Metronidazole Degradation by UV and UV/H2O2 Advanced Oxidation Processes: Kinetics, Mechanisms, and Effects of Natural Water Matrices
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Experimental Design
2.2. Analytical Methods
2.3. Statistics Analysis
3. Results and Discussion
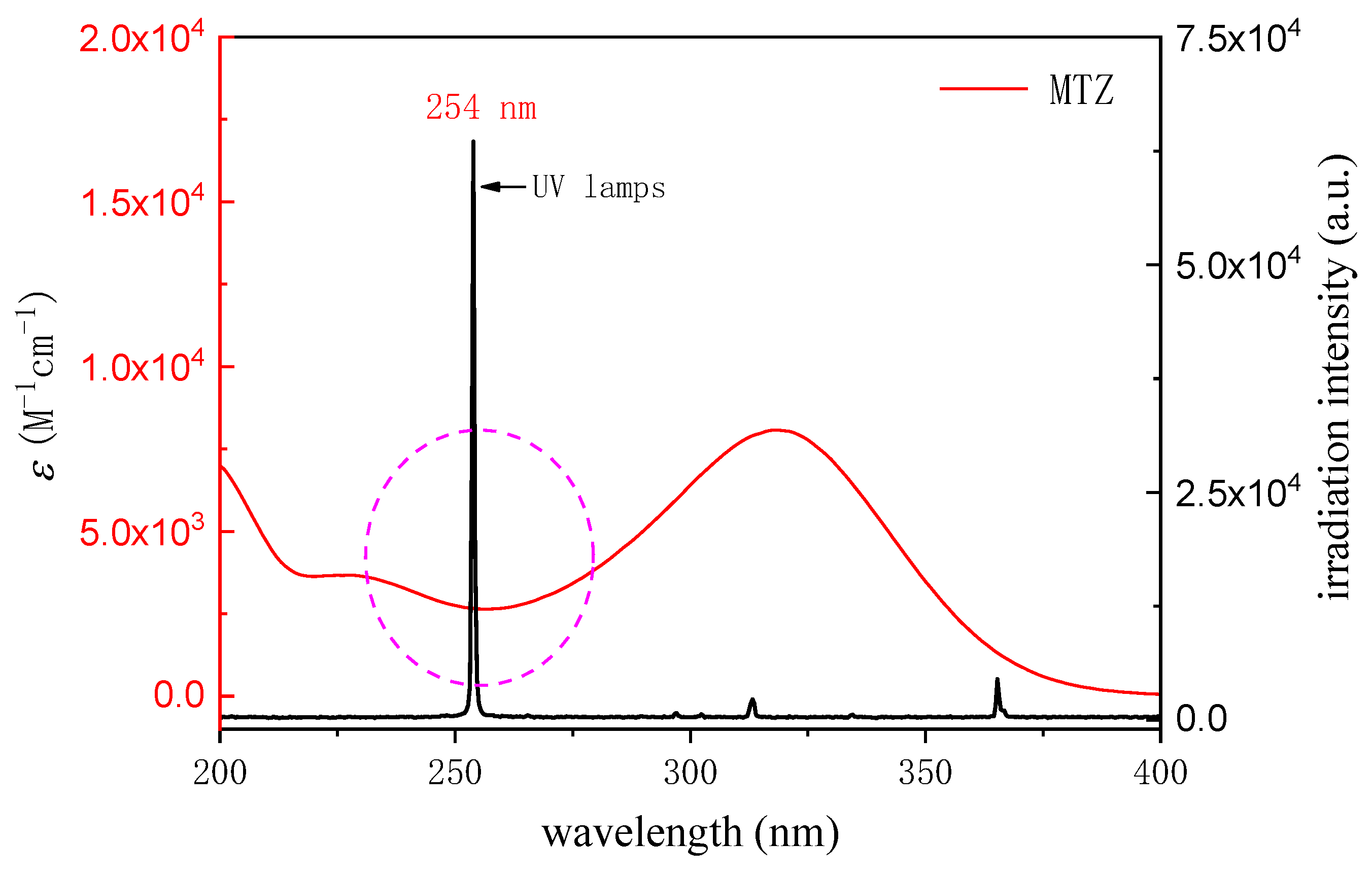
3.1. Effective Light Intensity and Optical Path of Photochemical Reaction System
3.1.1. Potassium Ferric Oxalate Chemical Photometer Method
- (1)
- Calculate the fitting equation of for UV irradiation time t, and obtain value.
- (2)
- Effective light intensity () can also be calculated by the following formula:
3.1.2. Hydrogen Peroxide Chemical Photometer Method
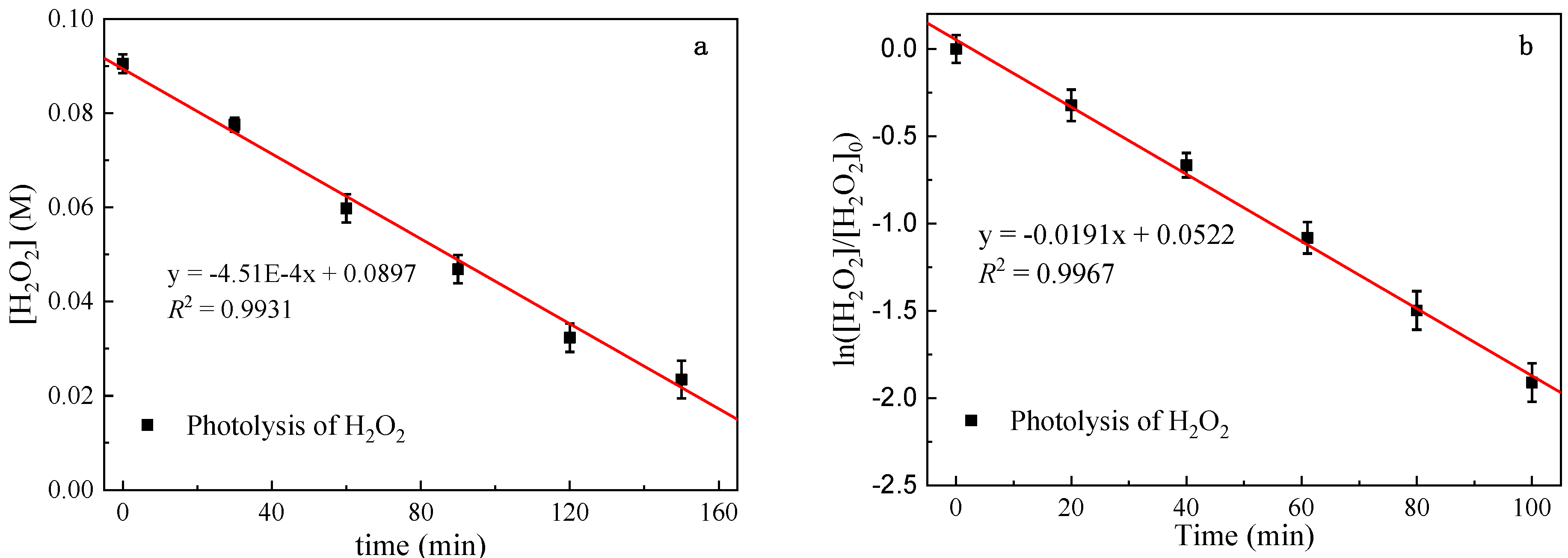
3.2. Direct Photolysis of Metronidazole
3.3. MTZ Degradation Kinetic in UV/H2O2 System
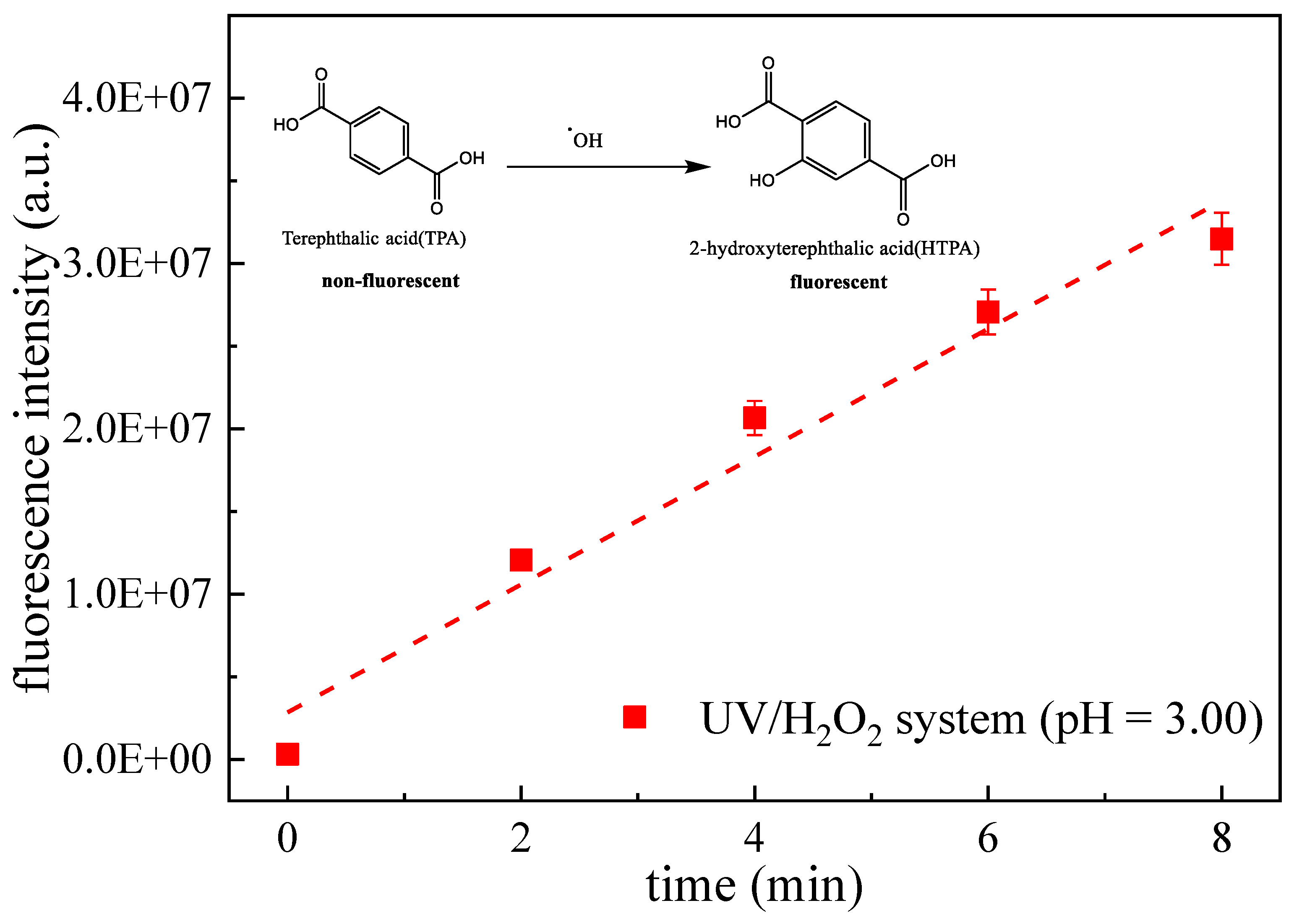
3.4. Competitive Kinetic
3.5. Pseudo First-Order Reaction Kinetic Model Based on Steady State Assumption
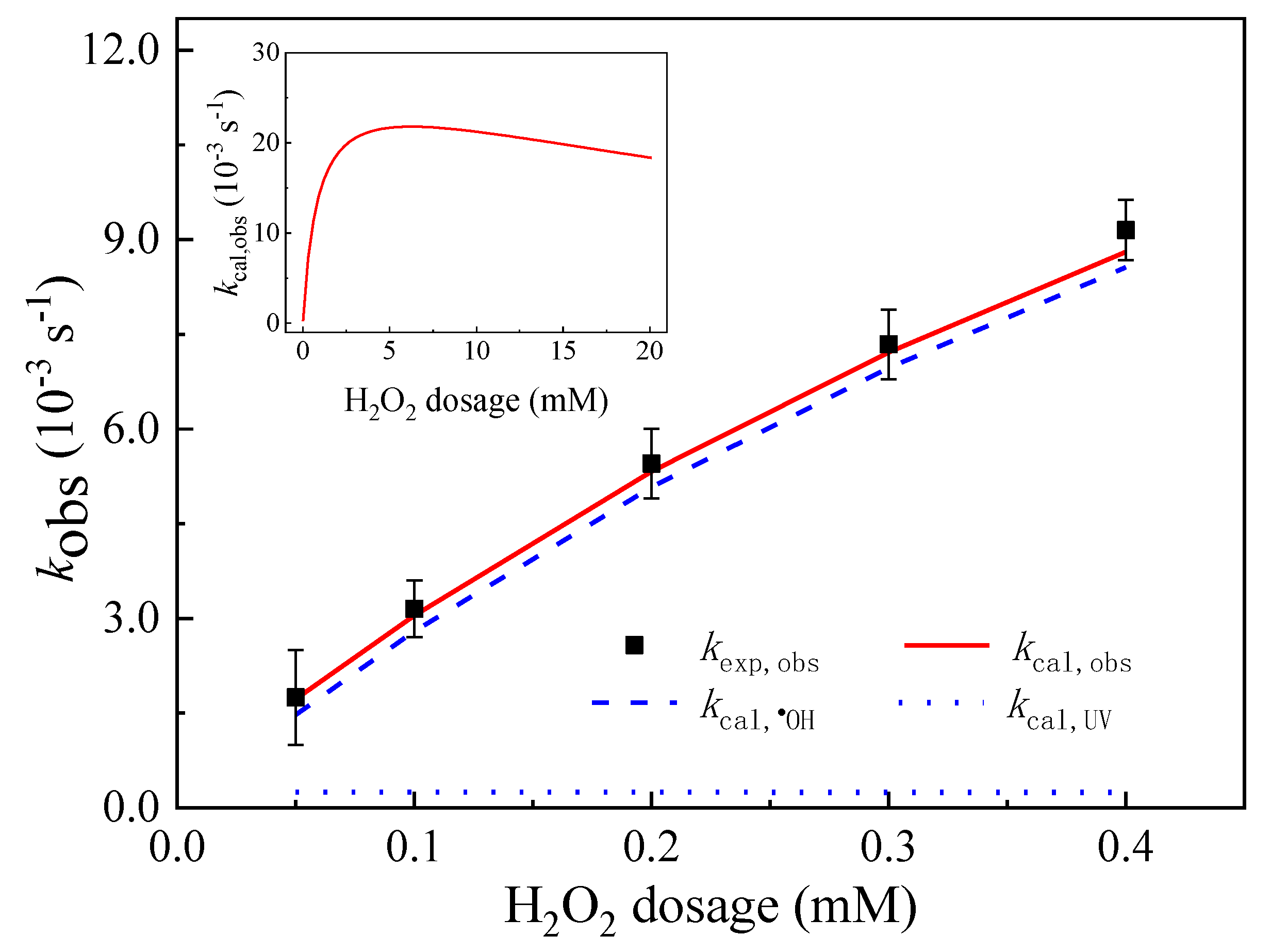
3.6. Influence of H2O2 Dosage and Complex Matrix
3.6.1. Effect of the Initial H2O2 Dosage
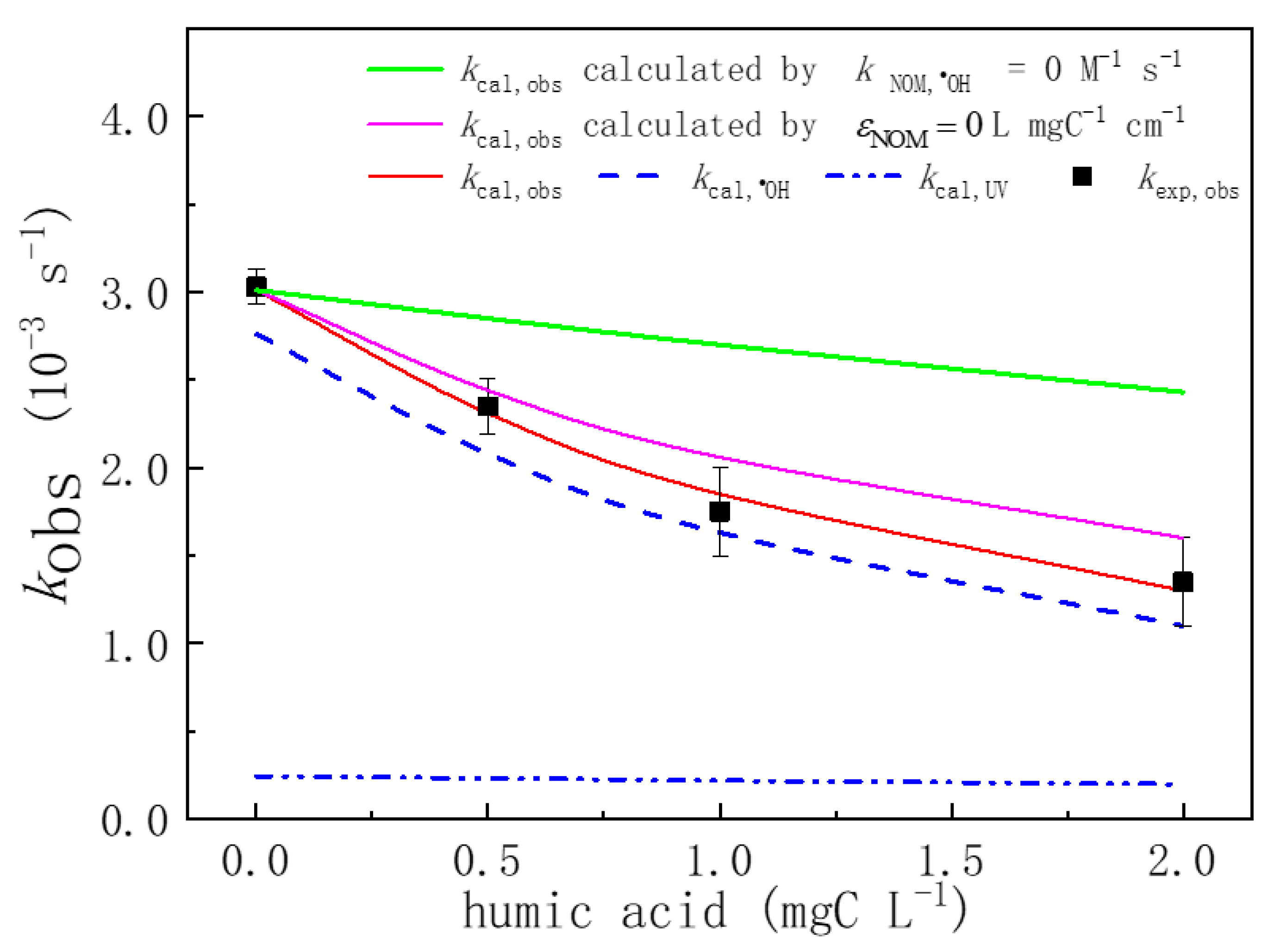
3.6.2. Effect of Organic Matter
3.6.3. Effect of Inorganic Anions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- K’Oreje, K.O.; Kandie, F.J.; Vergeynst, L.; Abira, M.A.; Van Langenhove, H.; Okoth, M.; Demeestere, K. Occurrence, fate and removal of pharmaceuticals, personal care products and pesticides in wastewater stabilization ponds and receiving rivers in the Nzoia Basin, Kenya. Sci. Total Environ. 2018, 637–638, 336–348. [Google Scholar] [CrossRef] [PubMed]
- Ben, Y.; Fu, C.; Hu, M.; Liu, L.; Wong, M.H.; Zheng, C. Human health risk assessment of antibiotic resistance associated with antibiotic residues in the environment: A review. Environ. Res. 2019, 169, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.Y.; Van Boeckel, T.P.; Martinez, E.M.; Pant, S.; Gandra, S.; Levin, S.A.; Goossens, H.; Laxminarayan, R. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc. Natl. Acad. Sci. USA 2018, 115, E3463–E3470. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhuan, R.; Chu, L. The occurrence, distribution and degradation of antibiotics by ionizing radiation: An overview. Sci. Total Environ. 2019, 646, 1385–1397. [Google Scholar] [CrossRef]
- Guo, X.; Yan, Z.; Zhang, Y.; Xu, W.; Kong, D.; Shan, Z.; Wang, N. Behavior of antibiotic resistance genes under extremely high-level antibiotic selection pressures in pharmaceutical wastewater treatment plants. Sci. Total Environ. 2018, 612, 119–128. [Google Scholar] [CrossRef]
- Zhao, R.; Feng, J.; Yin, X.; Jie, L.; Wenjie, F.; Berendonk, T.; Zhang, T.; Li, X.; Li, B. Antibiotic resistome in landfill leachate from different cities of China deciphered by metagenomic analysis. Water Res. 2018, 134, 126–139. [Google Scholar] [CrossRef]
- Zhi, S.; Zhou, J.; Yang, F.; Tian, L.; Zhang, K. Systematic analysis of occurrence and variation tendency about 58 typical veterinary antibiotics during animal wastewater disposal processes in Tianjin, China. Ecotoxicol. Environ. Saf. 2018, 165, 376–385. [Google Scholar] [CrossRef]
- Su, R.; Li, Y.; Min, M.-Y.; Ouyang, X.-H.; Song, R.-J.; Li, J.-H. Copper-catalyzed oxidative intermolecular 1,2-alkylarylation of styrenes with ethers and indoles. Chem. Commun. 2018, 54, 13511–13514. [Google Scholar] [CrossRef]
- Ben, Y.; Hu, M.; Zhang, X.; Wu, S.; Wong, M.H.; Wang, M.; Andrews, C.B.; Zheng, C. Efficient detection and assessment of human exposure to trace antibiotic residues in drinking water. Water Res. 2020, 175, 115699. [Google Scholar] [CrossRef]
- Zhang, Q.-Q.; Ying, G.-G.; Pan, C.-G.; Liu, Y.-S.; Zhao, J.-L. Comprehensive Evaluation of Antibiotics Emission and Fate in the River Basins of China: Source Analysis, Multimedia Modeling, and Linkage to Bacterial Resistance. Environ. Sci. Technol. 2015, 49, 6772–6782. [Google Scholar] [CrossRef]
- Krzeminski, P.; Tomei, M.C.; Karaolia, P.; Langenhoff, A.; Almeida, C.M.R.; Felis, E.; Gritten, F.; Andersen, H.R.; Fernandes, T.; Manaia, C.M.; et al. Performance of secondary wastewater treatment methods for the removal of contaminants of emerging concern implicated in crop uptake and antibiotic resistance spread: A review. Sci. Total Environ. 2019, 648, 1052–1081. [Google Scholar] [CrossRef] [PubMed]
- Su, R.; Ou, Q.; Wang, H.; Luo, Y.; Dai, X.; Wang, Y.; Chen, Y.; Shi, L. Comparison of phytoremediation potential of Nerium indicum with inorganic modifier calcium carbonate and organic modifier mushroom residue to lead-zinc tailings. Int. J. Environ. Res. Public Health 2022, 19, 10353. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; An, X.-L.; Chen, Q.-L.; Yang, X.; Christie, P.; Ke, X.; Wu, L.; Zhu, Y.-G. Antibiotics disturb the microbiome and increase the iIncidence of resistance genes in the gut of a common soil collembolan. Environ. Sci. Technol. 2018, 52, 3081–3090. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.-N.; Zhang, Y.; Cao, Z.-H.; Wang, X.-Y.; Ma, B.; Wu, W.-B.; Hu, N.; Huo, Z.-Y.; Yuan, Q.-B. Occurrence of super antibiotic resistance genes in the downstream of the Yangtze River in China: Prevalence and antibiotic resistance profiles. Sci. Total Environ. 2019, 651, 1946–1957. [Google Scholar] [CrossRef]
- He, L.; Su, R.; Chen, Y.; Zeng, P.; Du, L.; Cai, B.; Zhang, A.; Zhu, H. Integration of manganese accumulation, subcellular distribution, chemical forms, and physiological responses to understand manganese tolerance in Macleaya cordata. Environ. Sci. Pollut. Res. 2022, 29, 39017–39026. [Google Scholar] [CrossRef]
- Osińska, A.; Korzeniewska, E.; Harnisz, M.; Felis, E.; Bajkacz, S.; Jachimowicz, P.; Niestępski, S.; Konopka, I. Small-scale wastewater treatment plants as a source of the dissemination of antibiotic resistance genes in the aquatic environment. J. Hazard. Mater. 2020, 381, 121221. [Google Scholar] [CrossRef]
- Zhao, R.; Feng, J.; Liu, J.; Fu, W.; Li, X.; Li, B. Deciphering of microbial community and antibiotic resistance genes in activated sludge reactors under high selective pressure of different antibiotics. Water Res. 2019, 151, 388–402. [Google Scholar] [CrossRef]
- Su, R.; Xie, C.; Alhassan, S.I.; Huang, S.; Chen, R.; Xiang, S.; Wang, Z.; Huang, L. Oxygen reduction reaction in the field of water environment for application of nanomaterials. Nanomaterials 2020, 10, 1719. [Google Scholar] [CrossRef]
- Su, R.; Zhang, H.; Chen, F.; Wang, Z.; Huang, L. Applications of single atom catalysts for environmental management. Int. J. Environ. Res. Public Health 2022, 19, 11155. [Google Scholar] [CrossRef]
- Su, R.; Yang, X.-H.; Hu, M.; Wang, Q.-A.; Li, J.-H. Annulation Cascades of N-Allyl-N-((2-bromoaryl)ethynyl)amides Involving C–H Functionalization. Org. Lett. 2019, 21, 2786–2789. [Google Scholar] [CrossRef]
- Jia, A.; Wan, Y.; Xiao, Y.; Hu, J. Occurrence and fate of quinolone and fluoroquinolone antibiotics in a municipal sewage treatment plant. Water Res. 2012, 46, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Le, T.-H.; Ng, C.; Tran, N.H.; Chen, H.; Gin, K.Y.-H. Removal of antibiotic residues, antibiotic resistant bacteria and antibiotic resistance genes in municipal wastewater by membrane bioreactor systems. Water Res. 2018, 145, 498–508. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mozaz, S.; Chamorro, S.; Marti, E.; Huerta, B.; Gros, M.; Sànchez-Melsió, A.; Borrego, C.M.; Barceló, D.; Balcázar, J.L. Occurrence of antibiotics and antibiotic resistance genes in hospital and urban wastewaters and their impact on the receiving river. Water Res. 2015, 69, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Su, R.; Yang, H. Efficient copper(i)-catalyzed oxidative intermolecular 1,2-estersulfenylation of styrenes with peroxyesters and disulfides. Org. Biomol. Chem. 2020, 18, 5045–5049. [Google Scholar] [CrossRef]
- Huang, Y.; Kong, M.; Coffin, S.; Cochran, K.; Westerman, D.; Schlenk, D.; Richardson, S.; Lei, L.; Dionysiou, D. Degradation of contaminants of emerging concern by UV/H2O2 for water reuse: Kinetics, mechanisms, and cytotoxicity analysis. Water Res. 2020, 174, 115587. [Google Scholar] [CrossRef]
- Yang, Y.; Cao, Y.; Jiang, J.; Lu, X.; Ma, J.; Pang, S.; Li, J.; Liu, Y.; Zhou, Y.; Guan, C. Comparative study on degradation of propranolol and formation of oxidation products by UV/H2O2 and UV/persulfate (PDS). Water Res. 2019, 149, 543–552. [Google Scholar] [CrossRef]
- Starling, M.C.V.M.; Souza, P.P.; Le Person, A.; Amorim, C.C.; Criquet, J. Intensification of UV-C treatment to remove emerging contaminants by UV-C/H2O2 and UV-C/S2O82−: Susceptibility to photolysis and investigation of acute toxicity. Chem. Eng. J. 2019, 376, 120856. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, X.; Fang, Z.; Wang, S.; Shan, C.; Wei, S.; Pan, B. Unravelling molecular transformation of dissolved effluent organic matter in UV/H2O2, UV/persulfate, and UV/chlorine processes based on FT-ICR-MS analysis. Water Res. 2021, 199, 117158. [Google Scholar] [CrossRef]
- Lee, M.Y.; Wang, W.L.; Du, Y.; Jeon, T.W.; Shin, S.K.; Wu, Q.Y.; Dao, G.H.; Hu, H.Y. Applications of UV/H2O2, UV/persulfate, and UV/persulfate/Cu2+ for the elimination of reverse osmosis concentrate generated from municipal wastewater reclamation treatment plant: Toxicity, transformation products, and disinfection byproducts. Sci. Total Environ. 2021, 762, 144161. [Google Scholar] [CrossRef]
- Shad, A.; Chen, J.; Qu, R.J.; Dar, A.A.; Bin-Jumah, M.; Allam, A.A.; Wang, Z.Y. Degradation of sulfadimethoxine in phosphate buffer solution by UV alone, UV/PMS and UV/H2O2: Kinetics, degradation products, and reaction pathways. Chem. Eng. J. 2020, 398, 125357. [Google Scholar] [CrossRef]
- Chen, L.; Cai, T.; Cheng, C.; Xiong, Z.; Ding, D. Degradation of acetamiprid in UV/H2O2 and UV/persulfate systems: A comparative study. Chem. Eng. J. 2018, 351, 1137–1146. [Google Scholar] [CrossRef]
- Forouzesh, M.; Ebadi, A.; Aghaeinejad-Meybodi, A. Degradation of metronidazole antibiotic in aqueous medium using activated carbon as a persulfate activator. Sep. Purif. Technol. 2019, 210, 145–151. [Google Scholar] [CrossRef]
- Tyers, M.; Wright, G.D. Drug combinations: A strategy to extend the life of antibiotics in the 21st century. Nat. Rev. Microbiol. 2019, 17, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Lee, G.; Zoh, K.D. Benzophenone-3 degradation via UV/H2O2 and UV/persulfate reactions. J. Hazard. Mater. 2021, 403, 11. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Wu, Z.; Tang, C.; Guo, K.; Cao, Y.; Fang, J. Comparative study of naproxen degradation by the UV/chlorine and the UV/H2O2 advanced oxidation processes. Environ. Sci. Water Res. Technol. 2018, 4, 1219–1230. [Google Scholar] [CrossRef]
- Su, R.; Chai, L.; Tang, C.; Li, B.; Yang, Z. Comparison of the degradation of molecular and ionic ibuprofen in a UV/H2O2 system. Water Sci. Technol. 2018, 77, 2174–2183. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Su, R.; Yao, H.; Zhang, A.; Xiang, S.; Huang, L. Degradation of trimethoprim by sulfate radical-based advanced oxidation processes: Kinetics, mechanisms, and effects of natural water matrices. Environ. Sci. Pollut. Res. 2021, 28, 62572–62582. [Google Scholar] [CrossRef]
- Parker, C.A. A new sensitive chemical actinometer. I. Some trials with potassium ferrioxalate. Proc. R. Soc. A 1953, 220, 104–116. [Google Scholar]
- Hatchard, C.G.; Parker, C.A. A new sensitive chemical actinometer. II. potassium ferrioxalate as a standard chemical actinometer. Proc. R. Soc. A 1956, 235, 518–536. [Google Scholar]
- Beltran, F.J.; Ovejero, G.; Garcia-Araya, J.F.; Rivas, J. Oxidation of polynuclear aromatic hydrocarbons in water. 2. UV radiation and ozonation in the presence of UV radiation. Ind. Eng. Chem. Res. 1995, 34, 1607–1615. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, L.; Yue, J.; Webster, R.D.; Lim, T.T. Kinetic modeling and energy efficiency of UV/H2O2 treatment of iodinated trihalomethanes. Water Res. 2015, 75, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Sun, P.; Boyer, T.H.; Zhao, L.; Huang, C.H. Degradation of pharmaceuticals and metabolite in synthetic human urine by UV, UV/H2O2, and UV/PDS. Environ. Sci. Technol. 2015, 49, 3056–3066. [Google Scholar] [CrossRef] [PubMed]
- Leifer, A. The Kinetics of Environmental Aquatic Photochemistry: Theory and Practice; American Chemical Society: Washington, DC, USA, 1988. [Google Scholar]
- Pereira, V.J.; Weinberg, H.S.; Linden, K.G.; Singer, P.C. UV degradation kinetics and modeling of pharmaceutical compounds in laboratory grade and surface water via direct and indirect photolysis at 254 nm. Environ. Sci. Technol. 2007, 41, 1682–1688. [Google Scholar] [CrossRef] [PubMed]
- Szabó, R.K. Decomposition of some pharmaceuticals by Advanced Oxidation Processes. Ph.D. Thesis, University of Szeged Doctoral School of Environmental Sciences, Szeged, Hungary, 2010. [Google Scholar]
- Yang, Y.; Pignatello, J.J.; Ma, J.; Mitch, W.A. Effect of matrix components on UV/H2O2 and UV/S2O82− advanced oxidation processes for trace organic degradation in reverse osmosis brines from municipal wastewater reuse facilities. Water Res. 2016, 89, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.; Kim, S.; Yoon, Y.; Jung, Y.; Hwang, T.; Lee, J.; Kang, J. Comparative evaluation of ibuprofen removal by UV/H2O2 and UV/S2O82− processes for wastewater treatment. Chem. Eng. J. 2015, 269, 379–390. [Google Scholar] [CrossRef]
- Vogna, D.; Marotta, R.; Andreozzi, R.; Napolitano, A.; d’Ischia, M. Kinetic and chemical assessment of the UV/H2O2 treatment of antiepileptic drug carbamazepine. Chemosphere 2004, 54, 497–505. [Google Scholar] [CrossRef]
- Shemer, H.; Kunukcu, Y.K.; Linden, K.G. Degradation of the pharmaceutical Metronidazole via UV, Fenton and photo-Fenton processes. Chemosphere 2006, 63, 269–276. [Google Scholar] [CrossRef]
- Yuan, F.; Hu, C.; Hu, X.; Qu, J.; Yang, M. Degradation of selected pharmaceuticals in aqueous solution with UV and UV/H2O2. Water Res. 2009, 43, 1766–1774. [Google Scholar] [CrossRef]
- Barreto, J.C.; Smith, G.S.; Strobel, N.H.P.; McQuillin, P.A.; Miller, T.A. Terephthalic acid: A dosimeter for the detection of hydroxyl radicals in vitro. Life Sci. 1994, 56, PL89–PL96. [Google Scholar] [CrossRef]
- Qu, X.; Kirschenbaum, L.J.; Borish, E.T. Hydroxyterephthalate as a fluorescent probe for hydroxyl radicals: Application to hair melanin. Photochem. Photobiol. 2010, 71, 307–313. [Google Scholar] [CrossRef]
- Haag, W.R.; Yao, C.D. Rate constants for reaction of hydroxyl radicals with several drinking water contaminants. Environ. Sci. Technol. 1992, 26, 1005–1013. [Google Scholar] [CrossRef]
- Packer, J.L.; Werner, J.J.; Latch, D.E.; McNeill, K.; Arnold, W.A. Photochemical fate of pharmaceuticals in the environment: Naproxen, diclofenac, clofibric acid, and ibuprofen. Aquat. Sci. 2003, 65, 342–351. [Google Scholar] [CrossRef]
- Van Scherpenzeel, M.; van den Berg, R.J.; Donker-Koopman, W.E.; Liskamp, R.M.; Aerts, J.M.; Overkleeft, H.S.; Pieters, R.J. Nanomolar affinity, iminosugar-based chemical probes for specific labeling of lysosomal glucocerebrosidase. Bioorgan. Med. Chem. 2010, 18, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Lian, L.; Yao, B.; Hou, S.; Fang, J.; Yan, S.; Song, W. Kinetic study of hydroxyl and sulfate radical-mediated oxidation of pharmaceuticals in wastewater effluents. Environ. Sci. Technol. 2017, 51, 2954. [Google Scholar] [CrossRef]
- Dodd, M.C.; Buffle, M.-O.; von Gunten, U. Oxidation of antibacterial molecules by aqueous ozone: Moiety-specific reaction kinetics and application to ozone-based wastewater treatment. Environ. Sci. Technol. 2006, 40, 1969–1977. [Google Scholar] [CrossRef]
- Mahdi Ahmed, M.; Barbati, S.; Doumenq, P.; Chiron, S. Sulfate radical anion oxidation of diclofenac and sulfamethoxazole for water decontamination. Chem. Eng. J. 2012, 197, 440–447. [Google Scholar] [CrossRef]
- Crittenden, J.C.; Hu, S.; Hand, D.W.; Green, S.A. A kinetic model for H2O2/UV process in a completely mixed batch reactor. Water Res. 1999, 33, 2315–2328. [Google Scholar] [CrossRef]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (•OH/•O−) in aqueous solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef]
- Stumm, W.; Morgan, J. Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters; Wiley: New York, NY, USA, 1996. [Google Scholar]
- Lutze, H.V.; Bircher, S.; Rapp, I.; Kerlin, N.; Bakkour, R.; Geisler, M.; von Sonntag, C.; Schmidt, T.C. Degradation of chlorotriazine pesticides by sulfate radicals and the influence of organic matter. Environ. Sci. Technol. 2015, 49, 1673–1680. [Google Scholar] [CrossRef]
- Jayson, G.; Parsons, B.; Swallow, A.J. Some simple, highly reactive, inorganic chlorine derivatives in aqueous solution. Their formation using pulses of radiation and their role in the mechanism of the fricke dosimeter. J. Chem. Soc. Faraday Trans. 1973, 69, 1597–1607. [Google Scholar] [CrossRef]
- Kläning, U.K.; Wolff, T. Laser flash photolysis of HCIO, CIO−, HBrO, and BrO− in aqueous solution. reactions of Cl− and Br− Atoms. Ber. Bunsenges. Phys. Chem. 1985, 89, 243–245. [Google Scholar] [CrossRef]
- Yu, X.Y.; Bao, Z.C.; Barker, J.R. Free radical reactions involving Cl•, Cl2•−, and SO4•− in the 248 nm photolysis of aqueous solutions containing S2O82− and Cl. J. Phys. Chem. A 2004, 108, 295–308. [Google Scholar] [CrossRef]
- McElroy, W.J. A laser photolysis study of the reaction of SO4− with Cl− and the subsequent decay of Cl2−in aqueous solution. J. Phys. Chem. 1990, 94, 2434–2441. [Google Scholar] [CrossRef]
- Luo, C.; Jiang, J.; Ma, J.; Pang, S.; Liu, Y.; Song, Y.; Guan, C.; Li, J.; Jin, Y.; Wu, D. Oxidation of the odorous compound 2,4,6-trichloroanisole by UV activated persulfate: Kinetics, products, and pathways. Water Res. 2016, 96, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Grigor’ev, A.; Makarov, I.; Pikaev, A. Formation of Cl2− in the bulk of solution during radiolysis of concentrated aqueous solutions of chlorides. Khimiya Vysok. Ehnergij 1987, 21, 123–126. [Google Scholar]
- Keen, O.S.; Love, N.G.; Linden, K.G. The role of effluent nitrate in trace organic chemical oxidation during UV disinfection. Water Res. 2012, 46, 5224–5234. [Google Scholar] [CrossRef]
- Mack, J.; Bolton, J.R. Photochemistry of nitrite and nitrate in aqueous solution: A review. J. Photochem. Photobiol. A 1999, 128, 1–13. [Google Scholar] [CrossRef]
- Guo, H.G.; Gao, N.Y.; Chu, W.H.; Li, L.; Zhang, Y.J.; Gu, J.S.; Gu, Y.L. Photochemical degradation of ciprofloxacin in UV and UV/H2O2 process: Kinetics, parameters, and products. Environ. Sci. Pollut. Res. 2013, 20, 3202–3213. [Google Scholar] [CrossRef]
- Huie, R.E.; Clifton, C.L.; Kafafi, S.A. Rate constants for hydrogen abstraction reactions of the sulfate radical, SO4•−: Experimental and theoretical results for cyclic ethers. J. Phys. Chem. 1991, 95, 9336–9340. [Google Scholar] [CrossRef]
- Yang, Y.; Pignatello, J.J.; Ma, J.; Mitch, W.A. Comparison of halide impacts on the efficiency of contaminant degradation by sulfate and hydroxyl radical-based advanced oxidation processes (AOPs). Environ. Sci. Technol. 2014, 48, 2344–2351. [Google Scholar] [CrossRef]
- Grebel, J.E.; Pignatello, J.J.; Mitch, W.A. Effect of halide ions and carbonates on organic contaminant degradation by hydroxyl radical-based advanced oxidation processes in saline waters. Environ. Sci. Technol. 2010, 44, 6822–6828. [Google Scholar] [CrossRef] [PubMed]
- Beitz, T.; Bechmann, W.; Mitzner, R. Investigations of reactions of selected azaarenes with radicals in water. 2. chlorine and bromine radicals. J. Phys. Chem. A 1998, 102, 6766–6771. [Google Scholar] [CrossRef]
- Watts, M.J.; Rosenfeldt, E.J.; Linden, K.G. Comparative OH radical oxidation using UV-Cl2 and UV-H2O2 processes. J. Water Supply Res. Technol.-AQUA 2007, 56, 469. [Google Scholar] [CrossRef]
- Alegre, M.L.; Geronés, M.; Rosso, J.A.; Bertolotti, S.; Braun, A.; Mártir, D.O.; Gonzalez, M.C. Kinetic Study of the Reactions of Chlorine Atoms and Cl2•− Radical Anions in Aqueous Solutions. 1. Reaction with Benzene. J. Phys. Chem. A 2000, 104, 3117–3125. [Google Scholar] [CrossRef]
- Deng, J.; Shao, Y.; Gao, N.; Xia, S.; Tan, C.; Zhou, S.; Hu, X. Degradation of the antiepileptic drug carbamazepine upon different UV-based advanced oxidation processes in water. Chem. Eng. J. 2013, 222 (Suppl. C), 150–158. [Google Scholar] [CrossRef]



| Compound | Acetonitrile | Phosphate Buffer (20 mM, pH 3) | λ | Flow Rate | Injection Volume | Column Temperature |
|---|---|---|---|---|---|---|
| nm | mL min−1 | µL | °C | |||
| MTZ | 15% | 85% | 320 | 0.3 | 5 | 35 |
| pCBA | 30% | 70% | 238 |
| # | Reaction | k (M−1 s−1) | Reference |
|---|---|---|---|
| 1 | , s−1 | [59] | |
| 2 | k1 = 2.7 × 107 | [60] | |
| 3 | k2 = 7.5 × 109 | [59] | |
| 4 | k3 = 2.51 × 10−12 | [59] | |
| In the presence of Phosphates | |||
| 5 | H3PO4 ⇌ H+ + | pKa1 = 2.1 unitless | [61] |
| 6 | ⇌ H+ + | pKa2 = 7.2 unitless | [61] |
| 7 | ⇌ H+ + | pKa3 = 12.3 unitless | [61] |
| 8 | kH1 = 2.0 × 104 | [60] | |
| 9 | kH2 = 1.5 × 105 | [60] | |
| 10 | kH3 < 1.5 × 107 | [60] | |
| 11 | kH4 = 2.7 × 106 | [60] | |
| In the presence of NOM | [62] | ||
| 12 | k14 = 1.4 × 104 L mgC−1 s−1 | [62] | |
| In the presence of Cl− | |||
| 13 | k16 = 4.3 × 109 | [63] | |
| 14 | k17 = 1.8 × 1010 | [64] | |
| 15 | k18 = 2.5 × 105 | [63] | |
| 16 | k19 = 6.0 × 109 | [63] | |
| 17 | k20 = 2.1 × 1010 | [64] | |
| 18 | k21 = 8.5 × 109 | [65] | |
| 19 | k22 = 1.3 × 103 | [66,67] | |
| 20 | k23 = 4.5 × 107 | [68] | |
| 21 | k24 = 9.0 × 108 | [65] | |
| In the presence of Carbonates | |||
| 22 | k27 = 8.5 × 106 | [60] | |
| 23 | k28 = 3.9 × 108 | [60] | |
| In the presence of | |||
| 24 | k30 < 1.0 × 105 | [69] | |
| 25 | = 0.24 mol Einstein−1 | [69] | |
| 26 | [70] | ||
| 27 | / | [70] | |
| 28 | / | [69] | |
| 29 | / | [69] | |
| 30 | / | [69] | |
| Degradation of MTZ | |||
| 31 | rUV, M s−1 | * | |
| 32 | * | ||
| 33 | Secondary radical + →? | N.A. | N.A. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, R.; Dai, X.; Wang, H.; Wang, Z.; Li, Z.; Chen, Y.; Luo, Y.; Ouyang, D. Metronidazole Degradation by UV and UV/H2O2 Advanced Oxidation Processes: Kinetics, Mechanisms, and Effects of Natural Water Matrices. Int. J. Environ. Res. Public Health 2022, 19, 12354. https://doi.org/10.3390/ijerph191912354
Su R, Dai X, Wang H, Wang Z, Li Z, Chen Y, Luo Y, Ouyang D. Metronidazole Degradation by UV and UV/H2O2 Advanced Oxidation Processes: Kinetics, Mechanisms, and Effects of Natural Water Matrices. International Journal of Environmental Research and Public Health. 2022; 19(19):12354. https://doi.org/10.3390/ijerph191912354
Chicago/Turabian StyleSu, Rongkui, Xiangrong Dai, Hanqing Wang, Zhixiang Wang, Zishi Li, Yonghua Chen, Yiting Luo, and Danxia Ouyang. 2022. "Metronidazole Degradation by UV and UV/H2O2 Advanced Oxidation Processes: Kinetics, Mechanisms, and Effects of Natural Water Matrices" International Journal of Environmental Research and Public Health 19, no. 19: 12354. https://doi.org/10.3390/ijerph191912354
APA StyleSu, R., Dai, X., Wang, H., Wang, Z., Li, Z., Chen, Y., Luo, Y., & Ouyang, D. (2022). Metronidazole Degradation by UV and UV/H2O2 Advanced Oxidation Processes: Kinetics, Mechanisms, and Effects of Natural Water Matrices. International Journal of Environmental Research and Public Health, 19(19), 12354. https://doi.org/10.3390/ijerph191912354







