sativus L.) Cv. Cherry Belle. Environ. Sci. Pollut. Res. 2020, 27, 12476–12490. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Dun, Y.; Zhang, Z.; Li, M.; Wu, G. Foliar Application of Selenium and Zinc to Alleviate Wheat (Triticum aestivum L.) Cadmium Toxicity and Uptake from Cadmium-Contaminated Soil. Ecotoxicol. Environ. Saf. 2020, 190, 110091. [Google Scholar] [CrossRef] [PubMed]
- Kogan, S.; Sood, A.; Garnick, M.S. Zinc and Wound Healing: A Review of Zinc Physiology and Clinical Applications. Wounds 2017, 29, 102–106. [Google Scholar] [PubMed]
- Gammoh, N.Z.; Rink, L. Zinc in Infection and Inflammation. Nutrients 2017, 9, 624. [Google Scholar] [CrossRef]
- Maret, W.; Sandstead, H.H. Zinc Requirements and the Risks and Benefits of Zinc Supplementation. J. Trace Elem. Med. Biol. 2006, 20, 3–18. [Google Scholar] [CrossRef]
- Livingstone, C. Zinc: Physiology, deficiency, and parenteral nutrition. Nutr. Clin. Pract. 2015, 30, 371–382. [Google Scholar] [CrossRef]
- Yu, H.; Zhen, J.; Leng, J.; Cai, L.; Ji, H.; Keller, B.B. Zinc as a Countermeasure for Cadmium Toxicity. Acta Pharm. Sin. 2021, 42, 340–346. [Google Scholar] [CrossRef]
- Branca, J.J.V.; Morucci, G.; Maresca, M.; Tenci, B.; Cascella, R.; Paternostro, F.; Ghelardini, C.; Gulisano, M.; Di Cesare Mannelli, L.; Pacini, A. Selenium and Zinc: Two Key Players against Cadmium-Induced Neuronal Toxicity. Toxicol. Vitr. 2018, 48, 159–169. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, J.; Gao, J.; Shahzad, M.; Han, Z.; Wang, Z.; Li, J.; Sjölinder, H. Zinc Supplementation Protects against Cadmium Accumulation and Cytotoxicity in Madin-Darby Bovine Kidney Cells. PLoS ONE 2014, 9, e103427. [Google Scholar] [CrossRef]
- Pan, J.; Huang, X.; Li, Y.; Li, M.; Yao, N.; Zhou, Z.; Li, X. Zinc Protects against Cadmium-Induced Toxicity by Regulating Oxidative Stress, Ions Homeostasis and Protein Synthesis. Chemosphere 2017, 188, 265–273. [Google Scholar] [CrossRef]
- Ben Mimouna, S.; Chemek, M.; Boughammoura, S.; Haouas, Z.; Messaoudi, I. Protective Role of Zinc against the Neurotoxicity Induced by Exposure to Cadmium during Gestation and Lactation Periods on Hippocampal Volume of Pups Tested in Early Adulthood. Drug Chem. Toxicol. 2018, 41, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, E.; Jing, W.; Dahms, H.-U.; Murugan, K.; Wang, L. Mitigative Effects of Zinc on Cadmium-Induced Reproductive Toxicity in the Male Freshwater Crab Sinopotamon henanense. Environ. Sci. Pollut. Res. 2020, 27, 16282–16292. [Google Scholar] [CrossRef] [PubMed]
- Babaknejad, N.; Bahrami, S.; Moshtaghie, A.A.; Nayeri, H.; Rajabi, P.; Iranpour, F.G. Cadmium Testicular Toxicity in Male Wistar Rats: Protective Roles of Zinc and Magnesium. Biol. Trace Elem. Res. 2018, 185, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Chemek, M.; Mimouna, S.B.; Boughammoura, S.; Delbès, G.; Messaoudi, I. Protective Role of Zinc against the Toxicity Induced by Exposure to Cadmium during Gestation and Lactation on Testis Development. Reprod. Toxicol. 2016, 63, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Tarnawska, M.; Babczyńska, A.; Hassa, K.; Kafel, A.; Płachetka-Bożek, A.; Augustyniak, J.; Dziewięcka, M.; Flasz, B.; Augustyniak, M. Protective Role of Zinc in Spodoptera Exigua Larvae under 135-Generational Cadmium Exposure. Chemosphere 2019, 235, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Ali, S.; Rehman, M.Z.U.; Maqbool, A. A Critical Review on the Effects of Zinc at Toxic Levels of Cadmium in Plants. Environ. Sci. Pollut. Res. 2019, 26, 6279–6289. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, C.; Du, B.; Cui, H.; Fan, X.; Zhou, D.; Zhou, J. Effects of Zinc Application on Cadmium (Cd) Accumulation and Plant Growth through Modulation of the Antioxidant System and Translocation of Cd in Low- and High-Cd Wheat Cultivars. Environ. Pollut. 2020, 265, 115045. [Google Scholar] [CrossRef]
- Sharifan, H.; Moore, J.; Ma, X. Zinc Oxide (ZnO) Nanoparticles Elevated Iron and Copper Contents and Mitigated the Bioavailability of Lead and Cadmium in Different Leafy Greens. Ecotoxicol. Environ. Saf. 2020, 191, 110177. [Google Scholar] [CrossRef]
- Hejazy, M.; Koohi, M.K. Effects of Nano-Zinc on Biochemical Parameters in Cadmium-Exposed Rats. Biol. Trace Elem. Res. 2017, 180, 265–274. [Google Scholar] [CrossRef]
- Huang, D.; Gong, X.; Liu, Y.; Zeng, G.; Lai, C.; Bashir, H.; Zhou, L.; Wang, D.; Xu, P.; Cheng, M.; et al. Effects of Calcium at Toxic Concentrations of Cadmium in Plants. Planta 2017, 245, 863–873. [Google Scholar] [CrossRef]
- Farzadfar, S.; Zarinkamar, F.; Modarres-Sanavy, S.A.M.; Hojati, M. Exogenously Applied Calcium Alleviates Cadmium Toxicity in Matricaria chamomilla L. Plants. Environ. Sci. Pollut. Res. 2013, 20, 1413–1422. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhao, C.; Zhang, Y.; Wang, X.; Wang, X.; Wang, J.; Wang, F.; Bi, Y. Calcium Alleviates Cadmium-Induced Inhibition on Root Growth by Maintaining Auxin Homeostasis in Arabidopsis Seedlings. Protoplasma 2016, 253, 185–200. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Q.; Nazir, M.M.; Ali, S.; Ouyang, Y.; Ye, S.; Zeng, F. Calcium Plays a Double-Edged Role in Modulating Cadmium Uptake and Translocation in Rice. Int. J. Mol. Sci. 2020, 21, 8058. [Google Scholar] [CrossRef] [PubMed]
- Kanu, A.S.; Ashraf, U.; Mo, Z.; Sabir, S.-R.; Baggie, I.; Charley, C.S.; Tang, X. Calcium Amendment Improved the Performance of Fragrant Rice and Reduced Metal Uptake under Cadmium Toxicity. Environ. Sci. Pollut. Res. 2019, 26, 24748–24757. [Google Scholar] [CrossRef] [PubMed]
- Sakouhi, L.; Kharbech, O.; Massoud, M.B.; Gharsallah, C.; Hassine, S.B.; Munemasa, S.; Murata, Y.; Chaoui, A. Calcium and Ethylene Glycol Tetraacetic Acid Mitigate Toxicity and Alteration of Gene Expression Associated with Cadmium Stress in Chickpea (Cicer arietinum L.) Shoots. Protoplasma 2021, 258, 849–861. [Google Scholar] [CrossRef] [PubMed]
- Kováčik, J.; Dresler, S.; Babula, P.; Hladký, J.; Sowa, I. Calcium Has Protective Impact on Cadmium-Induced Toxicity in Lichens. Plant Physiol. Biochem. 2020, 156, 591–599. [Google Scholar] [CrossRef]
- Ruta, L.L.; Popa, V.C.; Nicolau, I.; Danet, A.F.; Iordache, V.; Neagoe, A.D.; Farcasanu, I.C. Calcium Signaling Mediates the Response to Cadmium Toxicity in Saccharomyces cerevisiae Cells. FEBS Lett. 2014, 588, 3202–3212. [Google Scholar] [CrossRef]
- Wu, Z.; Zheng, R.; Liu, G.; Liu, R.; Wu, S.; Sun, C. Calcium Protects Bacteria against Cadmium Stress via Reducing Nitric Oxide Production and Increasing Iron Acquisition. Environ. Microbiol. 2021, 23, 3541–3553. [Google Scholar] [CrossRef]
- Baldisserotto, B.; Kamunde, C.; Matsuo, A.; Wood, C.M. A Protective Effect of Dietary Calcium against Acute Waterborne Cadmium Uptake in Rainbow Trout. Aquat. Toxicol. 2004, 67, 57–73. [Google Scholar] [CrossRef]
- Franklin, N.M.; Glover, C.N.; Nicol, J.A.; Wood, C.M. Calcium/Cadmium Interactions at Uptake Surfaces in Rainbow Trout: Waterborne versus Dietary Routes of Exposure. Environ. Toxicol. Chem. 2005, 24, 2954–2964. [Google Scholar] [CrossRef]
- Pratap, H.B.; Wendelaar Bonga, S.E. Calcium homeostasis in low and high calcium water acclimatized Oreochromis mossambicus exposed to ambient and dietary cadmium. J. Environ. Biol. 2007, 28, 385–393. [Google Scholar] [PubMed]
- Matek Sarić, M.; Blanuša, M.; Piasek, M.; Varnai, V.M.; Jureša, D.; Kostial, K. Effect of Dietary Calcium on Cadmium Absorption and Retention in Suckling Rats. Biometals 2002, 15, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liu, T.; Zhao, M.; Fu, H.; Wang, J.; Xu, Q. Protective Effects of Moderate Ca Supplementation against Cd-Induced Bone Damage under Different Population-Relevant Doses in Young Female Rats. Nutrients 2019, 11, 849. [Google Scholar] [CrossRef]
- El-Boshy, M.; Refaat, B.; Almaimani, R.A.; Abdelghany, A.H.; Ahmad, J.; Idris, S.; Almasmoum, H.; Mahbub, A.A.; Ghaith, M.M.; BaSalamah, M.A. Vitamin D3 and Calcium Cosupplementation Alleviates Cadmium Hepatotoxicity in the Rat: Enhanced Antioxidative and Anti-Inflammatory Actions by Remodeling Cellular Calcium Pathways. J. Biochem. Mol. Toxicol. 2020, 34, e22440. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Ren, Z.; Zhao, J.; Peprah, F.A.; Xie, Y.; Cheng, D.; Wang, Y.; Liu, H.; Chu Wong, C.K.; Zhou, Y.; et al. Calcimimetic Compound NPS R-467 Protects against Chronic Cadmium-Induced Mouse Kidney Injury by Restoring Autophagy Process. Ecotoxicol. Environ. Saf. 2020, 189, 110052. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Liu, Y.; Gong, X.; Zeng, G.; Zheng, B.; Wang, D.; Sun, Z.; Zhou, L.; Zeng, X. Effects of Selenium and Silicon on Enhancing Antioxidative Capacity in Ramie (Boehmeria nivea (L.) Gaud.) under Cadmium Stress. Environ. Sci. Pollut. Res. 2015, 22, 9999–10008. [Google Scholar] [CrossRef]
- Hussain, B.; Lin, Q.; Hamid, Y.; Sanaullah, M.; Di, L.; Hashmi, M.L.U.R.; Khan, M.B.; He, Z.; Yang, X. Foliage Application of Selenium and Silicon Nanoparticles Alleviates Cd and Pb Toxicity in Rice (Oryza sativa L.). Sci. Total Environ. 2020, 712, 136497. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Y.; Chai, T.; Tan, J.; Wang, J.; Feng, S.; Liu, G. Manganese-Mitigation of Cadmium Toxicity to Seedling Growth of Phytolacca Acinosa Roxb. Is Controlled by the Manganese/Cadmium Molar Ratio under Hydroponic Conditions. Plant Physiol. Biochem. 2013, 73, 144–153. [Google Scholar] [CrossRef]
- Eybl, V.; Kotyzová, D. Protective Effect of Manganese in Cadmium-Induced Hepatic Oxidative Damage, Changes in Cadmium Distribution and Trace Elements Level in Mice. Interdiscip. Toxicol. 2010, 3, 68–72. [Google Scholar] [CrossRef]
- Martin, P.; Fareh, M.; Poggi, M.C.; Boulukos, K.E.; Pognonec, P. Manganese Is Highly Effective in Protecting Cells from Cadmium Intoxication. Biochem. Biophys. Res. Commun. 2006, 351, 294–299. [Google Scholar] [CrossRef]
- Buha, A.; Bulat, Z.; Đukić-Ćosić, D.; Matović, V. Effects of Oral and Intraperitoneal Magnesium Treatment against Cadmium-Induced Oxidative Stress in Plasma of Rats. Arch. Ind. Hyg. Toxicol. 2012, 63, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Babaknejad, N.; Moshtaghie, A.A.; Nayeri, H.; Hani, M.; Bahrami, S. Protective Role of Zinc and Magnesium against Cadmium Nephrotoxicity in Male Wistar Rats. Biol. Trace Elem. Res. 2016, 174, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Ghaffarian-Bahraman, A.; Shahroozian, I.; Jafari, A.; Ghazi-Khansari, M. Protective Effect of Magnesium and Selenium on Cadmium Toxicity in the Isolated Perfused Rat Liver System. Acta Med. Iran. 2014, 52, 872–878. [Google Scholar] [PubMed]
- Klepacka, J.; Fornal, Ł. Ferulic Acid and Its Position Among the Phenolic Compounds of Wheat. Crit. Rev. Food Sci. Nutr. 2006, 46, 639–647. [Google Scholar] [CrossRef]
- Li, X.; Jiang, X.; Sun, J.; Zhu, C.; Li, X.; Tian, L.; Liu, L.; Bai, W. Cytoprotective Effects of Dietary Flavonoids against Cadmium-Induced Toxicity. Ann. N. Y. Acad. Sci. 2017, 1398, 5–19. [Google Scholar] [CrossRef]
- Oboh, G.; Adebayo, A.A.; Ademosun, A.O.; Olowokere, O.G. Rutin Alleviates Cadmium-Induced Neurotoxicity in Wistar Rats: Involvement of Modulation of Nucleotide-Degrading Enzymes and Monoamine Oxidase. Metab. Brain Dis. 2019, 34, 1181–1190. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aleem, G.A.; Khaleel, E.F. Rutin Hydrate Ameliorates Cadmium Chloride-Induced Spatial Memory Loss and Neural Apoptosis in Rats by Enhancing Levels of Acetylcholine, Inhibiting JNK and ERK1/2 Activation and Activating MTOR Signalling. Arch. Physiol. Biochem. 2018, 124, 367–377. [Google Scholar] [CrossRef]
- Beyrami, M.; Karimi, E.; Oskoueian, E. Synthesized Chrysin-Loaded Nanoliposomes Improves Cadmium-Induced Toxicity in Mice. Environ. Sci. Pollut. Res. 2020, 27, 40643–40651. [Google Scholar] [CrossRef]
- Ağır, M.S.; Eraslan, G. The Effect of Diosmin against Liver Damage Caused by Cadmium in Rats. J. Food Biochem. 2019, 43, e12966. [Google Scholar] [CrossRef]
- Yuan, Y.; Ma, S.; Qi, Y.; Wei, X.; Cai, H.; Dong, L.; Lu, Y.; Zhang, Y.; Guo, Q. Quercetin Inhibited Cadmium-Induced Autophagy in the Mouse Kidney via Inhibition of Oxidative Stress. J. Toxicol. Pathol. 2016, 29, 247–252. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, H.; Wang, K.; Yang, Z.; Liu, Z. Protective Effect of Quercetin on Rat Testes against Cadmium Toxicity by Alleviating Oxidative Stress and Autophagy. Environ. Sci. Pollut. Res. 2020, 27, 25278–25286. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, Z.-F.; Zhang, D.; Wang, Z.-Y.; Wang, L. Quercetin Alleviates Cadmium-Induced Autophagy Inhibition via TFEB-Dependent Lysosomal Restoration in Primary Proximal Tubular Cells. Ecotoxicol. Environ. Saf. 2021, 208, 111743. [Google Scholar] [CrossRef]
- Guan, T.; Xin, Y.; Zheng, K.; Wang, R.; Zhang, X.; Jia, S.; Li, S.; Cao, C.; Zhao, X. Metabolomics Analysis of the Effects of Quercetin on Renal Toxicity Induced by Cadmium Exposure in Rats. Biometals 2021, 34, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Mao, T.; Han, C.; Wei, B.; Zhao, L.; Zhang, Q.; Deng, R.; Liu, J.; Luo, Y.; Zhang, Y. Protective Effects of Quercetin Against Cadmium Chloride-Induced Oxidative Injury in Goat Sperm and Zygotes. Biol. Trace Elem. Res. 2018, 185, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Halder, S.; Kar, R.; Chakraborty, S.; Bhattacharya, S.K.; Mediratta, P.K.; Banerjee, B.D. Cadmium Level in Brain Correlates with Memory Impairment in F1 and F2 Generation Mice: Improvement with Quercetin. Environ. Sci. Pollut. Res. 2019, 26, 9632–9639. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wei, W.; Li, Y.; Huang, J.; Ci, X. Hesperetin Relieves Cisplatin-Induced Acute Kidney Injury by Mitigating Oxidative Stress, Inflammation and Apoptosis. Chem. Biol. Interact. 2019, 308, 269–278. [Google Scholar] [CrossRef]
- Khan, A.; Ikram, M.; Hahm, J.R.; Kim, M.O. Antioxidant and Anti-Inflammatory Effects of Citrus Flavonoid Hesperetin: Special Focus on Neurological Disorders. Antioxidants 2020, 9, 609. [Google Scholar] [CrossRef]
- Shagirtha, K.; Bashir, N.; MiltonPrabu, S. Neuroprotective Efficacy of Hesperetin against Cadmium Induced Oxidative Stress in the Brain of Rats. Toxicol. Ind. Health 2017, 33, 454–468. [Google Scholar] [CrossRef]
- Lin, B.-W.; Gong, C.-C.; Song, H.-F.; Cui, Y.-Y. Effects of Anthocyanins on the Prevention and Treatment of Cancer. Br. J. Pharmacol. 2017, 174, 1226–1243. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and Anthocyanins: Colored Pigments as Food, Pharmaceutical Ingredients, and the Potential Health Benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef]
- Kowalczyk, E.; Kopff, A.; Fijałkowski, P.; Kopff, M.; Niedworok, J.; Błaszczyk, J.; Kedziora, J.; Tyślerowicz, P. Effect of Anthocyanins on Selected Biochemical Parameters in Rats Exposed to Cadmium. Acta Biochim. Pol. 2003, 50, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Guo, J.; Jiang, X.; Sun, J.; Tian, L.; Jiao, R.; Tang, Y.; Bai, W. Cyanidin-3-O-Glucoside Protects against Cadmium-Induced Dysfunction of Sex Hormone Secretion via the Regulation of Hypothalamus-Pituitary-Gonadal Axis in Male Pubertal Mice. Food Chem. Toxicol. 2019, 129, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.A.; Subhan, N.; Rahman, M.M.; Uddin, S.J.; Reza, H.M.; Sarker, S.D. Effect of Citrus Flavonoids, Naringin and Naringenin, on Metabolic Syndrome and Their Mechanisms of Action. Adv. Nutr. 2014, 5, 404–417. [Google Scholar] [CrossRef]
- Rathi, V.K.; Das, S.; Parampalli Raghavendra, A.; Rao, B.S.S. Naringin Abates Adverse Effects of Cadmium-Mediated Hepatotoxicity: An Experimental Study Using HepG2 Cells. J. Biochem. Mol. Toxicol. 2017, 31, e21915. [Google Scholar] [CrossRef]
- Yılmaz, D.; Aydemir, N.C.; Vatan, Ö.; Tüzün, E.; Bilaloğlu, R. Influence of Naringin on Cadmium-Induced Genomic Damage in Human Lymphocytes in vitro. Toxicol. Ind. Health 2012, 28, 114–121. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, B.M.; Kim, H.S. Potential Protective Roles of Curcumin against Cadmium-Induced Toxicity and Oxidative Stress. J. Toxicol. Environ. Health Part B 2021, 24, 95–118. [Google Scholar] [CrossRef] [PubMed]
- Eybl, V.; Kotyzová, D.; Lešetický, L.; Bludovská, M.; Koutenský, J. The Influence of Curcumin and Manganese Complex of Curcumin on Cadmium-Induced Oxidative Damage and Trace Elements Status in Tissues of Mice. J. Appl. Toxicol. 2006, 26, 207–212. [Google Scholar] [CrossRef]
- Momeni, H.; Eskandari, N. Curcumin Protects the Testis against Cadmium-Induced Histopathological Damages and Oxidative Stress in Mice. Hum. Exp. Toxicol. 2020, 39, 653–661. [Google Scholar] [CrossRef]
- Suntres, Z.E.; Coccimiglio, J.; Alipour, M. The Bioactivity and Toxicological Actions of Carvacrol. Crit. Rev. Food Sci. Nutr. 2015, 55, 304–318. [Google Scholar] [CrossRef]
- Banik, S.; Akter, M.; Corpus Bondad, S.E.; Saito, T.; Hosokawa, T.; Kurasaki, M. Carvacrol Inhibits Cadmium Toxicity through Combating against Caspase Dependent/Independent Apoptosis in PC12 cells. Food Chem. Toxicol. 2019, 134, 110835. [Google Scholar] [CrossRef]
- Sanjeev, S.; Bidanchi, R.M.; Murthy, M.K.; Gurusubramanian, G.; Roy, V.K. Influence of Ferulic Acid Consumption in Ameliorating the Cadmium-Induced Liver and Renal Oxidative Damage in Rats. Environ. Sci. Pollut. Res. 2019, 26, 20631–20653. [Google Scholar] [CrossRef] [PubMed]
- Ullah, R.; Ikram, M.; Park, T.J.; Ahmad, R.; Saeed, K.; Alam, S.I.; Rehman, I.U.; Khan, A.; Khan, I.; Jo, M.G.; et al. Vanillic Acid, a Bioactive Phenolic Compound, Counteracts LPS-Induced Neurotoxicity by Regulating c-Jun N-Terminal Kinase in Mouse Brain. Int. J. Mol. Sci. 2021, 22, 361. [Google Scholar] [CrossRef] [PubMed]
- Salau, V.F.; Erukainure, O.L.; Ibeji, C.U.; Olasehinde, T.A.; Koorbanally, N.A.; Islam, M.S. Vanillin and Vanillic Acid Modulate Antioxidant Defense System via Amelioration of Metabolic Complications Linked to Fe2+-Induced Brain Tissues Damage. Metab. Brain Dis. 2020, 35, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Punvittayagul, C.; Chariyakornkul, A.; Jarukamjorn, K.; Wongpoomchai, R. Protective Role of Vanillic Acid against Diethylnitrosamine- and 1,2-Dimethylhydrazine-Induced Hepatocarcinogenesis in Rats. Molecules 2021, 26, 2718. [Google Scholar] [CrossRef]
- Bhuyan, M.H.M.B.; Parvin, K.; Mohsin, S.M.; Mahmud, J.A.; Hasanuzzaman, M.; Fujita, M. Modulation of Cadmium Tolerance in Rice: Insight into Vanillic Acid-Induced Upregulation of Antioxidant Defense and Glyoxalase Systems. Plants 2020, 9, 188. [Google Scholar] [CrossRef]
- Guo, B.; Liu, C.; Liang, Y.; Li, N.; Fu, Q. Salicylic Acid Signals Plant Defence against Cadmium Toxicity. Int. J. Mol. Sci. 2019, 20, 2960. [Google Scholar] [CrossRef]
- Li, Q.; Wang, G.; Wang, Y.; Yang, D.; Guan, C.; Ji, J. Foliar Application of Salicylic Acid Alleviate the Cadmium Toxicity by Modulation the Reactive Oxygen Species in Potato. Ecotoxicol. Environ. Saf. 2019, 172, 317–325. [Google Scholar] [CrossRef]
- Sah, S.K.; Reddy, K.R.; Li, J. Abscisic Acid and Abiotic Stress Tolerance in Crop Plants. Front. Plant Sci. 2016, 7, 571. [Google Scholar] [CrossRef]
- Dawuda, M.M.; Liao, W.; Hu, L.; Yu, J.; Xie, J.; Calderón-Urrea, A.; Wu, Y.; Tang, Z. Foliar Application of Abscisic Acid Mitigates Cadmium Stress and Increases Food Safety of Cadmium-Sensitive Lettuce (Lactuca sativa L.) Genotype. PeerJ 2020, 8, e9270. [Google Scholar] [CrossRef]
- Reiter, R.J.; Rosales-Corral, S.; Tan, D.X.; Jou, M.J.; Galano, A.; Xu, B. Melatonin as a Mitochondria-Targeted Antioxidant: One of Evolution’s Best Ideas. Cell. Mol. Life Sci. 2017, 74, 3863–3881. [Google Scholar] [CrossRef]
- Sami, A.; Shah, F.A.; Abdullah, M.; Zhou, X.; Yan, Y.; Zhu, Z.; Zhou, K. Melatonin Mitigates Cadmium and Aluminium Toxicity through Modulation of Antioxidant Potential in Brassica napus L. Plant Biol. 2020, 22, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Tousi, S.; Zoufan, P.; Ghahfarrokhie, A.R. Alleviation of Cadmium-Induced Phytotoxicity and Growth Improvement by Exogenous Melatonin Pretreatment in Mallow (Malva parviflora) Plants. Ecotoxicol. Environ. Saf. 2020, 206, 111403. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhuang, X.; Zhou, J.; Sun, L.; Wan, H.; Li, H.; Lyu, D. Exogenous Melatonin Alleviates Cadmium Uptake and Toxicity in Apple Rootstocks. Tree Physiol. 2020, 40, 746–761. [Google Scholar] [CrossRef]
- Ni, J.; Wang, Q.; Shah, F.A.; Liu, W.; Wang, D.; Huang, S.; Fu, S.; Wu, L. Exogenous Melatonin Confers Cadmium Tolerance by Counterbalancing the Hydrogen Peroxide Homeostasis in Wheat Seedlings. Molecules 2018, 23, 799. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Song, J.; Liu, Z.; Liu, Z.; Cui, J. Melatonin Alleviates Cadmium Toxicity by Reducing Nitric Oxide Accumulation and IRT1 Expression in Chinese Cabbage Seedlings. Environ. Sci. Pollut. Res. 2021, 28, 15394–15405. [Google Scholar] [CrossRef] [PubMed]
- Nabaei, M.; Amooaghaie, R. Melatonin and Nitric Oxide Enhance Cadmium Tolerance and Phytoremediation Efficiency in Catharanthus roseus (L.) G. Don. Environ. Sci. Pollut. Res. 2020, 27, 6981–6994. [Google Scholar] [CrossRef]
- Hasan, M.K.; Ahammed, G.J.; Sun, S.; Li, M.; Yin, H.; Zhou, J. Melatonin Inhibits Cadmium Translocation and Enhances Plant Tolerance by Regulating Sulfur Uptake and Assimilation in Solanum lycopersicum L. J. Agric. Food Chem. 2019, 67, 10563–10576. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, Y.; Qian, J.; Si, W.; Tan, Q.; Xu, J.; Zhao, Y. Melatonin Enhances the Cadmium Tolerance of Mushrooms through Antioxidant-Related Metabolites and Enzymes. Food Chem. 2020, 330, 127263. [Google Scholar] [CrossRef]
- Yang, Q.; Zhu, J.; Luo, X.; Li, F.; Cong, L.; Wang, Y.; Sun, Y. Melatonin Attenuates Cadmium-Induced Ovulatory Dysfunction by Suppressing Endoplasmic Reticulum Stress and Cell Apoptosis. Reprod. Biol. Endocrinol. 2019, 17, 61. [Google Scholar] [CrossRef]
- Ataei, N.; Aghaei, M.; Panjehpour, M. The Protective Role of Melatonin in Cadmium-Induced Proliferation of Ovarian Cancer Cells. Res. Pharm. Sci. 2018, 13, 159–167. [Google Scholar] [CrossRef]
- Knani, L.; Bartolini, D.; Kechiche, S.; Tortoioli, C.; Murdolo, G.; Moretti, M.; Messaoudi, I.; Reiter, R.J.; Galli, F. Melatonin Prevents Cadmium-Induced Bone Damage: First Evidence on an Improved Osteogenic/Adipogenic Differentiation Balance of Mesenchymal Stem Cells as Underlying Mechanism. J. Pineal Res. 2019, 67, e12597. [Google Scholar] [CrossRef] [PubMed]
- Lamtai, M.; Azirar, S.; Zghari, O.; Ouakki, S.; El Hessni, A.; Mesfioui, A.; Ouichou, A. Melatonin Ameliorates Cadmium-Induced Affective and Cognitive Impairments and Hippocampal Oxidative Stress in Rat. Biol. Trace Elem. Res. 2021, 199, 1445–1455. [Google Scholar] [CrossRef]
- Cao, Z.; Fang, Y.; Lu, Y.; Tan, D.; Du, C.; Li, Y.; Ma, Q.; Yu, J.; Chen, M.; Zhou, C.; et al. Melatonin Alleviates Cadmium-Induced Liver Injury by Inhibiting the TXNIP-NLRP3 Inflammasome. J. Pineal Res. 2017, 62, e12389. [Google Scholar] [CrossRef]
- Winkler, J.; Ghosh, S. Therapeutic Potential of Fulvic Acid in Chronic Inflammatory Diseases and Diabetes. J. Diabetes Res. 2018, 2018, e5391014. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.-W.; Zeng, G.-M.; Gong, J.-L.; Liang, J.; Xu, P.; Zhang, C.; Huang, B.-B. Impact of Humic/Fulvic Acid on the Removal of Heavy Metals from Aqueous Solutions Using Nanomaterials: A Review. Sci. Total Environ. 2014, 468–469, 1014–1027. [Google Scholar] [CrossRef] [PubMed]
- Van Rensburg, C.E.J. The Antiinflammatory Properties of Humic Substances: A Mini Review. Phytother. Res. 2015, 29, 791–795. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, R.; Zheng, J.; Shen, Z.; Xu, X. Exogenous Foliar Application of Fulvic Acid Alleviate Cadmium Toxicity in Lettuce (Lactuca sativa L.). Ecotoxicol. Environ. Saf. 2019, 167, 10–19. [Google Scholar] [CrossRef]
- Wang, X.; Wang, T.; Pan, T.; Huang, M.; Ren, W.; Xu, G.; Amin, H.K.; Kassab, R.B.; Abdel Moneim, A.E. Senna Alexandrina Extract Supplementation Reverses Hepatic Oxidative, Inflammatory, and Apoptotic Effects of Cadmium Chloride Administration in Rats. Environ. Sci. Pollut. Res. 2020, 27, 5981–5992. [Google Scholar] [CrossRef]
- Seif, M.M.; Madboli, A.-N.; Marrez, D.A.; Aboulthana, W.M.K. Hepato-Renal Protective Effects of Egyptian Purslane Extract against Experimental Cadmium Toxicity in Rats with Special Emphasis on the Functional and Histopathological Changes. Toxicol. Rep. 2019, 6, 625–631. [Google Scholar] [CrossRef]
- Mężyńska, M.; Brzóska, M.M.; Rogalska, J.; Piłat-Marcinkiewicz, B. Extract from Aronia melanocarpa L. Berries Prevents Cadmium-Induced Oxidative Stress in the Liver: A Study in A Rat Model of Low-Level and Moderate Lifetime Human Exposure to This Toxic Metal. Nutrients 2019, 11, 21. [Google Scholar] [CrossRef]
- Kozłowska, M.; Brzóska, M.M.; Rogalska, J.; Galicka, A. The Impact of a Polyphenol-Rich Extract from the Berries of Aronia melanocarpa L. on Collagen Metabolism in the Liver: A Study in an in vivo Model of Human Environmental Exposure to Cadmium. Nutrients 2020, 12, 2766. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowski, A.; Onopiuk, B.M.; Car, H.; Onopiuk, P.; Dąbrowska, Z.N.; Rogalska, J.; Brzóska, M.M.; Dąbrowska, E. Beneficial Impact of an Extract from the Berries of Aronia melanocarpa L. on the Oxidative-Reductive Status of the Submandibular Gland of Rats Exposed to Cadmium. Antioxidants 2020, 9, 185. [Google Scholar] [CrossRef] [PubMed]
- Borowska, S.; Brzóska, M.M.; Gałażyn-Sidorczuk, M.; Rogalska, J. Effect of an Extract from Aronia melanocarpa L. Berries on the Body Status of Zinc and Copper under Chronic Exposure to Cadmium: An in vivo Experimental Study. Nutrients 2017, 9, 1374. [Google Scholar] [CrossRef]
- Dkhil, M.A.; Al-Quraishy, S.; Diab, M.M.S.; Othman, M.S.; Aref, A.M.; Abdel Moneim, A.E. The Potential Protective Role of Physalis peruviana L. Fruit in Cadmium-Induced Hepatotoxicity and Nephrotoxicity. Food Chem. Toxicol. 2014, 74, 98–106. [Google Scholar] [CrossRef]
- Silva, M.J.D.; Vilegas, W.; da Silva, M.A.; de Moura, C.F.G.; Ribeiro, F.A.P.; da Silva, V.H.P.; Ribeiro, D.A. Mimosa (Mimosa caesalpiniifolia) Prevents Oxidative DNA Damage Induced by Cadmium Exposure in Wistar Rats. Toxicol. Mech. Methods 2014, 24, 567–574. [Google Scholar] [CrossRef]
- Aly, F.M.; Kotb, A.M.; Hammad, S. Effects of Spirulina platensis on DNA Damage and Chromosomal Aberration against Cadmium Chloride-Induced Genotoxicity in Rats. Environ. Sci. Pollut. Res. 2018, 25, 10829–10836. [Google Scholar] [CrossRef] [PubMed]
- Claudio, S.R.; Gollucke, A.P.B.; Yamamura, H.; Morais, D.R.; Bataglion, G.A.; Eberlin, M.N.; Peres, R.C.; Oshima, C.T.F.; Ribeiro, D.A. Purple Carrot Extract Protects against Cadmium Intoxication in Multiple Organs of Rats: Genotoxicity, Oxidative Stress and Tissue Morphology Analyses. J. Trace Elem. Med. Biol. 2016, 33, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Morsi, A.A.; Shawky, L.M.; Bana, E.A.E. The Potential Gonadoprotective Effects of Grape Seed Extract against the Histopathological Alterations Elicited in an Animal Model of Cadmium-Induced Testicular Toxicity. Folia Morphol. 2020, 79, 767–776. [Google Scholar] [CrossRef]
- Alkhedaide, A.; Alshehri, Z.S.; Sabry, A.; Abdel-Ghaffar, T.; Soliman, M.M.; Attia, H. Protective Effect of Grape Seed Extract against Cadmium-Induced Testicular Dysfunction. Mol. Med. Rep. 2016, 13, 3101–3109. [Google Scholar] [CrossRef]
- Sönmez, M.F.; Tascioglu, S. Protective Effects of Grape Seed Extract on Cadmium-Induced Testicular Damage, Apoptosis, and Endothelial Nitric Oxide Synthases Expression in Rats. Toxicol. Ind. Health 2016, 32, 1486–1494. [Google Scholar] [CrossRef]
- Bashir, N.; Shagirtha, K.; Manoharan, V.; Miltonprabu, S. The Molecular and Biochemical Insight View of Grape Seed Proanthocyanidins in Ameliorating Cadmium-Induced Testes-Toxicity in Rat Model: Implication of PI3K/Akt/Nrf-2 Signaling. Biosci. Rep. 2019, 39, BSR20180515. [Google Scholar] [CrossRef] [PubMed]
- El-Tarras, A.E.-S.; Attia, H.F.; Soliman, M.M.; El Awady, M.A.; Amin, A.A. Neuroprotective Effect of Grape Seed Extract against Cadmium Toxicity in Male Albino Rats. Int. J. Immunopathol. Pharm. 2016, 29, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Bashir, N.; Manoharan, V.; Miltonprabu, S. Grape Seed Proanthocyanidins Protects against Cadmium Induced Oxidative Pancreatitis in Rats by Attenuating Oxidative Stress, Inflammation and Apoptosis via Nrf-2/HO-1 Signaling. J. Nutr. Biochem. 2016, 32, 128–141. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Chen, Q.; Chen, J.; Liu, D. Potential Ameliorative Effects of Grape Seed-Derived Polyphenols against Cadmium Induced Prostatic Deficits. Biomed. Pharmacother. 2017, 91, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhang, R.; Li, W.; Niu, Y.; Guo, H.; Liu, X.; Hou, Y.; Zhao, L. The Protective Effect of Grape Seed Procyanidin Extract against Cadmium-Induced Renal Oxidative Damage in Mice. Environ. Toxicol. Pharmacol. 2013, 36, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Hou, F.; Xiao, M.; Li, J.; Cook, D.W.; Zeng, W.; Zhang, C.; Mi, Y. Ameliorative Effect of Grape Seed Proanthocyanidin Extract on Cadmium-Induced Meiosis Inhibition During Oogenesis in Chicken Embryos. Anat. Rec. 2016, 299, 450–460. [Google Scholar] [CrossRef]

| Substances | Model | Dose of Substances | Dose of Cd | Detrimental Response to Cd Exposure and the Curative Effect of the Substance | Ref. |
|---|---|---|---|---|---|
| Vitamin C | Zea mays L. | 0.1–0.5 mM (4 weeks foliar application) | 50 mg kg−1 soil | Plant growth, photosynthesis, protein content, MDA content, H2O2 accumulation, CAT, SOD, GR activities, grains Cd uptake | Zhang et al., 2019 [51] |
| Brassica napus L. | 250 and 500 mg kg−1 (single foliar application) | 10 μM (equal dose) | Ascorbate—glutathione cycle, ROS content | Jung et al., 2020 [53] | |
| Triticum aestivum L. | 1 mM (seedlings preincubation) | 100 μM (equal dose) | AsA-GSH-NADPH cycle homeostasis, ROS generation | Wang et al., 2017 [52] | |
| Schizosaccharomyces pombe L. | 10 mM (cell treatment for 30 min) | 10–400 μM (equal dose) | Ionome balance, CAT and SOD activity, MDA content, cell morphology | Navratilova et al., 2021 [54] | |
| Rabbit | 150 mg kg−1 b.w. (oral administration for 28 days) | 1.5 mg kg−1 b.w. (oral administration for 28 days) | Cd accumulation, blood biochemical parameters | Mumtaz et al., 2019 [55]; Ali et al., 2020 [56] | |
| Mouse | 50 and 100 mg kg−1 b.w. | 100 mg L−1 (in drinking water for 8 weeks) | Vascular disfunction, hypertension, oxidative damage | Donpunha et al., 2011 [57] | |
| Vitamin E | Tilapia | 50 mg kg−1 diet for 12 weeks | 20 and 50 mg kg−1 diet | Serum creatine, ALT and AST activity, residual Cd content | Ayyat et al., 2017 [61] |
| Ctenopharyngodon idellus | 20 IU kg−1 (single dose at day 4 after Cd injection) | 20 μM kg−1 single i.p. injection | Hepatocytes effect, MDA content, hepatocytes apoptosis and apoptosis-related gene, liver morphology, SOD, CAT, GPx activity | Duan et al., 2018 [62]; Huang et al., 2020 [63] | |
| Mice | 100 mg kg−1 or 50 IU kg−1 | 5 mg kg−1 b.w. for 28 days, or 30 ppm for 7 weeks in drinking water | Liver weight and Cd content, ALT and AST content, hepatocellular necrosis, rupture in hepatocytes, inhibition of oxidative stress, antioxidant enzymes and expression of Nrf2 genes | Fang et al., 2021 [64]; Al-Attar 2011 [65] | |
| Rat | 20 mg kg−1 b.w., 100 mg kg−1 b.w., 250 mg kg−1 b.w., or 40 and 400 mg kg−1 b.w., and 100 UI kg−1) | 20 mg kg−1 b.w. for 30 days, and 5 mg kg−1 b.w. for 28 days in the daily gavage; or i.p. 2 mg kg−1 b.w. for 8 or 28 days, or in drinking water 50 ppm for 20 weeks) | CAT, SOD, GR, GPx and GST, LPX, body weight effect, serum urea and creatinine, renal histological alterations, angiotensin converting enzyme, blood pressure, heart rate | Adi et al., 2016 [66], Fang et al., 2021 [67], Karabulut-Bulan et al., 2008 [68], Choi and Rhee 2003 [69] |
| Substances | Model | Dose of Substances | Dose of Cd | Detrimental Response to Cd Exposure and the Curative Effect of the Substance | Ref. |
|---|---|---|---|---|---|
| Selenium | Chicken | 2 mg kg−1 or 0.5 mg kg−1 Se in the diet | 150 mg kg−1 for 90 or 120 days | Ionome alterations, amino acid content profile, MDA, DNA and protein crosslink, protein carbonyl content, CYP450, b5, GSH, AND, ERND, AH, cytochrome C reductase, CAT, SOD, GPX, caspase8, MAPK signaling pathway | Qu et al., 2020 [79], Cong et al., 2019 [81], Wang et al., 2020 [82] |
| Avian leghorn male hepatoma cells | 1.25 or 2.5 μM | 2.5 μM (equal dose) for 24 h | Ca2+ homeostasis, calmodulin, alteration of the cadherin/calreticulin cycle, ER stress, autophagy leading to cell death | Zhang et al., 2020 [80] | |
| porcine jejunal epithelial cells | 0.4 ppm in the cell culture medium for 24 h | 0.5–1 ppm in the media for 24 h | Cell viability, DNA damage, cell death | Lynch et al., 2017 [83] | |
| Festuca pratensis L. | 0.1 mg L−1 dissolved in water | 30 mg L−1 for 7 days | Plant height, root length, MDA content, electrolyte leakage, antioxidant enzyme activities, photosynthetic efficiency, chlorophyll, and soluble protein content | Li et al., 2020 [84] | |
| Raphanus sativus L. | 2–8 mg L−1 for 30 days | 5–10 mg L−1 for 30 days | Cd uptake, chlorophyll biosynthesis, micronutrients content, enzymatic antioxidant protective system | Auobi Amirabad et al., 2020 [85] | |
| Triticum aestivum L. | 10–40 mg L−1 (foliar application for 6 months) | 0.3 mg kg−1 soil | Photosynthesis, plant biomass production, antioxidant enzymes activity | Wu et al., 2020 [86] | |
| Zinc | SH-SY5Y catecholaminergic neuroblastoma cells | 50 μM (equal dose for 24 h) | 10 μM (equal dose for 24 h) | Attenuate the Cd-induced neurotoxicity | Branca et al., 2018 [92] |
| MDBK epithelial cells | 10 and 50 μM (equal dose for 24 h) | 10 and 50 μM (equal dose for 24 h) | Cd uptake, Cd-mediated apoptotic cell death, mitochondrial damage and oxidative stress | Zhang et al., 2014 [93] | |
| Saccharomyces cerevisiae | 40–640 μM (equal dose for 2 h) | 40–320 μM (equal dose for 2 h) | Genes expression alternation, ionome homeostasis and mitochondrial membrane potential, sulfur and GSH metabolism, ribosomal proteins, S-containing amino acids, S-rich proteins and antioxidant enzymes | Pan et al., 2017 [94] | |
| Rats | 60 mg L−1 in drinking water during gestation and lactation | 50 mg L−1 in drinking water | Hippocampal volume, CA1, CA3 pyramidal cell layer and the dentate gyrus, SOD, metallothionein level, prenatal Zn metabolism | Mimouna et al., 2018 [95], Chemek et al., 2016 [98] | |
| Sinopotamon henanense | 0.1–1 mg L−1 for 14 days | 0.05–0.5 mg L−1 for 14 days | Sperm count and motility, histological damage, morphological lesions, relative testis weight, SOD, CAT and GPx activity, MDA | Liu et al., 2020 [96], Babaknejad et al., 2018 [97] | |
| Spodoptera exigua | 100–400 μg g−1 of food for 135 generations | 44 μg g−1 of food for 135 generations | DNA damage, ADP/ATP ratio, ATP and HSP70 concentrations, growth rate | Tarnawska et al., 2019 [99] | |
| Triticum aestivum L. | 0.2% foliar spray, or 41.2 mg kg−1 soil | naturally contaminated water | Influx transporter gene TaNramp5, efflux transporters TaTM20 and TaHMA3, leaf TaHMA2, root TaLCT1 gene | Zhou et al., 2020 [101] | |
| Spinaciae oleracea | 100 mg L−1 water for 2 weeks | 1 mg L−1 water for 2 weeks | Cu and Fe content | Sharifan et al., 2020 [102] | |
| Petroselinum sativum | 100 mg L−1 water for 2 weeks | 1 mg L−1 water for 2 weeks | Cu and Fe content | Sharifan et al., 2020 [102] | |
| Coriandrum sativum L. | 100 mg L−1 water for 2 weeks | 1 mg L−1 water for 2 weeks | Cu and Fe content | Sharifan et al., 2020 [102] | |
| Matricaria chamomilla L. | 0.1–5 mM in hydroponic solution for 2 weeks | 120 and 180 μM in hydroponic solution for 2 weeks | ROS accumulation, MDA, antioxidant enzyme, reduced influx of Cd, biomass accumulation | Farzadfar et al., 2013 [105] | |
| Arabidopsis thaliana L. | 3 mM (seedlings exposure for 5 days) | 50 μM (seedlings exposure for 5 days) | Oxidative stress, cell H2O2 content, lipid peroxidation, auxin content and distribution, root length | Li et al., 2016 [106] | |
| Oryza sativa L. | 2.5 and 5 mg kg−1 soil (3 days), and 0.05–5 mM in BMS solution (till ripening stage) | 50 and 100 mg kg−1 soil (3 days), and 50 μM in BMS solution (till ripening stage) | Cd intake, oxidative stress, SOD, CAT and POD activity, photosynthesis, carotenoids, proline and protein content, grain yield and yield components, OsNRAMP1 and OsNRAMP5, OsHMA2, OsHMA3 transporter genes | Zhang et al., 2020 [107], Kanu et al., 2019 [108] | |
| Calcium | Cicer arietinum L. | 100 mM (seeds exposure for 6 days) | 200 μM (seeds exposure for 6 days) | Oxidative stress, thioredoxin and thioredoxin reductase activities, SOD, CAT and APX | Sakouhi et al., 2021 [109] |
| Hypogymnia physodes (L.) Nyl. | 100 and 1000 μM in water for 24 h | 10 and 100 μM in water for 24 h | ROS and MDA, thiol, glutathione, and ascorbic acid levels | Kováčik et al., 2020 [110] | |
| Saccharomyces cerevisiae L. | 10 mM (in culture media for 60 min or 2–3 days) | 50–500 μM (in culture media for 60 min or 2–3 days) | Ca release from vacuole, Yvc1p transporter, Cch1p/Mid1p channel | Ruta et al., 2014 [111] | |
| Bacillus sp. 98 | 5–30 mM (in culture media) | 0.2–4 mM (in culture media) | Intracellular NO production, NO synthase, NO dioxygenase, Fe uptake | Wu et al., 2021 [112] | |
| Oncorhynchus mykiss | 30 and 60 mg g−1 food, or 20–60 mg kg−1 in diet | 50 μg L−1 in water for 7 days, or 3 μg L−1 in water, or 500 mg kg−1 diet for 28 days | Cd uptake, Cd transport, morphology | Baldisserotto et al., 2004 [113], Franklin et al., 2005 [114] | |
| Oreochromis mossambicus | 0.2–0.8 mM freshwater concentration | 10 μg L−1 freshwater, or 10 μg per fish for 35 days | Cd transport, Cd accumulation in gill and gut, morphology | Pratap and Wendelaar Bonga 2007 [115] | |
| Rat | 1–6% in milk or 0.4% solution or 100 mg kg−1 orally administered | 0.5 mg kg−1 b.w. for 10 days 1–50 mg kg−1 diet for 90 days or 44 mg L−1 in drinking water for 4 weeks | Cd content, ionome, bone formation, expression of osteogenic gene markers, fibroblast growth factor 23/Klotho-associated gene expression Cd-induced hepatotoxicity, MDA, H2O2, protein carbonyls, interleukin (IL) 1β, IL-6, IL17A, tumor necrosis factor-α, anti-inflammatory IL-10, IL-22 markers, GSH, GPx, CAT activities | Sarić et al., 2002 [116], Huang et al., 2019 [117], El-Boshy et al., 2020 [118] | |
| Mice | 100 mg kg−1 diet | 10–1000 ppm in the diet for 28 days | Cd-induced nephrotoxicity, glomerular atrophy, renal proximal tubule injury, MDA, urine protein, KIM-1, apoptosi | Gu et al., 2020 [119] | |
| Silicon | Boehmeria nivea (L.) Gaud | 1 mM in hydrophonic solution for 7 days | 5 mg L−1 in hydrophonic solution for 7 days | Cd translocation, SOD, CAT, POD, APX, ROS, MDA, H2O2, glutathione, ascorbate, vitamin E | Tang et al., 2015 [120] |
| Oryza sativa L. | 5–20 mg L−1 foliar application | 0.84 mg kg−1 soil | Cd content, phytic acid | Hussain et al., 2020 [121] | |
| Manganese | Phytolacca acinosa Roxb | 0.5–12 mM in hydrophonic solution for 17 days | 50–200 μM in hydrophonic solution for 17 days | Mn/Cd ratio, lipid peroxidation and plant water-loss, photosynthesis | Liu et al., 2013 [122] |
| Mice | 20 mg kg−1 b.w. single i.p. injection | 7 mg kg−1 b.w. single s.c. injection | Cd content, GSH-Px activity, lipid peroxidation, GSH, CAT | Eybl and Kotyzová 2010 [123] | |
| Magnesium | Rat blood plasma | 3 and 50 mg kg−1 b.w. i.p. or oral exposure 10 min or 1 h prior to Cd exposure | 30 mg kg−1 b.w. single dose administered by orogastric tube or 1.5 mg kg−1 b.w. i.p. injection of single dose | SOD activity, superoxide anion, total oxidative status, MDA and oxidation protein content | Buha et al., 2012 [125] |
| Rat | 0.5 or 1.5 mg kg−1 b.w. i.p. injection for 21 days | 1 mg kg−1 b.w. i.p. injection for 21 days | Cd-induced nephrotoxicity, MDA, serum sodium, potassium, and urea levels, creatinine, and protein levels | Babaknejad et al., 2016 [126] | |
| Isolated perfused rat liver model system | 1.2 mM for 90 min | 15 μM for 90 min | Glutathione level, enhanced MDA content and aminotransferase activity | Ghaffarian-Bahraman et al., 2014 [127] |
| Substances | Model | Dose of Substances | Dose of Cd | Detrimental Response to Cd Exposure and the Curative Effect of the Substance | Ref. |
|---|---|---|---|---|---|
| Rutin | Rat | 25–100 mg kg−1 b.w. (oral administration for 14 or 30 days) | 5 mg kg−1 b.w. (oral administration for 14 or 30 days) | Neurotoxicity and cognitive disturbance, ERK1/2 and JNK apoptotic pathways, PTEN derived regulation of mTOR survival pathway, ectonucleotidases, adenosine deaminase and MAO activities | Oboh et al., 2019 [130], Abdel-Aleem and Khaleel 2018 [131] |
| Chrysin | Mice | 2.5 and 5 mg kg−1 b.w. in drinking water for 30 days | 2 mg kg−1 b.w. in drinking water for 30 days | Hepatic stress, liver enzymes enhancement, morphological changes | Beyrami et al., 2020 [132] |
| Diosmin | Rat | 100 mg kg−1 b.w. in drinking water for 30 days | 200 ppm in drinking water for 30 days | Hepatotoxicity, liver enzyme, antioxidant parameters, histopathological parameters | Ağır and Eraslan 2019 [133] |
| Quercetin | Mice | 5–100 mg kg−1 b.w. by i.p. injection for 3 days | 0.4 mg kg−1 b.w. by i.p. injection for 3 days | Autophagosome formation, LC3-II/β-actin ratio, ROS, MDA content, antioxidant capacity | Yuan et al., 2016 [134] |
| Primary rat proximal tubular cells | 1 μg mL−1 for 12 h | 2.5 μM for 12 h | Autophagy marker proteins, TFEB-dependent recovery of lysosomal function, v-ATPases | Zhao et al., 2021 [136] | |
| Rat | 10 and 50 mg kg−1 b.w. by oral gavage for 12 weeks 50 mg kg−1 b.w. i.p. injection for 4 weeks | 4.89 mg kg−1 b.w. in drinking water for 12 weeks 2 mg kg−1 b.w. i.p. injection for 4 weeks | Nephrotoxicity, metabolic alterations, lipids, amino acids, and purine metabolism Decreased body and testicular weight, pathological changes in testes, oxidative stress, autophagy | Wang et al., 2020 [135] Guan et al., 2021 [137] | |
| Goat sperm | 10 μM for up to 12 h at 38.5 °C | 60 μM for up to 12 h at 38.5 °C | Oxidative stress, sperm motility, survival rates, membrane integrity, mitochondrial activity, altered embryo development | Mao et al., 2018 [138] | |
| Mice | 20–100 mg kg−1 b.w. day−1 for 1 week | 1.2 mg kg−1 b.w. day−1 for 1 week | Memory impairment of the F1-F2 generation, brain activity, expression of GST and CAT, Cd uptake | Halder et al., 2019 [139] | |
| Hesperetin | Rat | 40 mg kg−1 b.w. by oral administration | 3 mg kg−1 by s.c. injection for 21 days | Acetylcholinesterase, ROS, protein carbonylation, SOD, CAT and DPx, GSH, vitamin C, total sulfhydryl groups, apoptotic markers, mitochondrial dysfunction | Shagirtha et al., 2016 [142] |
| Anthocyanins | Rat | 10 mg kg−1 b.w. by stomach tube for 30 days | 4 μg kg−1 b.w. by stomach tube for 30 days | Cd accumulation in the liver and kidney | Kowalczyk et al., 2003 [145] |
| Mice | 500 mg kg−1 day−1 for up to 30 days | 5 mg kg−1 day−1 for up to 30 days | Levels of gonadotropins, luteinizing hormone and follicle stimulating hormone, Gnrh1 gene expression | Li et al., 2019 [146] | |
| Naringin | HepG2 cells | 5 μM for 24 h | 50 μM for 24 h | Alteration of antioxidant system, cytotoxicity, redox homeostasis, mitochondrial membrane potential, apoptosis, SOD, GST, CAT activities, lipid peroxidation | Rathi et al., 2017 [148] |
| Human lymphocytes | 1–2 mg mL−1 for 24 h | 20 and 40 μM for 24 h | Chromosomal aberrations, ROS, antioxidant metabolism | Yılmaz et al., 2012 [149] | |
| Curcumin | Mice | 0.14 mM kg−1 b.w. by gavage for 3 days, or 100 mg kg−1 b.w. by single i.p. injection | 33 μM kg−1 or 5 mg kg−1 b.w. by s.c. injection 1 or 24 h after curcumin treatment | Antioxidant enzymes activity, levels of total glutathione and thiol, MDA, H2O2, | Eybl et al., 2006 [151], Momeni et al., 2020 [152] |
| Carvacrol | PC12 cells | 100 μM for 48 h | 10 μM for 48 h | Growth retardation, glutathione and glutathione reductase, caspase 3, cytochrome c, apoptosis inducing factor, mTOR, protein kinase B, nuclear factor kappa-light-chain-enhancer of activated B cells, extracellular signal-regulated kinase-1, nuclear factor erythroid 2-related factor 2 | Banik et al., 2019 [154] |
| Ferulic acid | Rat | 50 mg kg−1 b.w. orally administered for 15 or 30 days | 10 mg kg−1 b.w. for 15 and 30 days by s.c. injection | Body weight and serum total protein contents, histopathological damage, AST, ALT, ALP and LDH, uric acid, urea, urea nitrogen, and creatinine content), lipid hydroperoxides, MDA, protein carbonyl content, total oxidant status, and oxidative stress index, total thiols, total antioxidant concentration, SOD, CAT, and GPx, GSH and total free sulfhydryl groups, TNF-α, COX-2, and HSP70 proteins | Sanjeev et al., 2019 [155] |
| Vanillic acid | Oryza sativa L. | 50 μM seedlings exposure for 72 h | 1 and 2 mM seedlings exposure for 72 h | Photosynthetic pigment, osmotic status, biomass accumulation, growth, ROS, ascorbate pool size, antioxidant, and glyoxalase systems, phytochelatin content | Bhuyan et al., 2020 [159] |
| Salicylic acid | Solanum tuberosum L. | 600 μM foliar spray exposure for 10 days | 200 μM foliar spray exposure for 10 days | RWC, antioxidant enzymatic mechanism pathway, chlorophyll, proline content, MDA, H2O2, and superoxide anion radicals | Li et al., 2019 [161] |
| Abscisic acid | Lactuca sativa L. | 10 μg L−1 sprayed on leaves | 100 μM in hydroponic nutrient solution | Oxidative stress, H2O2, MDA, photosynthesis, mineral nutrients content, plant biomass | Dawuda et al., 2020 [163] |
| Melatonin | Brassica napus L. Malva parviflora L. | 15, 50 or 100 µM in the nutrition solution | 25 µM for 5 days and 50 µM for 8 days | Photosynthesis, SOD, CAT, peroxidase, ascorbate peroxidase, proline, chlorophyll and anthocyanin content, MDA, H2O2 | Sami et al., 2020 [165], Tousi et al., 2020 [166] |
| Malus domestica L. | 100 µM for up to 20 days | 30 µM for up to 20 days | Cd translocation, antioxidant enzymes activity, root Cd uptake, and leaf Cd accumulation | He et al., 2020 [167] | |
| Brassica rapa subs. pekinensis | 100 µM sprayed of leaves once a day | 20 µM in the nutrient solution for 8 days | Nitric oxide, expression of the transporter gene IRT1, Cd absorption | Ni et al., 2018 [168] | |
| Catharanthus roseus (L.) G. Don | 100 μM foliar spray | 50, 100, 200 mg kg−1 soil for 30 days | Phytoremediation efficiency, shoot biomass and chlorophyll content, POD and CAT activities, electrolyte leakage | Nabaei and Amooaghaie 2020 [170] | |
| Solanum lycopersicum L. | 100 μM foliar spray for 15 days every 5th day | 100 μM for 15 days | Sulfur metabolism, caffeic acid O-methyltransferase (COMT) gene | Hasan et al., 2019 [171] | |
| Mushrooms | 50, 100, or 200 μM in the growth medium for 5 days | 2, 5, and 8 μM in the growth medium for 5 days | Amino acid and glutathione metabolism, oxidation-reduction processes, metal, and ROS | Gao et al., 2020 [172] | |
| Mice | 25 mg kg−1 for 14 days by i.p. injection | 5 mg kg−1 for 14 days by i.p. injection | Ovulation dysfunction and ovarian injury, pathohistological damage | Yang et al., 2019 [173] | |
| Mice | 10 mg kg−1 by i.p. injection for 3 days before Cd administration | 2 mg kg−1 by single i.p. injection | Hepatocellular damage, ALT/AST enzymes, antioxidant activity, thioredoxin-interacting protein, TXNIP-NLRP3 inflammasome pathway | Cao et al., 2017 [177] | |
| Ovarian cancer cells | 1 μM for 48 h in growth medium | 1–100 nM for 48 h in growth medium | Estradiol (E2)-derived proliferation | Ataei et al., 2018 [174] | |
| Rat | 3 mg L−1 in drinking water for 1 month | 50 mg L−1 in drinking water for 1 month | Mineral and organic components, Ca2+ level, bone damage and histological alterations | Knani et al., 2019 [175] | |
| Rat | 4 mg kg−1 30 min prior to Cd administration | 1 mg kg−1 by i.p. injection for 8 weeks | Memory and learning disabilities, NO and lipid peroxidation, CAT and SOD, Cd-induced neuronal loss | Lamtai et al., 2021 [176] | |
| Human adipose cells | 10 nmol L−1–50 μmol L−1 in growth medium for 4 to 72 h | 0.25 to 10 μmol L−1 in growth medium for 4 to 72 h | Osteogenic differentiation properties, adipogenic differentiation | Knani et al., 2019 [175] | |
| Fulvic acid | Lactuca sativa L. | 0.5 g L−1 in hydroponics for 2 weeks | 20 μM in hydroponics for 2 weeks | Nutrient elemental imbalance, pigment content, photosynthesis, photosystem PSII, ROS, antioxidant capacity | Wang et al., 2019 [181] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Požgajová, M.; Navrátilová, A.; Kovár, M. Curative Potential of Substances with Bioactive Properties to Alleviate Cd Toxicity: A Review. Int. J. Environ. Res. Public Health 2022, 19, 12380. https://doi.org/10.3390/ijerph191912380
Požgajová M, Navrátilová A, Kovár M. Curative Potential of Substances with Bioactive Properties to Alleviate Cd Toxicity: A Review. International Journal of Environmental Research and Public Health. 2022; 19(19):12380. https://doi.org/10.3390/ijerph191912380
Chicago/Turabian StylePožgajová, Miroslava, Alica Navrátilová, and Marek Kovár. 2022. "Curative Potential of Substances with Bioactive Properties to Alleviate Cd Toxicity: A Review" International Journal of Environmental Research and Public Health 19, no. 19: 12380. https://doi.org/10.3390/ijerph191912380
APA StylePožgajová, M., Navrátilová, A., & Kovár, M. (2022). Curative Potential of Substances with Bioactive Properties to Alleviate Cd Toxicity: A Review. International Journal of Environmental Research and Public Health, 19(19), 12380. https://doi.org/10.3390/ijerph191912380










