1. Introduction
The fight against climate change, good health and well-being are, respectively, the thirteenth and the third Sustainable Development Goals (SDGs) of the United Nations.
Section 3.3. of the third SDG aims to end the HIV/AIDS epidemic, tuberculosis, malaria and neglected tropical diseases and to combat hepatitis, water-borne diseases and other communicable diseases by 2030.
Global warming is the phenomenon of increasing average ocean and air temperatures, induced by the quantity of heat trapped on the earth’s surface. In humanitarian terms, an increase in extreme weather events leads to torrential rains, storms, droughts, as well as an increase in diseases with epidemic potential such as malaria, diarrheal and respiratory diseases.
Malaria is a vector-borne disease whose existence and transmission depend on three main factors: the Plasmodium parasite, the Anopheles vector and the human host. Beyond these essential factors, the risk of malaria transmission can be maintained or increased by environmental or climatic conditions as well as socio-economic factors [
1].
In the past, several authors had already highlighted the relationship between climate variations and malaria endemicity [
2,
3,
4].
Malaria constitutes a global burden in terms of morbidity and mortality. In 2019, 229 million malaria cases in 91 countries were recorded, an increase from 211 million cases in 2015. Globally, the number of deaths from malaria was estimated at 409,000 in 2019, compared with 446,000 in 2015. The WHO African Region carries a disproportionately high share of the global malaria burden with 94% of malaria cases and deaths occurring in 2019 [
5].
Environmentally related morbidity and mortality still remain high globally, although they have declined significantly in recent decades [
6].
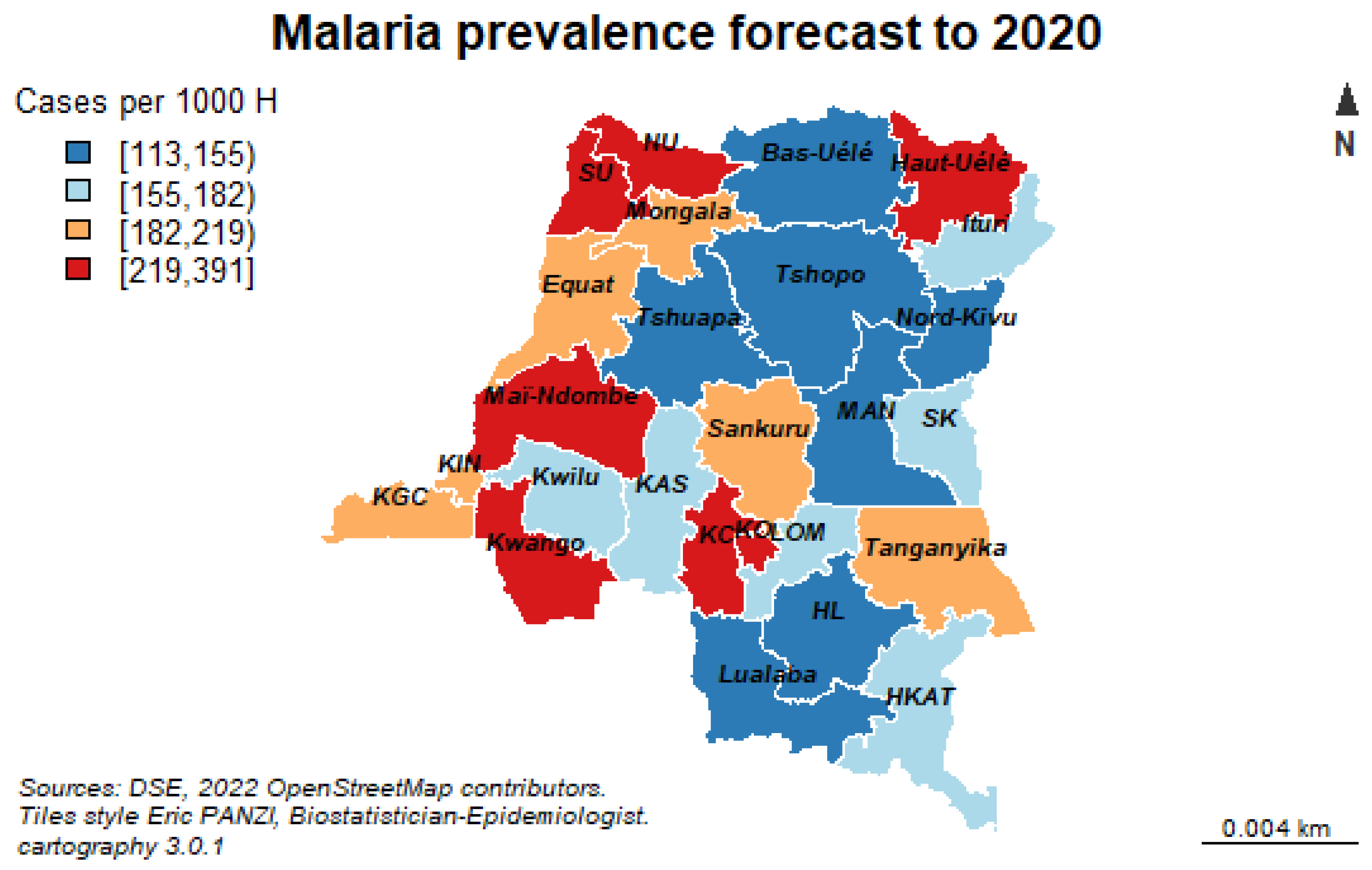
In sub-Saharan Africa in general and in the Democratic Republic of Congo (DRC) in particular, where malaria represents the greatest burden of disease, studies on forecasting malaria prevalence based on an environmental and longitudinal approach are rare.
Much published research on the relationship between malaria and climate change has emphasized on the increased risk posed by the expansion of malaria-prone areas. In some parts of Africa, the number of malaria cases is decreasing and scientists believe that this is due not only to prevention measures but also to the effects of climate change on rainfall [
7,
8,
9].
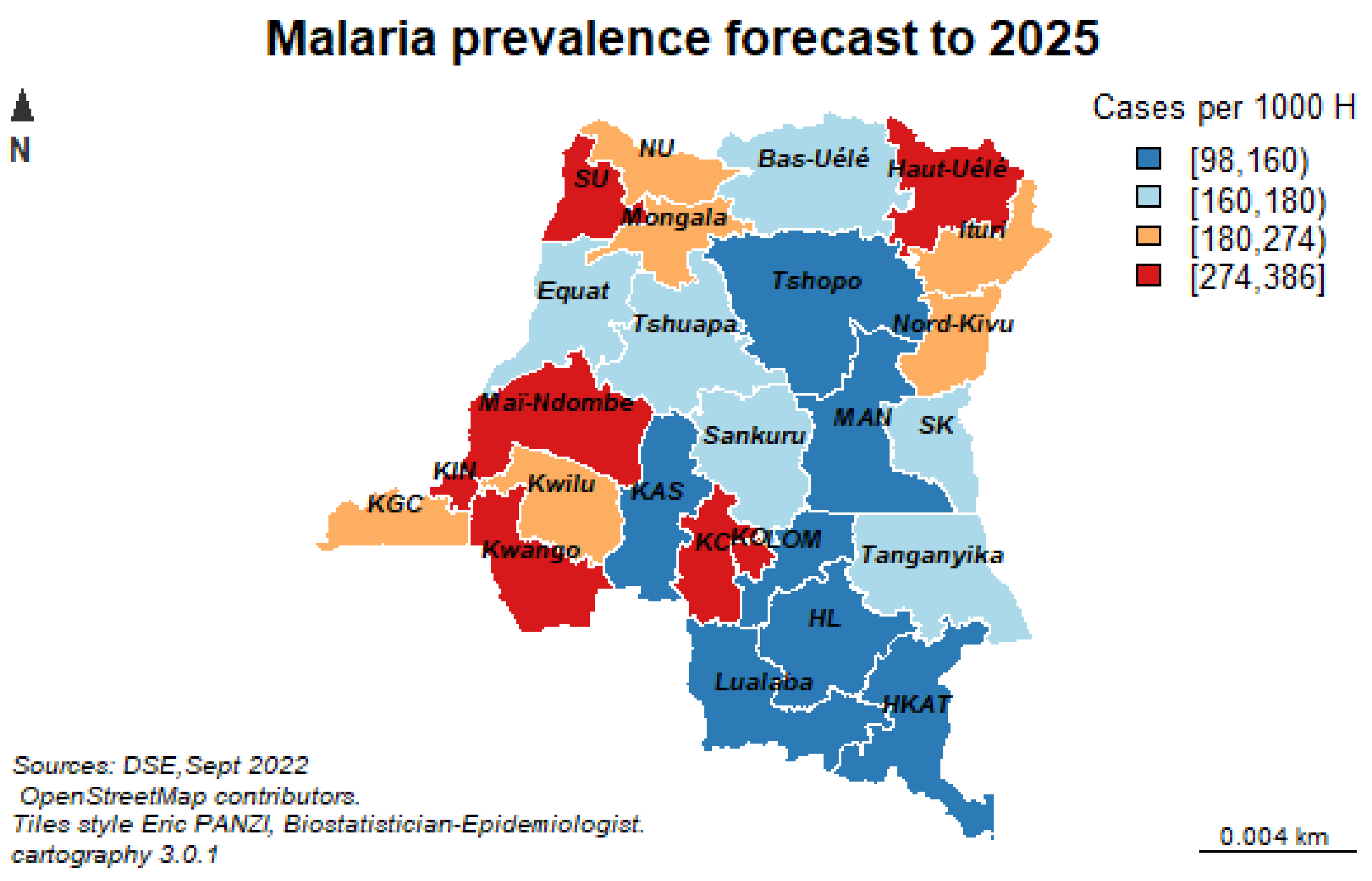
The purpose of this study is to forecast malaria episodes to 2036 using time series forecasting models in order to strengthen the surveillance and response to diseases with epidemic potential throughout the DRC.
4. Discussion
Malaria is caused by infection with protozoan parasites of the species Plasmodium. Plasmodium falciparum is widespread in Africa, while
P. vivax,
P. ovale and
P. malariae infections are less common and geographically limited [
14,
15].
Globally, the number of malaria cases is estimated to be 228 million in 2018 (95% confidence interval [CI]: 206–258 million), compared with 251 million in 2010 (95% CI: 231–278 million) and 231 million in 2017 (95% CI: 211–259 million) [
16].
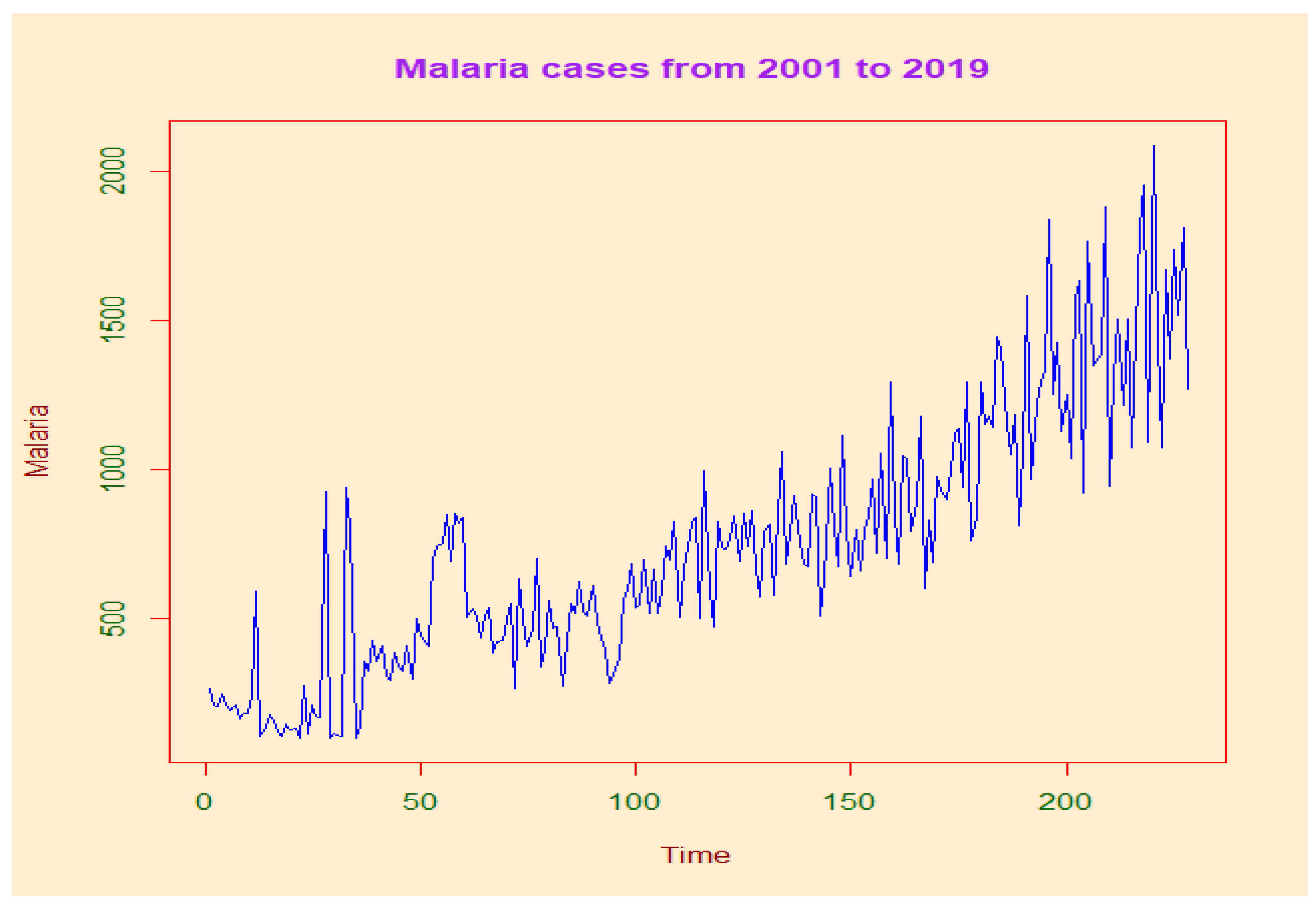
In DRC, our results show an average malaria prevalence of the last 19 years of 13,246 (11,783.83–1417.83) cases per 100,000 people at risk. This prevalence increases significantly during the whole study period. The year 2002 was more morbid with 29,137.99 (12,094.51–38,304.56) cases per 100,000 persons at risk.
Most cases in 2018 (213 million or 93%) were recorded in the WHO African region, far ahead of the Southeast Asian region (3.4%) and the Eastern Mediterranean region (2.1%) [
16]. Our study showed that malaria-attributable mortality had reached 117.33 (71.08–163.58) malaria deaths per 100,000 people exposed to malaria in 2002. In 2008, the lowest level recorded was 19.04 (14.14–23.35) malaria deaths per 100,000 people exposed to malaria. In general, malaria mortality has been declining dramatically over the past 19 years.
The same observations were made by WHO 2019 showing that on a global scale, malaria incidence declined between 2010 and 2018 from 71 cases per 1000 people at risk of malaria to 57 per 1000. Nevertheless, this decline slowed considerably between 2014 and 2018, with incidence decreasing to 57 per 1000 in 2014 and remaining at a similar level through 2018 [
16].
The WHO Africa region alone accounted for 94% of global malaria deaths in 2018. Yet, it also accounted for 85% of the 180,000 fewer deaths from the disease compared to 2010 [
16]. Nearly 85% of global malaria deaths in 2018 were concentrated in 20 countries in the WHO African region and India. Nigeria alone accounted for nearly 24% of these deaths, followed by the Democratic Republic of Congo (11%), the United Republic of Tanzania (5%), and Angola, Mozambique, and Niger (4% each) [
16].
In our study, Spearman’s correlation analysis indicates a statistically significant association between evaporation at two meters from the ground and the number of annual malaria cases. A statistically insignificant association was observed between the rest of the climate variables and the number of annual malaria cases. In contrast, in South Africa, a statistically significant association between all climate variables studied and monthly malaria cases was reported where minimum temperature had the highest correlation (R = 0.39;
p < 0.001), followed by total quantity of rainfall at two meters altitude and mean temperature (r = 0.35;
p < 0.001, r = 0.35;
p < 0.001), then relative humidity and maximum temperature with (R = 0.29;
p < 0.001, r = 0.25;
p < 0.001), respectively [
17].
This difference could be related to the disparity in transmission levels in Africa, both in intensity and in seasonality and regularity. This disparity has consequences at several levels: biological, immunological, pathological, etc. Subjected to a strong and permanent plasmodial infection, the population develops a premunition, a temporary immunity, non-sterilizing and maintained by the infection, itself maintained by the transmission.
It should be noted that South Africa is located in an area where transmission is regular every year, with a long seasonal interruption of about 6 months, linked to the rhythm of the rains. The populations of these areas have a very high degree of resistance to malaria but are not totally free of “moderate” malaria attacks and may be susceptible to severe malaria attacks in case of plasmodial infections by foreign strains [
18].
Wilson’s classifications of epidemiological facies as “models” reflect the dynamics of host-vector-parasite relationships in different environments, and he points out that all intermediate situations can be found within and between these basic models.
There are seasonal variations in the intensity of transmission, but no interruption, however brief. Transmission is generally by An. gambiae and An. funestus. This mode of transmission is well illustrated by the degraded forest areas of Central Africa (DRC, Congo, Cameroon, etc.).
This approach by Wilson highlights the transmission/malaria disease relationships, taking into consideration the intensity of transmission (chance of infection or frequency of infection), the duration of transmission with its regularity from one year to the next (transmission continues nearly all-year round or annually recurring season or seasons of malaria transmission), the ecological variables (altitude, temperature, humidity) with the relative development of immunity. Variations in these different variables are reflected in variations in the classical malaria indices (splenic index, plasmodic index) but above all in malaria morbidity according to age groups and general mortality in the event of an epidemic.
Some authors agree with Wilson’s categorization of epidemiological facies where the DRC/Zaire is located in the Degraded Forest Zone: permanent transmission (=Wilson’s Group I): where malaria is endemic with intense and permanent transmission [
19].
The results of our study reveal a negative but not statistically significant association observed between the remaining number of annual malaria cases and wind speed, number of rainy days, maximum and minimum temperature. Our results concur with those found by Lindsay et al. showing a negative but statistically significant association between rainfall and malaria cases, which could be due to the fact that the breeding sites were washed away by heavy rains [
20].
The significant relationships observed with a lag of more than one month between malaria onset and associated climatic variables (p < 0.05), such as rainfall and minimum temperature at two months, suggest that climatic conditions in a given year (early onset of rains, especially those associated with La Niña conditions and tropical cyclones) may affect malaria transmission in the following year.
Periods of heavy rainfall combined with low temperatures and high relative humidity promote saturated soil moisture, which prolongs the life of water pockets and may result in the persistence of larval habitats [
21,
22].
Several forecasting models were used in this study to predict future malaria episodes. Some authors used the SARIMAX model to forecast monthly time series of malaria cases using the SARIMA Box-Jenkins approach and multiple linear regression (MLR). The forecast shows that the number of malaria cases remains high until July, which is normally a period of low malaria transmission, indicating a change in the malaria season [
17].
For Kumar et al. [
23], the autoregressive integrated moving average, ARIMA (0,1,1) (0,1,0) 12, was the best fitting model and could explain 72.5% variability in the time series data. Rainfall (
p = 0.004) and relative humidity (
p = 0.001) were found to be significant predictors for malaria transmission in the study area. The seasonal adjustment factor (SAF) for malaria cases showed a peak during August and September. Although ARIMA time series analysis models are a simple and reliable tool for producing reliable forecasts for malaria in Delhi, India, in our study however, the combination of several forecasts led to higher forecast accuracy.
The combination of multiple model forecasts (ETS, ARIMA, STL-ETS, NNAR, and BATS) fit better and led to greater accuracy in modeling and forecasting for future malaria infestations over the next 200 months. The results were virtually unanimous: the combined model approach predicts an average increase of 309,198 new malaria infestations per year per province over the next 16 years in DRC. However, the NNAR predictive model was particularly well suited in this series.
Wangdi et al. [
24] also found that ARIMA (2,1,1) (0,1,1) 12 was the best possible model for predicting malaria cases in Bhutan. In the same vein, The ARIMA model has also been used for malaria case prediction in Sri Lanka [
25] and Ethiopia [
26]. To evaluate the predictive performance of our model, we divided our data set into a training set and a test set of sizes 90% and 10%, respectively.
The mean absolute scaled error (MASE) as a measure of the quality of the predictions made by our models showed that the naive seasonal method was the best of the three methods for this data set with a MASE of 0.894. Recall that the closer the MASE is to zero, the better the forecast.
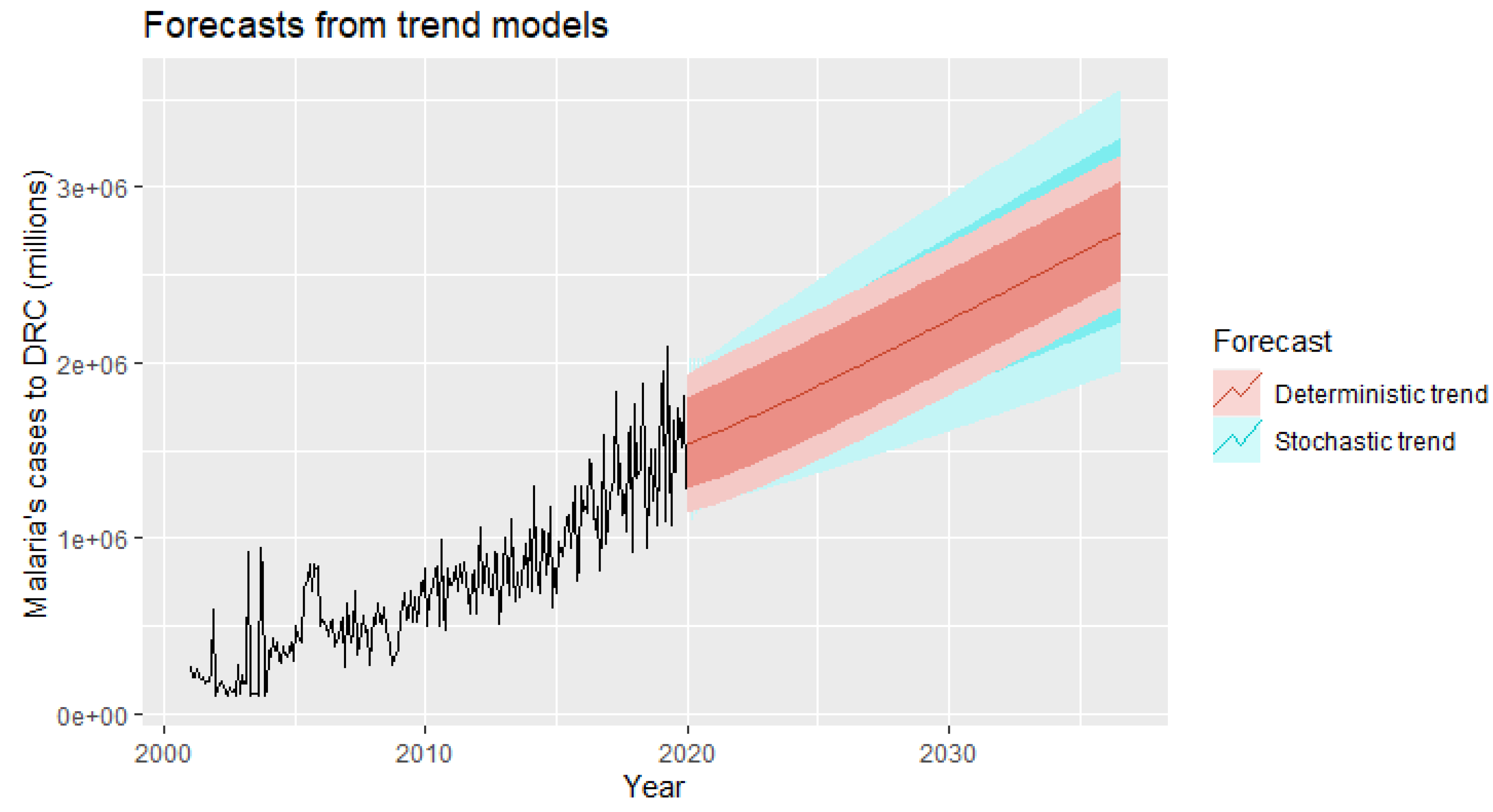
Our forecasting horizon was set at 2036, and it was desirable and wise to finally retain the stochastic model, as it is safer to forecast with stochastic trends, especially for longer forecasting horizons, as the forecasting intervals allow for greater uncertainty about future growth.
Our stochastic model predicts an average annual increase of 6024.07 new malaria cases per facility.
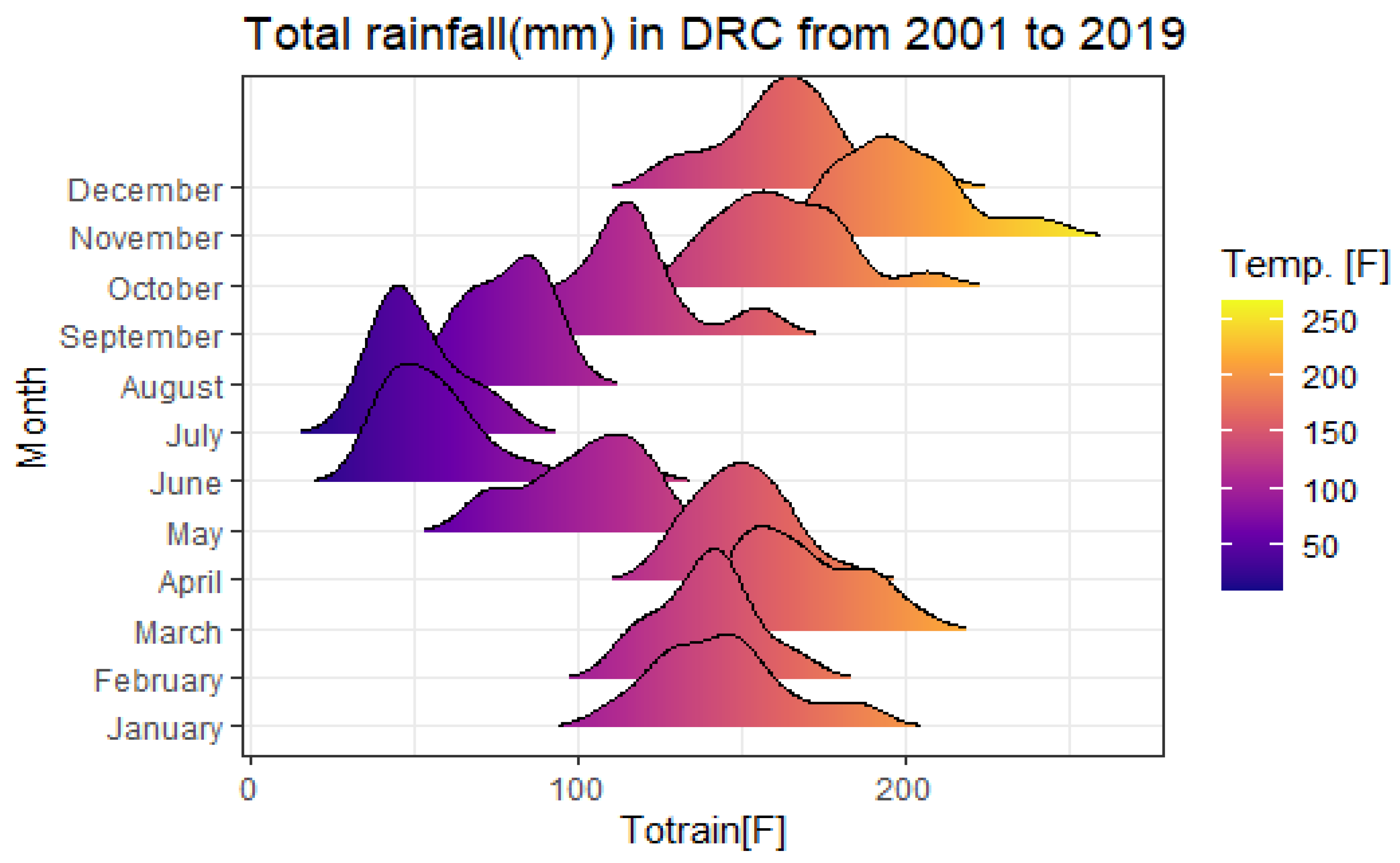
As for the estimated seasonal component, this study shows a slight decrease in malaria cases from January to February throughout the study period, rising again in March and reaching the peak in April. It declines to lowest values in June, but rises again to the peak in July, September and November. In addition, malaria is present almost year-round in the DRC due to the tropical climate and the country’s extensive river system. The DRC has about 30 major rivers with at least 20,000 km of shoreline and 15 lakes with about 180,000 km of shoreline.
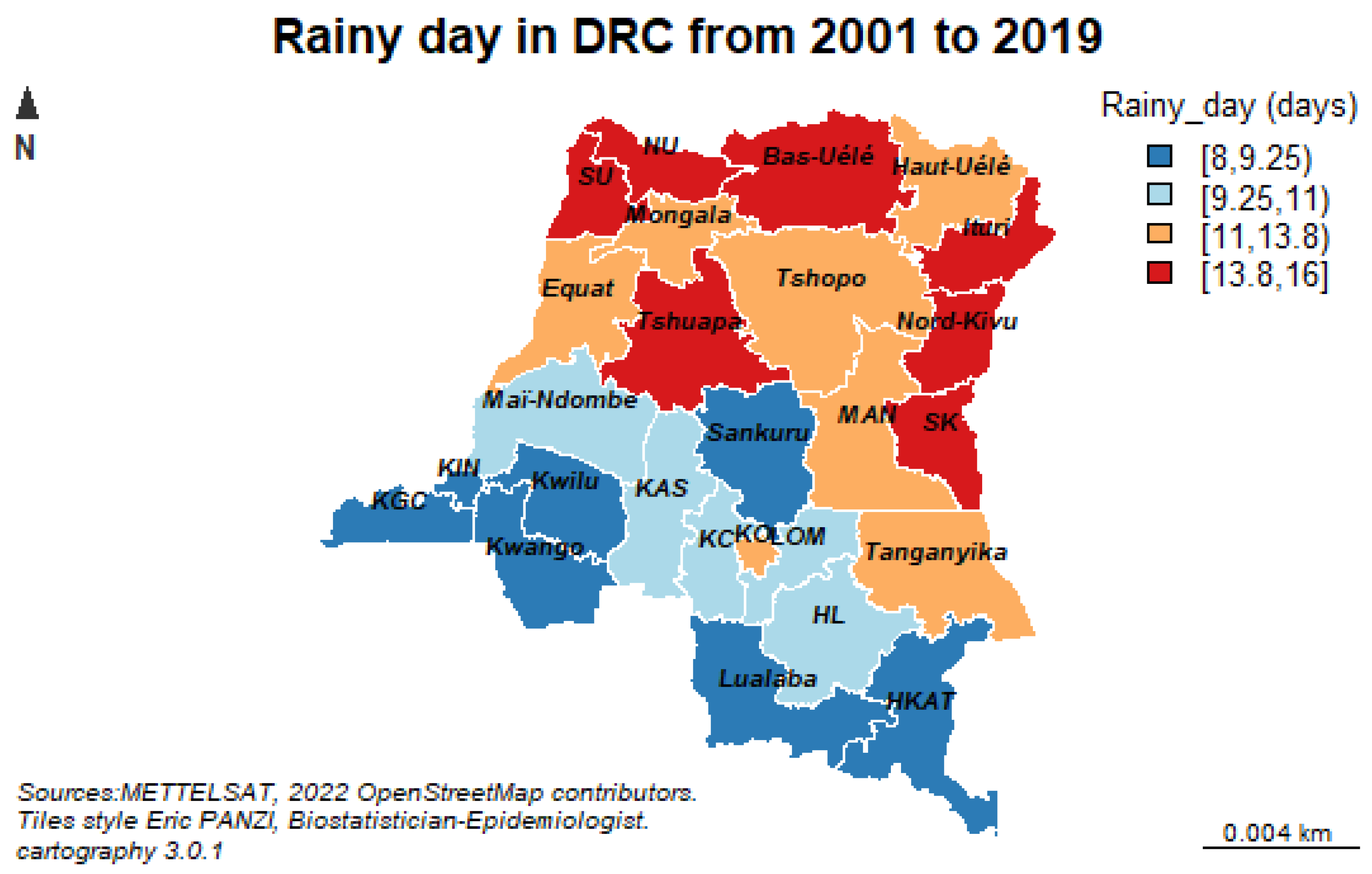
As for the “seasonality” component, this study reveals that the “Palmet” series will have variation between months simply due to the different number of days in each month, in addition to the seasonal variation in future years. This disparity is closely related to the variability of precipitation during the study period showing very intense palustrine episodes from January to June and then from September to December. On the other hand, a decrease in rainfall is observed between July and October (dry season).
The relief of the DRC can also have an impact on the seasonality of malaria. There is a vast low-lying area in the center of the country, which is a plateau shaped by the westward flowing river basin and covered by a large tropical forest. This area is surrounded by mountainous terraces such as the Mitumba Mountains in the east and the Virunga Mountains in the north, savanna-covered plateaus in the south and southwest, and the north is bordered beyond the river by dense forest. High mountains are found at the eastern end of the country (Great Rift region).
A study conducted in Kenya shows an increase in malaria cases in the high and medium altitude areas while in the riverine and lowland areas, the number of malaria cases had significantly decreased as of 2011, during the study period [
27].
Finally, our results of the multivariate time series analysis showed that over the next 16 years, the number of malaria cases will increase from its historical average value of 653,071 cases per year due to increase in geo-climatic factors such as evaporation under shelter at two meters from the ground, relative soil moisture, high rainfall and population growth with a density of one inhabitant per square kilometer will significantly increase the number of malaria cases from its historical mean value each year. On the other hand, increasing the minimum temperature by one degree Celsius each year will lead to an average decrease in malaria cases by 23.287 of its historical average value per year.
Our previous study conducted in 2022 showed that the number of rainy days resulted in a statistically significant 7% increase in malaria cases with malaria morbidity significantly associated with geographic location, total evaporation under shelter, maximum daily temperature at two meters altitude, and humidity at two meters altitude [
28].
Our results are similar to those of Solomon Kibret et al. in their study on environmental and meteorological factors related to malaria transmission around large dams in three ecological settings in Ethiopia, which showed that Rainfall (lagged 1 and 2 months) was significantly associated with malaria incidence, but only at the lowland dam, while minimum and maximum air temperatures (lagged 1 and 2 months) were significant factors only at the highland dam [
29].
The polynomial model Mopuri R et al. had predicted malaria cases with high predictive power and revealed that first lag malaria cases and population play a vital role in malaria transmission. Similarly, mean temperature, rainfall and normalized vegetation index had significant impact on malaria cases [
30].
In the same perspective, the results of Nkiruka et al. suggest that, although the exact association between malaria incidence and climate variability varies across geographic regions, non-seasonal changes in three climatic factors (rainfall, temperature, and surface radiation) contribute significantly to malaria occurrence [
31].
The results of Sahai et al. agree with ours. The authors note that changes in rainfall and temperature regimes probably play a major role in increasing the incidence of these diseases in different geographical locations. A detailed analysis was conducted on the incidence of malaria and diarrhea in two districts of Maharashtra State. It is found that the increased likelihood of high (low) rainfall, high (low) minimum temperatures and low (moderate) maximum temperatures are more (less) conducive to both diseases in these areas, but with different thresholds [
32].