Tricuspid Valve Damage Related to Transvenous Lead Extraction
Abstract
:1. Introduction
2. Methods
2.1. Study Population
2.2. Lead Extraction Procedure-Definitions
2.3. Indications for Lead Extraction
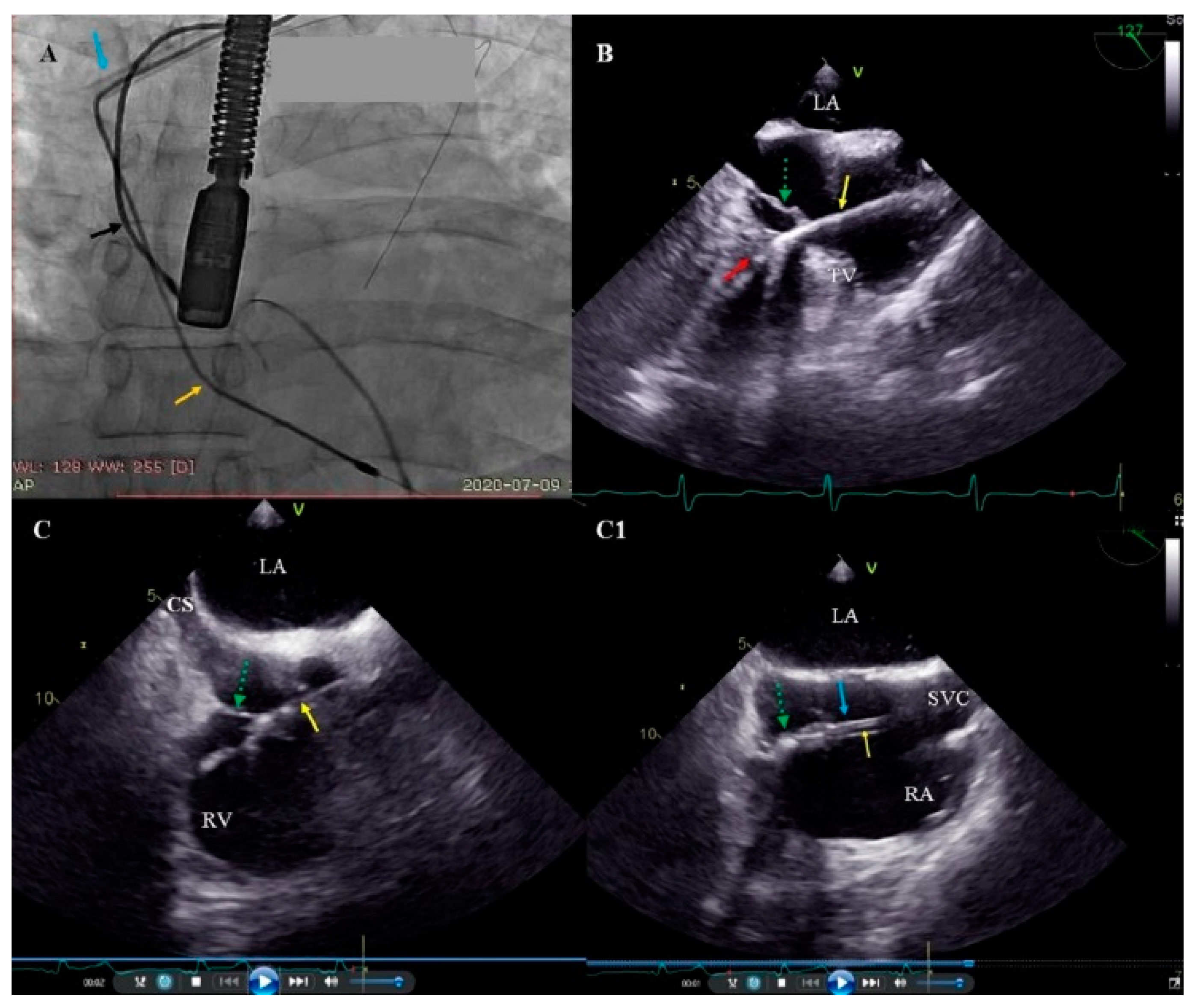
2.4. Lead Extraction-Techniques
2.5. Echocardiographic Examinations
2.6. Tricuspid Valve Damage Evaluation
2.7. Statistical Analysis
2.8. Approval of the Bioethics Committee
3. Results
3.1. Clinical Data of Study Group
3.2. Analysis of Data on the Function of the Tricuspid Valve
4. Discussion
5. Conclusions
Study Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Love, C.J.; Wilkoff, B.L.; Byrd, C.L.; Belott, P.H.; Brinker, J.A.; Fearnot, N.E.; Friedman, R.A.; Furman, S.; Goode, L.B.; Hayes, D.L.; et al. Recommendations for extraction of chronically implanted transvenous pacing and defibrillator leads: Indications, facilities, training. North American Society of Pacing and Electrophysiology Lead Extraction Conference Faculty. Pacing Clin. Electrophysiol. 2000, 23, 544–551. [Google Scholar]
- Wilkoff, B.L.; Love, C.J.; Byrd, C.L.; Bongiorni, M.G.; Carrillo, R.G.; Crossley, G.H., 3rd; Epstein, L.M.; Friedman, R.A.; Kennergren, C.E.; Mitkowski, P.; et al. Transvenous lead extraction: Heart Rhythm Society expert consensus on facilities, training, indications, and patient management: This document was endorsed by the American Heart Association (AHA). Heart Rhythm 2009, 6, 1085–1104. [Google Scholar] [CrossRef] [PubMed]
- Deharo, J.C.; Bongiorni, M.G.; Rozkovec, A.; Bracke, F.; Defaye, P.; Fernandez-Lozano, I.; Golzio, P.G.; Hansky, B.; Kennergren, C.; Manolis, A.S.; et al. Pathways for training and accreditation for transvenous lead extraction: A European Heart Rhythm Association position paper. Europace 2012, 14, 124–134. [Google Scholar] [PubMed]
- Kusumoto, F.M.; Schoenfeld, M.H.; Wilkoff, B.; Berul, C.I.; Birgersdotter-Green, U.M.; Carrillo, R.; Cha, Y.M.; Clancy, J.; Deharo, J.C.; Ellenbogen KAExner, D.; et al. 2017 HRS expert consensus statement on cardiovascular implantable electronic device lead management and extraction. Heart Rhythm 2017, 14, e503–e551. [Google Scholar] [CrossRef] [PubMed]
- Bongiorni, M.G.; Burri, H.; Deharo, J.C.; Starck, C.; Kennergren, C.; Saghy, L.; Rao, A.; Tascini, C.; Lever, N.; Kutarski, A.; et al. 2018 EHRA expert consensus statement on lead extraction: Recommendations on definitions, endpoints, research trial design, and data collection requirements for clinical scientific studies and registries: Endorsed by APHRS/HRS/LAHRS. Europace 2012, 14, 994–1001. [Google Scholar] [CrossRef] [PubMed]
- Nowosielecka, D.; Polewczyk, A.; Jacheć, W.; Tułecki, Ł.; Kleinrok, A.; Kutarski, A. Echocardiographic findings in patients with cardiac implantable electronic devices-analysis of factors predisposing to lead-associated changes. Clin. Physiol. Funct. Imaging 2021, 41, 25–41. [Google Scholar] [CrossRef]
- Brunner, M.P.; Cronin, E.M.; Wazni, O.; Baranowski, B.; Saliba, W.I.; Sabik, J.F.; Lindsay, B.D.; Wilkoff, B.L.; Tarakji, K.G. Outcomes of patients requiring emergent surgical or endovascular intervention for catastrophic complications during transvenous lead extraction. Heart Rhythm 2014, 11, 419–425. [Google Scholar] [CrossRef]
- Stefańczyk, P.; Nowosielecka, D.; Tułecki, Ł.; Tomków, K.; Polewczyk, A.; Jacheć, W.; Kleinrok, A.; Borzęcki, W.; Kutarski, A. Transvenous Lead Extraction without Procedure-Related Deaths in 1000 Consecutive Patients: A Single-Center Experience. Vasc. Health Risk Manag. 2021, 17, 445–459. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Rozen, G.; Kaadan, M.I.; Galvin, J.; Ruskin, J.N. Safety and In-Hospital Outcomes of Transvenous Lead Extraction for Cardiac Implantable Device-Related Infections: Analysis of 13 Years of Inpatient Data in the United States. JACC Clin. Electrophysiol. 2019, 5, 1450–1458. [Google Scholar] [CrossRef]
- Tułecki, Ł.; Polewczyk, A.; Jacheć, W.; Nowosielecka, D.; Tomków, K.; Stefańczyk, P.; Kosior, J.; Duda, K.; Polewczyk, M.; Kutarski, A. Study of Major and Minor Complications of 1500 Transvenous Lead Extraction Procedures Performed with Optimal Safety at Two High-Volume Referral Centers. Int. J. Environ. Res. Public Health 2021, 18, 10416. [Google Scholar] [CrossRef]
- Assayag, P.; Thuaire, C.; Benamer, H.; Sebbah, J.; Leport, C.; Brochet, E. Partial rupture of the tricuspid valve after extraction of permanent pacemaker leads: Detection by transesophageal echocardiography. Pacing Clin. Electrophysiol. 1999, 22, 971–974. [Google Scholar] [CrossRef] [PubMed]
- Roeffel, S.; Bracke, F.; Meijer, A.; Van Gelder, B.; Van Dantzig, J.M.; Botman, C.J.; Peels, K. Transesophageal echocardiographic evaluation of tricuspid valve regurgitation during pacemaker and implantable cardioverter defibrillator lead extraction. Pacing Clin. Electrophysiol. 2002, 25, 1583–1586. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Gentry, J.L., 3rd; Varma, N.; Wazni, O.; Tarakji, K.G.; Mehta, A.; Mick, S.; Grimm, R.; Wilkoff, B.L. Transvenous Extraction of Pacemaker and Defibrillator Leads and the Risk of Tricuspid Valve Regurgitation. JACC Clin. Electrophysiol. 2018, 4, 1421–1428. [Google Scholar] [CrossRef]
- Pecha, S.; Castro, L.; Gosau, N.; Linder, M.; Vogler, J.; Willems, S.; Reichenspurner, H.; Hakmi, S. Evaluation of tricuspid valve regurgitation following laser lead extraction†. Eur. J. Cardiothorac. Surg. 2017, 51, 1108–1111. [Google Scholar] [CrossRef]
- Givon, A.; Vedernikova, N.; Luria, D.; Vatury, O.; Kuperstein, R.; Feinberg, M.S.; Eldar, M.; Glikson, M.; Nof, E. Tricuspid Regurgitation following Lead Extraction: Risk Factors and Clinical Course. Isr. Med. Assoc. J. 2016, 18, 18–22. [Google Scholar]
- Regoli, F.; Caputo, M.; Conte, G.; Faletra, F.F.; Moccetti, T.; Pasotti, E.; Cassina, T.; Casso, G.; Schlotterbeck, H.; Engeler, A.; et al. Clinical utility of routine use of continuous transesophageal echocardiography monitoring during transvenous lead extraction procedure. Heart Rhythm 2015, 12, 313–320. [Google Scholar] [CrossRef]
- Coffey, J.O.; Sager, S.J.; Gangireddy, S.; Levine, A.; Viles-Gonzalez, J.F.; Fischer, A. The impact of transvenous lead extraction on tricuspid valve function. Pacing Clin. Electrophysiol. 2014, 37, 19–24. [Google Scholar] [CrossRef]
- Rodriguez, Y.; Mesa, J.; Arguelles, E.; Carrillo, R.G. Tricuspid insufficiency after laser lead extraction. Pacing Clin. Electrophysiol. 2013, 36, 939–944. [Google Scholar] [CrossRef]
- Franceschi, F.; Thuny, F.; Giorgi, R.; Sanaa, I.; Peyrouse, E.; Assouan, X.; Prévôt, S.; Bastard, E.; Habib, G.; Deharo, J.C. Incidence, risk factors, and outcome of traumatic tricuspid regurgitation after percutaneous ventricular lead removal. J. Am. Coll. Cardiol. 2009, 53, 2168–2174. [Google Scholar] [CrossRef]
- Tułecki, Ł.; Polewczyk, A.; Jacheć, W.; Nowosielecka, D.; Tomków, K.; Stefańczyk, P.; Kosior, J.; Duda, K.; Polewczyk, M.; Kutarski, A. Analysis of Risk Factors for Major Complications of 1500 Transvenous Lead Extraction Procedures with Especial Attention to Tricuspid Valve Damage. Int. J. Environ. Res. Public Health 2021, 18, 9100. [Google Scholar] [CrossRef]
- Zucchelli, G.; Di Cori, A.; Segreti, L.; Laroche, C.; Blomstrom-Lundqvist, C.; Kutarski, A.; Regoli, F.; Butter, C.; Defaye, P.; Pasquié, J.L.; et al. Major cardiac and vascular complications after transvenous lead extraction: Acute outcome and predictive factors from the ESC-EHRA ELECTRa (European Lead Extraction ConTRolled) registry. Europace 2019, 21, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Jacheć, W.; Polewczyk, A.; Polewczyk, M.; Tomasik, A.; Janion, M.; Kutarski, A. Risk Factors Predicting Complications of Transvenous Lead Extraction. Biomed. Res. Int. 2018, 2018, 8796704. [Google Scholar] [CrossRef] [PubMed]
- Brunner, M.P.; Cronin, E.M.; Duarte, V.E.; Yu, C.; Tarakji, K.G.; Martin, D.O.; Callahan, T.; Cantillon, D.J.; Niebauer, M.J.; Saliba, W.I.; et al. Clinical predictors of adverse patient outcomes in an experience of more than 5000 chronic endovascular pacemaker and defibrillator lead extractions. Heart Rhythm 2014, 11, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Wazni, O.; Epstein, L.M.; Carrillo, R.G.; Love, C.; Adler, S.W.; Riggio, D.W.; Karim, S.S.; Bashir, J.; Greenspon, A.J.; DiMarco, J.P.; et al. Lead extraction in the contemporary setting: The LExICon study: An observational retrospective study of consecutive laser lead extractions. J. Am. Coll. Cardiol. 2010, 55, 579–586. [Google Scholar] [CrossRef]
- Jacheć, W.; Polewczyk, A.; Polewczyk, M.; Tomasik, A.; Kutarski, A. Transvenous Lead Extraction SAFeTY Score for Risk Stratification and Proper Patient Selection for Removal Procedures Using Mechanical Tools. J. Clin. Med. 2020, 9, 356–361. [Google Scholar] [CrossRef]
- Sidhu, B.S.; Ayis, S.; Gould, J.; Elliott, M.K.; Mehta, V.; Kennergren, C.; Butter, C.; Deharo, J.C.; Kutarski, A.; Maggioni, A.P.; et al. Risk stratification of patients undergoing transvenous lead extraction with the ELECTRa Registry Outcome Score (EROS): An ESC EHRA EORP European lead extraction ConTRolled ELECTRa registry analysis. Europace 2021, 23, 1462–1471. [Google Scholar] [CrossRef]
- Kancharla, K.; Acker, N.G.; Li, Z.; Samineni, S.; Cai, C.; Espinosa, R.E.; Osborn, M.; Mulpuru, S.K.; Asirvatham, S.J.; Friedman, P.A.; et al. Efficacy and safety of transvenous lead extraction in the device laboratory and operating room guided by a novel risk stratification scheme. JACC Clin. Electrophysiol. 2019, 5, 174–182. [Google Scholar] [CrossRef]
- Bontempi, L.; Vassanelli, F.; Cerini, M.; D’Aloia, A.; Vizzardi, E.; Gargaro, A.; Chiusso, F.; Mamedouv, R.; Lipari, A.; Curnis, A. Predicting the difficulty of a lead extraction procedure: The LED index. J. Cardiovasc. Med. 2014, 15, 668–673. [Google Scholar] [CrossRef]
- Fu, H.X.; Huang, X.M.; Zhong, L.I.; Osborn, M.J.; Asirvatham, S.J.; Espinosa, R.E.; Brady, P.A.; Lee, H.C.; Greason, K.L.; Baddour, L.M.; et al. Outcomes and complications of lead removal: Can we establish a risk stratification schema for a collaborative and effective approach? Pacing Clin. Electrophysiol. 2015, 38, 1439–1447. [Google Scholar] [CrossRef]
- Kutarski, A.; Czajkowski, M.; Pietura, R.; Obszanski, B.; Polewczyk, A.; Jachec, W.; Polewczyk, M.; Mlynarczyk, K.; Grabowski, M.; Opolski, G. Effectiveness, safety, and long-term outcomes of non-powered mechanical sheaths for transvenous lead extraction. Europace 2018, 20, 1324–1333. [Google Scholar] [CrossRef]
- Nowosielecka, D.; Jacheć, W.; Polewczyk, A.; Tułecki, Ł.; Tomków, K.; Stefańczyk, P.; Tomaszewski, A.; Brzozowski, W.; Szcześniak-Stańczyk, D.; Kleinrok, A.; et al. Transesophageal Echocardiography as a Monitoring Tool during Transvenous Lead Extraction—Does It Improve Procedure Effectiveness? J. Clin. Med. 2020, 9, 1382. [Google Scholar] [CrossRef]
- Nowosielecka, D.; Polewczyk, A.; Jacheć, W.; Tułecki, Ł.; Tomków, K.; Stefańczyk, P.; Kleinrok, A.; Kutarski, A. A new approach to the continuous monitoring of transvenous lead extraction using transesophageal echocardiography—Analysis of 936 procedures. Echocardiography 2020, 37, 601–611. [Google Scholar] [CrossRef]
- Nowosielecka, D.; Polewczyk, A.; Jacheć, W.; Kleinrok, A.; Tułecki, Ł.; Kutarski, A. Transesophageal echocardiography for the monitoring of transvenous lead extraction. Kardiol. Pol. 2020, 78, 1206–1214. [Google Scholar] [CrossRef]
- Nowosielecka, D.; Jacheć, W.; Polewczyk, A.; Kleinrok, A.; Tułecki, Ł.; Kutarski, A. The prognostic value of transesophageal echocardiography after transvenous lead extraction: Landscape after battle. Cardiovasc. Diagn. Ther. 2021, 11, 394–410. [Google Scholar] [CrossRef]
- Lancellotti, P.; Moura, L.; Pierard, L.A.; Agricola, E.; Popescu, B.A.; Tribouilloy Ch Hagendorff, A.; Monin, J.L.; Badano, L.; Zamorano, J.L.; Sicari, R.; et al. European Association of Echocardiography recommendations for the assessment of valvular regurgitation. Part 2: Mitral and tricuspid regurgitation (native valve disease). Eur. J. Echocardiogr. 2010, 11, 307–332. [Google Scholar] [CrossRef]
- Birnie, D.H.; Wang, J.; Alings, M.; Philippon, F.; Parkash, R.; Manlucu, J.; Angaran, P.; Rinne, C.; Coutu, B.; Low, R.A.; et al. Risk Factors for Infections Involving Cardiac Implanted Electronic Devices. J. Am. Coll. Cardiol. 2019, 10, 2845–2854. [Google Scholar] [CrossRef]
- Schaller, R.D.; Sadek, M.M.; Cooper, J.M. Simultaneous lead traction from above and below: A novel technique to reduce the risk of superior vena cava injury during transvenous lead extraction. Heart Rhythm 2018, 15, 1655–1663. [Google Scholar] [CrossRef]
- Mehrotra, D.; Kejriwal, N.K. Tricuspid valve repair for torrential tricuspid regurgitation after permanent pacemaker lead extraction. Tex. Heart Inst. J. 2011, 38, 305–307. [Google Scholar]



| TR Before TLE | TR After TLE | Number of Patients | Percentage of 2631 Patoents | Worsening of TR in Degrees | |
|---|---|---|---|---|---|
| All patients without worsening of TV function | 0–4 | 0–4 | 2376 | 90.31% | 0 |
| Non-significant (for 1 degree) Impairment of TV function (7.16%) | 0 | 1 | 27 | 1.03% | 1 |
| 1 | 2 | 87 | 3.31% | 1 | |
| 2 | 3 | 52 | 1.98% | 1 | |
| 3 | 4 | 22 | 0.84% | 1 | |
| Significant (for 2 or 3 degrees) worsening of TV function 67 (2.55%) | 0 | 2 | 3 | 0.11% | 2 |
| 1 | 3 | 43 | 1.63% | 2 | |
| 2 | 4 | 9 | 0.34% | 2 | |
| 0 | 3 | 1 | 0.04% | 3 | |
| 1 | 4 | 11 | 0.42% | 3 | |
| All patients with worsening of TV function | 255 | 9.69% | |||
| All examined patients | 2631 | 100.0% | |||
| Severe damage of TV during TLE reaching indications for cardiac surgery | |||||
| Tricuspid valve plastic repair performed immediately after TLE us rescue procedure | 2 | 0.08% | |||
| Tricuspid valve plastic repair performed as planned procedure | 8 | 0.30% | |||
| Tricuspid valve replacement | 2 | 0.08% | |||
| Borderline indications-observation only | 8 | 0.30% | |||
| Refused TV plastic repair-conservative treatment | 3 | 0.11% | |||
| Disqualification from TV plastic repair (cancer) | 1 | 0.04% | |||
| Other patients | 2607 | 99.09% | |||
| Patient-Related Potential Risk Factors of TVDrTLE | TR Increased for 2 or 3 Degrees | TR Increased for 1 Degree | TR Unchanged or Decreased | “U” Mann-Whitney Ch2 Tests | |||||
|---|---|---|---|---|---|---|---|---|---|
| Number of Patients/Number of the Group | 67 | A | 188 | B | 2376 | C | p Values | p Values | p Values |
| Presented values | N/mean | Sd/% | N/mean | Sd/% | N/mean | Sd/% | A vs. B | A vs. C | B vs. C |
| Clinical characteristics | |||||||||
| Patient’s age during TLE [y] | 62.42 | 19.28 | 66.58 | 14.46 | 67.01 | 14.83 | 0.038 | 0.397 | 0.756 |
| Patient’s age during first system implantation [y] | 47.34 | 21.77 | 55.97 | 16.17 | 58.67 | 15.82 | 0.029 | <0.001 | 0.034 |
| Sex (female) | 31 | 46.27% | 77 | 40.96% | 935 | 39.32% | 0.541 | 0.310 | 0.722 |
| NYHA class III & IV | 3 | 4.48% | 20 | 10.64% | 381 | 16.04% | 0.207 | 0.017 | 0.063 |
| LVEF < 40% | 10 | 14.93% | 43 | 22.72% | 754 | 31.75% | 0.230 | 0.005 | 0.012 |
| Permanent AF | 7 | 10.44% | 49 | 26.06% | 549 | 23.11% | 0.013 | 0.022 | 0.382 |
| Charlson’s index [points] | 3.43 | 3.55 | 4.59 | 3.51 | 4.87 | 3.69 | 0.005 | 0.001 | 0.775 |
| All infections indications | 16 | 23.88% | 68 | 36.17% | 742 | 31.23% | 0.092 | 0.251 | 0.186 |
| PADIT score | 5.62 | 2.72 | 5.24 | 2.86 | 4.76 | 2.81 | 0.316 | 0.021 | 0.010 |
| All non-infective indications | 51 | 76.12% | 120 | 63.83% | 1634 | 68.77% | 0.092 | 0.251 | 0.186 |
| System and history of pacing | |||||||||
| Device type-PM (AAI, VVI, DDD, CRT-P) | 61 | 91.05% | 149 | 79.26% | 1648 | 69.36% | 0.047 | <0.001 | 0.006 |
| Device type-ICD (VVI, DDD) | 4 | 5.97% | 32 | 17.02% | 536 | 22.54% | 0.043 | 0.001 | 0.095 |
| Device type-CRT-D | 2 | 2.99% | 7 | 3.72% | 192 | 8.08% | 0.917 | 0.196 | 0.045 |
| Presence of abandoned lead before TLE | 15 | 22.39% | 30 | 15.96% | 234 | 9.85% | 0.318 | 0.001 | 0.012 |
| Number of abandoned leads before TLE | 0.28 | 0.60 | 0.22 | 0.56 | 0.13 | 0.42 | 0.437 | 0.071 | 0.134 |
| 4 and >4 leads before TLE | 3 | 4.48% | 11 | 5.85% | 64 | 2.69% | 0.913 | 0.615 | 0.025 |
| Dwell time of oldest lead in the patient before TLE [months] | 181.3 | 87.23 | 127.7 | 72.41 | 100.9 | 74.64 | <0.001 | <0.001 | <0.001 |
| TLE Procedure Complexity, Efficacy, Complications, Outcomes and Long-Term Mortality After TLE | TR Increased for 2 or 3 Degrees | TR Increased for 1 Degree | TR Unchanged or Decreased | “U” Mann-Whitney Ch2 Tests | |||||
|---|---|---|---|---|---|---|---|---|---|
| Number of patients number of the group | 67 | A | 188 | B | 2376 | C | p | p | p |
| Presented values | N/mean | Sd/% | N/mean | Sd/% | N/mean | Sd/% | A vs. B | A vs. C | B vs. C |
| TLE procedure complexity | |||||||||
| Procedure duration (sheath to sheath) [minutes] | 31.00 | 36.14 | 22.19 | 33.79 | 13.72 | 20.46 | <0.001 | <0.001 | <0.001 |
| Average time of single lead extraction (sheath-to sheath/number of extracted leads) [minutes] | 18.39 | 24.14 | 11.59 | 14.77 | 8.02 | 11.37 | 0.005 | <0.001 | <0.001 |
| Technical problem during TLE (any); n (%) | 32 | 47.76% | 55 | 29.26% | 462 | 19.44% | 0.010 | <0.001 | 0.002 |
| Two or more technical problems; n (%) | 11 | 16.42% | 10 | 5.32% | 103 | 4.34% | 0.005 | <0.001 | 0.527 |
| Lead to lead strong connection (intraprocedural diagnosis); n (%) | 15 | 22.39% | 22 | 11.70% | 153 | 6.44% | 0.033 | <0.001 | 0.006 |
| Procedure-related potential risk factors of major complications | |||||||||
| Extraction of ICD leads; n (%) | 7 | 10.45% | 38 | 20.21% | 687 | 28.92% | 0.072 | <0.001 | 0.011 |
| Extraction of abandoned lead; n (%) | 15 | 22.39% | 30 | 15.96% | 214 | 9.01% | 0.010 | <0.001 | <0.001 |
| Extraction of UP lead; n (%) | 24 | 35.82% | 32 | 17.02% | 229 | 9.64% | <0.001 | <0.001 | <0.001 |
| Passive fixation | 53 | 79.10 | 135 | 71.81 | 1316 | 55.38 | 0.316 | <0.001 | <0.001 |
| Dwell time of oldest lead extracted in the patient [months] | 178.8 | 89.48 | 124.8 | 71.20 | 99.44 | 73.64 | <0.001 | <0.001 | <0.001 |
| Cumulative dwell time of extracted leads; [y] | 26.05 | 16.95 | 18.19 | 13.54 | 13.61 | 12.53 | <0.001 | <0.001 | <0.001 |
| Mean duration of extracted lead (in the whole group); [months] | 163.6 | 89.61 | 121.5 | 69.10 | 97.14 | 71.61 | <0.001 | <0.001 | <0.001 |
| Risk of major complications calculated by SEFETY TLE calculator (%) | 4.03 | 4.33 | 2.71 | 3.81 | 1.71 | 3.02 | 0.012 | <0.001 | <0.001 |
| Utility of additional tools | |||||||||
| Evolution (old and new) or TightRail; n (%) | 5 | 7.46% | 7 | 3.72% | 30 | 1.26% | 0.356 | 0.002 | 0.016 |
| Lasso catheter/snare; n (%) | 15 | 22.39% | 9 | 4.79% | 76 | 3.20% | <0.001 | <0.001 | 0.333 |
| TLE efficacy and complications | |||||||||
| Major complications (any); n (%) | 20 | 29.95% | 5 | 2.66% | 24 | 1.01% | <0.001 | <0.001 | 0.089 |
| Hemopericardium; n (%) | 4 | 5.97 | 4 | 2.13% | 21 | 0.86% | 0.254 | <0.001 | 0.199 |
| Death procedure related (intra-, post-procedural); n (%) | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% | |||
| Death indication-related (intra, post-procedural); n (%) | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% | |||
| Partial radiological success (remained tip or <4 cm lead fragment); n (%) | 11 | 16.42% | 5 | 2.66% | 94 | 3.96% | <0.001 | <0.001 | 0.489 |
| Full clinical success; n (%) | 49 | 73.13% | 185 | 98.40% | 2342 | 98.57% | <0.001 | <0.001 | 0.892 |
| Full procedural success; n (%) | 41 | 61.19% | 182 | 96.81% | 2287 | 96.25% | <0.001 | <0.001 | 0.852 |
| Death during whole FU; n (%) | 10 | 14.93% | 62 | 32.98% | 770 | 30.41% | 0.008 | 0.004 | 0.936 |
| Echocardiographic Findings/Abnormalities Recorded in Patients Undergoing TLE | TR Increased for 2 or 3 Degrees | TR Increased for 1 Degree | TR Unchanged or Even Decreased | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Number of patients number of the group | 67 | A | 188 | B | 2376 | C | p | p | p |
| Presented values | Count/average | %/Sd | Count/average | %/Sd | Count/average | %/Sd | A vs. B | A vs. C | B vs. C |
| ECHO before and after TLE | |||||||||
| LVEF (average) | 55.93 | 11.40 | 51.88 | 13.54 | 49.13 | 15.51 | 0.030 | <0.001 | 0.018 |
| TR significant (3 and 4 degree) | 0/67 | 0.00% | 22/188 | 11.70% | 511/2376 | 21.51% | 0.003 | <0.001 | 0.001 |
| Any shadows on the leads before TLE | |||||||||
| Any shadows on leads before TLE | 42/67 | 62.69% | 114/188 | 60.64% | 1172/2376 | 49.33% | 0.768 | 0.031 | 0.003 |
| Connecting tissue surrounding the lead | 10/67 | 14.93% | 23/188 | 12.23% | 237/2376 | 9.98% | 0.573 | 0.185 | 0.323 |
| Thickening of the lead | 26/67 | 38.81% | 46/188 | 24.47% | 431/2376 | 18.14% | 0.025 | <0.001 | 0.032 |
| Strong connective tissue scar connection of the lead with heart structures (any) | 31/64 | 48.44% | 46/188 | 24.47% | 261/2301 | 11.34% | <0.001 | <0.001 | <0.001 |
| Strong connective tissue scar connection of the lead with tricuspid apparatus | 26/57 | 45.61% | 26/188 | 13.83% | 88/2376 | 3.70% | <0.001 | <0.001 | <0.001 |
| Strong connective tissue scar connection of the lead with RA wall | 5/67 | 7.46% | 10/188 | 5.32% | 95/2376 | 4.00% | 0.522 | 0.158 | 0.379 |
| Strong connective tissue scar connection of the lead with SVC | 12/67 | 17.91% | 10/188 | 5.32% | 91/2376 | 3.83% | 0.002 | <0.001 | 0.312 |
| Strong connective tissue scar connection of the lead with RV wall | 27/67 | 40.30% | 24/188 | 12.77% | 120/2376 | 5.05% | <0.001 | <0.001 | <0.001 |
| Strong connection of the lead with another lead with connecting tissue scar | 14/67 | 20.90% | 26/188 | 13.83% | 214/2376 | 9.01% | 0.172 | <0.001 | 0.029 |
| Phenomena observed during monitoring of TLE procedure with TEE (only in patients monitored by TEE during TLE) | |||||||||
| Drawing of RA/RAA during mechanical lead extraction | 22/48 | 45.83% | 50/106 | 47.17% | 431/2376 | 18.14% | 0.877 | <0.001 | <0.001 |
| Drawing of tricuspid leflet during mechanical lead extraction | 33/48 | 68.75% | 28/106 | 26.42% | 64/2376 | 2.69% | <0.001 | <0.001 | <0.001 |
| Drawing of RV wall during mechanical lead extraction | 32/48 | 66.67% | 48/106 | 45.28% | 271/2376 | 11.41% | 0.014 | <0.001 | <0.001 |
| Abnormal lead loops visible in preoperative TTE/TEE | |||||||||
| Lead loops in the heart (any)/ECHO | 19/66 | 28.79% | 54/188 | 28.72% | 408/2376 | 17.17% | 0.992 | 0.014 | <0.001 |
| Loop in the RA | 12/67 | 17.91% | 39/187 | 20.86% | 300/2376 | 12.63% | 0.606 | 0.201 | 0.001 |
| Loop in the TV | 5/67 | 7.46% | 16/186 | 8.60% | 98/2376 | 4.13% | 0.772 | 0.180 | 0.004 |
| Loop in the RV | 8/67 | 11.94% | 14/187 | 7.49% | 118/2376 | 4.97% | 0.266 | 0.012 | 0.133 |
| Univariable Regression | Multivariable Regression | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| Mild TLE-related tricuspid valve damage risk factors | ||||||
| Presence of abandoned lead before TLE [yes/no] | 1.762 | 1.176–2.642 | 0.006 | 1.624 | 1.002–2.632 | 0.048 |
| TR before TLE [by one degree] | 0.610 | 0.501–0.743 | 0.001 | 0.546 | 0.443–0.674 | <0.001 |
| Strong CTS connection of the lead with tricuspid apparatus [yes/no] | 4.443 | 2.779–7.106 | <0.001 | 3.452 | 1.964–6.640 | <0.001 |
| Lead loops in the heart (any)/ECHO [yes/no] | 2.001 | 1.430–2.799 | <0.001 | 1.726 | 1.203–2.476 | 0.003 |
| Severe TLE-related tricuspid valve damage risk factors | ||||||
| Device type (AAI, VVI, DDD, CRT-P) [yes/no] | 5.289 | 2.112–13.24 | 0.001 | 5.264 | 1.182–23.45 | 0.029 |
| Dwell time of oldest one extracted lead [by one year] | 1.124 | 1.091–1.157 | <0.001 | 1.076 | 1.006–1.151 | 0.032 |
| Extraction of UP lead [yes/no] | 2.581 | 1.788–3.756 | <0.001 | 1.776 | 0.971–3.245 | 0.062 |
| TR before TLE [by one degree] | 0.306 | 0.193–0.485 | 0.001 | 0.155 | 0.078–0.305 | <0.001 |
| Strong CTS connection of the lead with tricuspid apparatus [yes/no] | 15.43 | 8.830–26.96 | <0.001 | 5.720 | 2.378–13.75 | <0.001 |
| Strong CTS connection of the lead with RV wall [yes/no] | 13.79 | 8.064–23.58 | <0.001 | 8.312 | 3.484–19.83 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polewczyk, A.; Jacheć, W.; Nowosielecka, D.; Tomaszewski, A.; Brzozowski, W.; Szczęśniak-Stańczyk, D.; Duda, K.; Kutarski, A. Tricuspid Valve Damage Related to Transvenous Lead Extraction. Int. J. Environ. Res. Public Health 2022, 19, 12279. https://doi.org/10.3390/ijerph191912279
Polewczyk A, Jacheć W, Nowosielecka D, Tomaszewski A, Brzozowski W, Szczęśniak-Stańczyk D, Duda K, Kutarski A. Tricuspid Valve Damage Related to Transvenous Lead Extraction. International Journal of Environmental Research and Public Health. 2022; 19(19):12279. https://doi.org/10.3390/ijerph191912279
Chicago/Turabian StylePolewczyk, Anna, Wojciech Jacheć, Dorota Nowosielecka, Andrzej Tomaszewski, Wojciech Brzozowski, Dorota Szczęśniak-Stańczyk, Krzysztof Duda, and Andrzej Kutarski. 2022. "Tricuspid Valve Damage Related to Transvenous Lead Extraction" International Journal of Environmental Research and Public Health 19, no. 19: 12279. https://doi.org/10.3390/ijerph191912279
APA StylePolewczyk, A., Jacheć, W., Nowosielecka, D., Tomaszewski, A., Brzozowski, W., Szczęśniak-Stańczyk, D., Duda, K., & Kutarski, A. (2022). Tricuspid Valve Damage Related to Transvenous Lead Extraction. International Journal of Environmental Research and Public Health, 19(19), 12279. https://doi.org/10.3390/ijerph191912279






