Dynamic Changes in Soil Phosphorus Accumulation and Bioavailability in Phosphorus-Contaminated Protected Fields
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Soils
- Soils for research on the characteristics of accumulated phosphorus: typical plastic greenhouses cultivated on dark brown soil in Longjing City, Yanbian Prefecture, Jilin Province were chosen, and at each, soil samples were collected at depths of 0–20 cm and 20–40 cm. Three protected field soil samples were collected from Longchi village (No. 1, 2, and 3), four from Longhai village (No. 4, 5, 7, and 8), and one from Longfeng village (No. 6). Simultaneously, a farmland (i.e., non-protected, open-air vegetable field) soil sample was collected from Longhai village as a control (No. 9). For each sample, the soil total phosphorus, available phosphorus, different forms of inorganic phosphorus, and organic phosphorus were measured, and for the protected fields, the characteristics of the soil accumulated phosphorus were also analyzed.
- Soils for the continuous spinach planting without phosphate fertilization experiment: Soil was collected from 17 greenhouses in Longjing City (7 in Longchi village and 10 in Longfeng village). These are protected-field soil samples with an obvious phosphorus accumulation gradient and similar texture. At the same time, there were three places in Longfeng village (No. A, B, and C) and two places in Longchi village (No. D and E). A farmland soil sample was used as a control (No. F). Table 1 lists the basic properties.
2.2. Experimental Crops
2.3. Experimental Design and Methods
2.4. Analysis of Soil Samples
2.5. Data Analysis
3. Results
3.1. Soil Accumulated Phosphorus Characteristics in Protected Fields
3.1.1. Soil Phosphorus Composition in Protected Fields
3.1.2. Composition of Inorganic Phosphorus in Protected Fields
3.2. Changes to Inorganic Phosphorus Content over Successive Planting Stubbles
3.2.1. Changes to Water-Soluble and Loosely Combined Phosphorus (WSLC-P) Content over Successive Planting Stubbles
3.2.2. Changes to Aluminum P (Al-P) Content over Successive Planting Stubbles
3.2.3. Changes to Iron P (Fe-P) Content over Successive Planting Stubbles
3.2.4. Changes to Calcium P (Ca-P) Content over Successive Planting Stubbles
3.2.5. Changes to Occluded P (O-P) Content over Successive Planting Stubbles
3.2.6. Changes to Total Inorganic Phosphorus Content over Successive Planting Stubbles
3.3. Changes to Available Phosphorus Content over Successive Planting Stubbles
3.4. Determination of the Critical Value of Phosphorus Deficiency and Establishment of a Prediction Model of Planting Stubbles
4. Discussion
4.1. Soil Phosphorus Accumulation
4.2. Changes in Inorganic Phosphorus Content
4.3. Model Building
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chang, J.; Wu, X.; Wang, Y.; Meyerson, L.A.; Gu, B.J.; Min, Y.; Xue, H.; Peng, C.H.; Ge, Y. Does growing vegetables in plastic greenhouses enhance regional ecosystem services beyond the food supply? Front. Ecol. Environ. 2013, 11, 43–49. [Google Scholar] [CrossRef]
- Liang, L.; Ridoutt, B.G.; Lal, R.; Wang, D.P.; Wu, W.L.; Peng, P.; Hang, S.; Wang, L.Y.; Zhao, G.S. Nitrogen footprint and nitrogen use efficiency of greenhouse tomato production in North China. J. Clean. Prod. 2019, 208, 285–296. [Google Scholar] [CrossRef]
- Kalkhajeh, Y.K.; Sorensen, H.; Huang, B.; Guan, D.-X.; Luo, J.; Hu, W.; Holm, P.E.; Hansen, H.C.B. DGT technique to assess P mobilization from greenhouse vegetable soils in China: A novel approach. Sci. Total Environ. 2018, 630, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Kalkhajeh, Y.K.; Huang, B.; Hu, W.Y.; Holm, P.E.; Hansen, H.C.B. Phosphorus saturation and mobilization in two typical Chinese greenhouse vegetable soils. Chemosphere 2017, 187, 440–441. [Google Scholar] [CrossRef] [PubMed]
- Su, D.C.; Yang, F.H.; Zhang, F.S. Profile Characteristics and Potential Environmental Effect of Accumulated Phosphorus in Soils of Vegetable Fields in Beijing. Pedosphere 2002, 12, 179–184. [Google Scholar]
- Yan, Z.J.; Liu, P.P.; Li, Y.H.; Ma, L.; Alva, A.; Dou, Z.X.; Chen, Q.; Zhang, F.S. Phosphorus in China’s Intensive Vegetable Production Systems: Overfertilization, Soil Enrichment, and Environmental Implications. J. Environ. Qual. 2013, 42, 982–989. [Google Scholar] [CrossRef]
- Liao, W.; Liu, J.; Huang, X.; Gao, Z.; Yang, L. Responses of vegetable yield and changes of phosphorus fractions in cinnamon soil to long-term excess phosphorus application. J. Plant Nutr. Fertitizer 2017, 23, 894–903. [Google Scholar]
- Condron, L.M. Phosphorus-Surplus and deficiency. In Managing Soil Quality: Challenges in Modern Agriculture; CAB International: Wallingford, UK, 2004; pp. 69–84. [Google Scholar]
- Wang, Y.T.; Zhang, T.Q.; O’Halloran, I.P.; Hu, Q.C.; Tan, C.S.; Speranzini, D.; Macdonald, I.; Patterson, G. Agronomic and environmental soil phosphorus tests for predicting potential phosphorus loss from Ontario soils. Geoderma 2015, 241, 51–58. [Google Scholar] [CrossRef]
- Dhillon, J.; Torres, G.; Driver, E.; Figueiredo, B.; Raun, W.R. World Phosphorus Use Efficiency in Cereal Crops. Agron. J. 2017, 109, 1670–1677. [Google Scholar] [CrossRef]
- Wang, J.; Wang, D.; Zhang, G.; Wang, Y.; Wang, C.; Teng, Y.; Christie, P. Nitrogen and phosphorus leaching losses from intensively managed paddy fields with straw retention. Agric. Water Manag. 2014, 141, 66–73. [Google Scholar] [CrossRef]
- Park, H.J.; Kim, S.U.; Jung, K.Y.; Lee, S.; Choi, Y.D.; Owens, V.N.; Kumar, S.; Yun, S.W.; Hong, C.O. Cadmium phytoavailability from 1976 through 2016: Changes in soil amended with phosphate fertilizer and compost. Sci. Total Environ. 2021, 762, 143132. [Google Scholar] [CrossRef] [PubMed]
- Kalkhajeh, Y.K.; Huang, B.A.; Sorensen, H.; Holm, P.E.; Hansen, H.C.B. Phosphorus accumulation and leaching risk of greenhouse vegetable soils in Southeast China. Pedosphere 2021, 31, 683–693. [Google Scholar] [CrossRef]
- Azeez, M.O.; Rubaek, G.H.; Pedersen, I.F.; Christensen, B.T. Depletion, accumulation and availability of soil phosphorus in the Askov long-term field experiment. Soil Res. 2020, 58, 117–124. [Google Scholar] [CrossRef]
- Wang, J.; Liu, W.Z.; Mu, H.F.; Dang, T.H. Inorganic Phosphorus Fractions and Phosphorus Availability in a Calcareous Soil Receiving 21-Year Superphosphate Application. Pedosphere 2010, 20, 304–310. [Google Scholar] [CrossRef]
- Fei, C.; Zhang, S.; Wei, W.; Liang, B.; Li, J.; Ding, X. Straw and optimized nitrogen fertilizer decreases phosphorus leaching risks in a long-term greenhouse soil. J. Soils Sediments 2020, 20, 1199–1207. [Google Scholar] [CrossRef]
- Srinivasarao, C.; Vittal, K.P.R.; Kundu, S.; Gajbhiye, P.N.; Babu, M.V. Continuous Cropping, Fertilization, and Organic Manure Application Effects on Potassium in an Alfisol under Arid Conditions. Commun. Soil Sci. Plant Anal. 2010, 41, 783–796. [Google Scholar] [CrossRef]
- Liao, D.; Zhang, C.C.; Li, H.G.; Lambers, H.; Zhang, F.S. Changes in soil phosphorus fractions following sole cropped and intercropped maize and faba bean grown on calcareous soil. Plant Soil 2020, 448, 587–601. [Google Scholar] [CrossRef]
- Dodd, R.J.; McDowell, R.W.; Condron, L.M. Changes in soil phosphorus availability and potential phosphorus loss following cessation of phosphorus fertiliser inputs. Soil Res. 2013, 51, 427–436. [Google Scholar] [CrossRef]
- Dodd, R.J.; McDowell, R.W.; Condron, L.M. Is tillage an effective method to decrease phosphorus loss from phosphorus enriched pastoral soils? Soil Tillage Res. 2014, 135, 1–8. [Google Scholar] [CrossRef]
- Ejraei, A.; Ghehsareh, A.M.; Hodaji, M.; Besalatpor, A.A. Regression-based phosphorus recommendation model for Washington Navel. J. Plant Nutr. 2019, 42, 2189–2198. [Google Scholar] [CrossRef]
- Ma, Y.B.; Li, J.M.; Li, X.Y.; Tang, X.; Liang, Y.C.; Huang, S.M.; Wang, B.R.; Liu, H.; Yang, X.Y. Phosphorus accumulation and depletion in soils in wheat-maize cropping systems: Modeling and validation. Field Crops Res. 2009, 110, 207–212. [Google Scholar] [CrossRef]
- Gagnon, B.; Ziadi, N.; Belanger, G.; Parent, G. Validation and use of critical phosphorus concentration in maize. Eur. J. Agron. 2020, 120, 126147. [Google Scholar] [CrossRef]
- Morel, C.; Plenet, D.; Mollier, A. Calibration of maize phosphorus status by plant-available soil P assessed by common and process-based approaches. Is it soil-specific or not? Eur. J. Agron. 2021, 122, 126174. [Google Scholar] [CrossRef]
- Zhang, C.; Tian, H.; Liu, J.; Wang, S.; Liu, M.; Pan, S.; Shi, X. Pools and distributions of soil phosphorus in China. Glob. Biogeochem. Cycles 2005, 19. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, R.; Liu, C.; Zhang, R.; Chen, F.; Liu, Y. Phosphorus spatial distribution and pollution risk assessment in agricultural soil around the Danjiangkou reservoir, China. Sci Total Environ. 2020, 699, 134417. [Google Scholar] [CrossRef]
- Rodrigues, M.; Pavinato, P.S.; Withers, P.J.A.; Teles, A.P.B.; Herrera, W.F.B. Legacy phosphorus and no tillage agriculture in tropical oxisols of the Brazilian savanna. Sci. Total Environ. 2016, 542, 1050–1061. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, X.; Li, R.; Zheng, F.; Zhang, M.; Li, S.; Song, X. Effect of Applying Chicken Manure and Phosphate Fertilizer on Soil Phosphorus Under Drip Irrigation in Greenhouse. Sci. Agric. Sin. 2019, 52, 3637–3647. [Google Scholar]
- Mardaninejad, S.; Zareabyaneh, H.; Tabatabaei, S.H.; Pessarakli, M.; Mohamadkhani, A. Root water uptake of pepper plants (Capsicum annuum L.) under deficit irrigation system. J. Plant Nutr. 2017, 40, 1569–1579. [Google Scholar] [CrossRef]
- Lu, R. Analysis Methods of Soil Agricultural Chemistry; China Agricultural Science and Technology Press: Beijing, China, 2000; Volume 107, pp. 147–150. [Google Scholar]
- Kleinman, P.J.A.; Sharpley, A.N.; Withers, P.J.A.; Bergstrom, L.; Johnson, L.T.; Doody, D.G. Implementing agricultural phosphorus science and management to combat eutrophication. Ambio 2015, 44, S297–S310. [Google Scholar] [CrossRef]
- Lv, H.F.; Zhao, Y.M.; Wang, Y.F.; Wan, L.; Wang, J.G.; Butterbach, K.; Lin, S. Conventional flooding irrigation and over fertilization drives soil pH decrease not only in the top- but also in subsoil layers in solar greenhouse vegetable production systems. Geoderma 2020, 363, 114156. [Google Scholar] [CrossRef]
- Wang, X.; Hou, Y. Effect of Continuous Vegetable Cultivation on Phosphorus Levels of Fluvo-Aquic Soils. Pedosphere 2004, 14, 171–176. [Google Scholar]
- Song, D.; Li, H.; Li, J.; Li, X.; Luo, Y. Experimental simulation on migration and conversion of organophosphor by pH value in aquitard. World Geol. 2011, 30, 121–127. [Google Scholar]
- Dou, Z.X.; Ramberg, C.F.; Toth, J.D.; Wang, Y.; Sharpley, A.N.; Boyd, S.E.; Chen, C.R.; Williams, D.; Xu, Z.H. Phosphorus Speciation and Sorption-Desorption Characteristics in Heavily Manured Soils. Soil Sci. Soc. Am. J. 2009, 73, 93–101. [Google Scholar] [CrossRef]
- Fan, B.; Fenton, O.; Daly, K.; Ding, J.; Chen, S.; Chen, Q. Alum split applications strengthened phosphorus fixation and phosphate sorption in high legacy phosphorus calcareous soil. J. Environ. Sci. 2021, 101, 87–97. [Google Scholar] [CrossRef]
- Shen, Y.; Duan, Y.H.; McLaughlin, N.; Huang, S.M.; Guo, D.D.; Xu, M.H. Phosphorus desorption from calcareous soils with different initial Olsen-P levels and relation to phosphate fractions. J. Soils Sediments 2019, 19, 2997–3007. [Google Scholar] [CrossRef]
- Dodd, J.R.; Mallarino, A.P. Soil-test phosphorus and crop grain yield responses to long-term phosphorus fertilization for corn-soybean rotations. Soil Sci. Soc. Am. J. 2005, 69, 1118–1128. [Google Scholar] [CrossRef]
- Sandana, P.; Orena, S.; Rojas, J.S.; Kalazich, J.; Uribe, M. Critical value of soil Olsen-P for potato production systems in volcanic soils of Chile. J. Soil Sci. Plant Nutr. 2018, 18, 965–976. [Google Scholar] [CrossRef]
- Appelhans, S.C.; Carciochi, W.D.; Correndo, A.; Boem, F.H.G.; Salvagiotti, F.; Garcia, F.O.; Melchiori, R.J.M.; Barbagelata, P.A.; Ventimiglia, L.A.; Ferraris, G.N.; et al. Predicting soil test phosphorus decrease in non-P-fertilized conditions. Eur. J. Soil Sci. 2021, 72, 254–264. [Google Scholar] [CrossRef]
- Sucunza, F.A.; Boem, F.H.G.; Garcia, F.O.; Boxler, M.; Rubio, G. Long-term phosphorus fertilization of wheat, soybean and maize on Mollisols: Soil test trends, critical levels and balances. Eur. J. Agron. 2018, 96, 87–95. [Google Scholar] [CrossRef]
- Hirte, J.; Richner, W.; Orth, B.; Liebisch, F.; Flisch, R. Yield response to soil test phosphorus in Switzerland: Pedoclimatic drivers of critical concentrations for optimal crop yields using multilevel modelling. Sci. Total Environ. 2021, 755, 143453. [Google Scholar] [CrossRef]
- Rubio, G.; Cabello, M.J.; Gutiérrez Boem, F.H.; Munaro, E. Estimating Available Soil Phosphorus Increases after Phosphorus Additions in Mollisols. Soil Sci. Soc. Am. J. 2008, 72, 1721–1727. [Google Scholar] [CrossRef]
- Wolf, A.M.; Baker, D. Comparisons of soil test phosphorus by Olsen, Bray P1, Mehlich I and Mehlich III methods. Commun. Soil Sci. Plant Anal. 1985, 16, 467–484. [Google Scholar] [CrossRef]
- Valkama, E.; Uusitalo, R.; Turtola, E. Yield response models to phosphorus application: A research synthesis of Finnish field trials to optimize fertilizer P use of cereals. Nutr. Cycl. Agroecosystems 2011, 91, 1–15. [Google Scholar] [CrossRef] [Green Version]

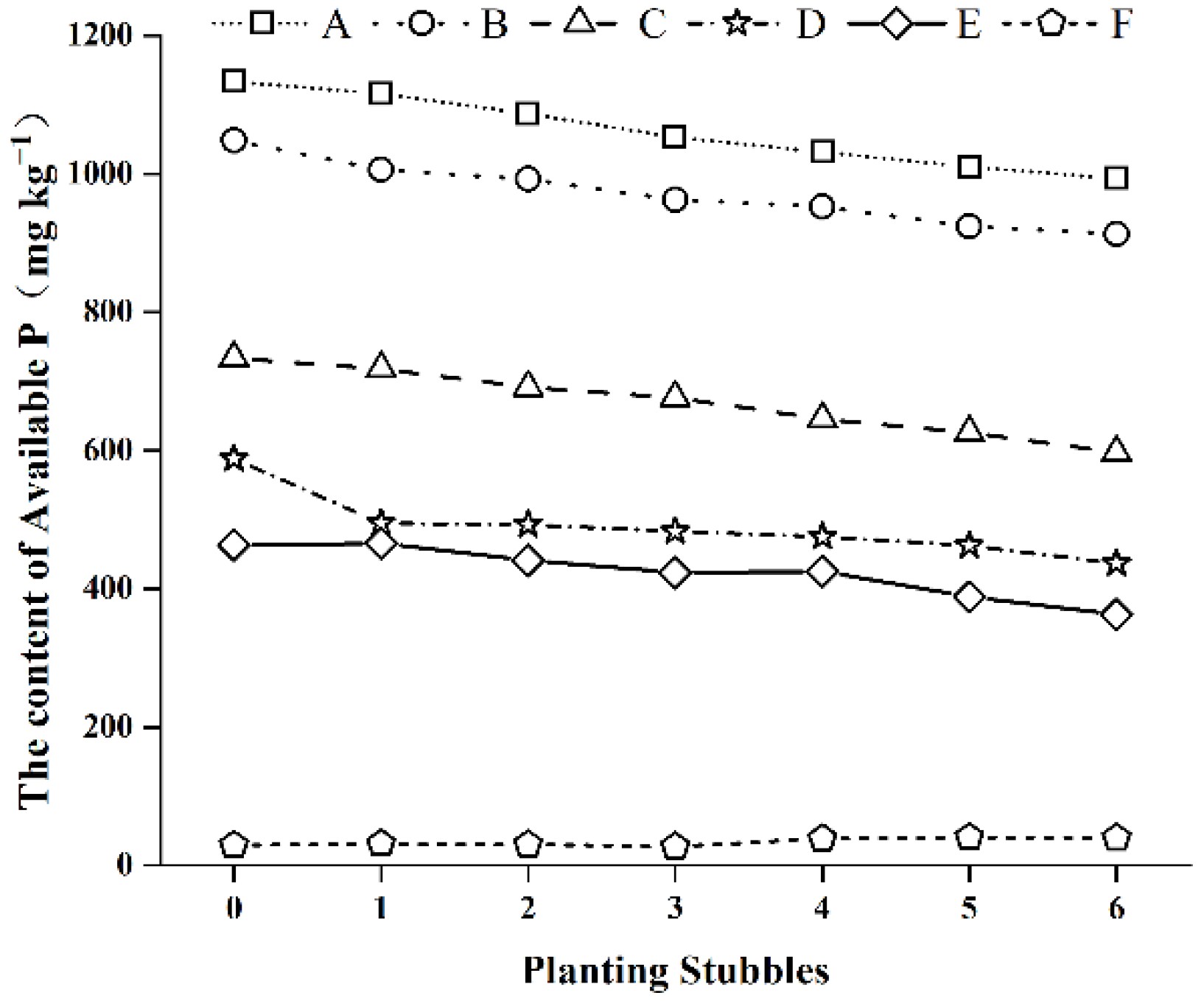
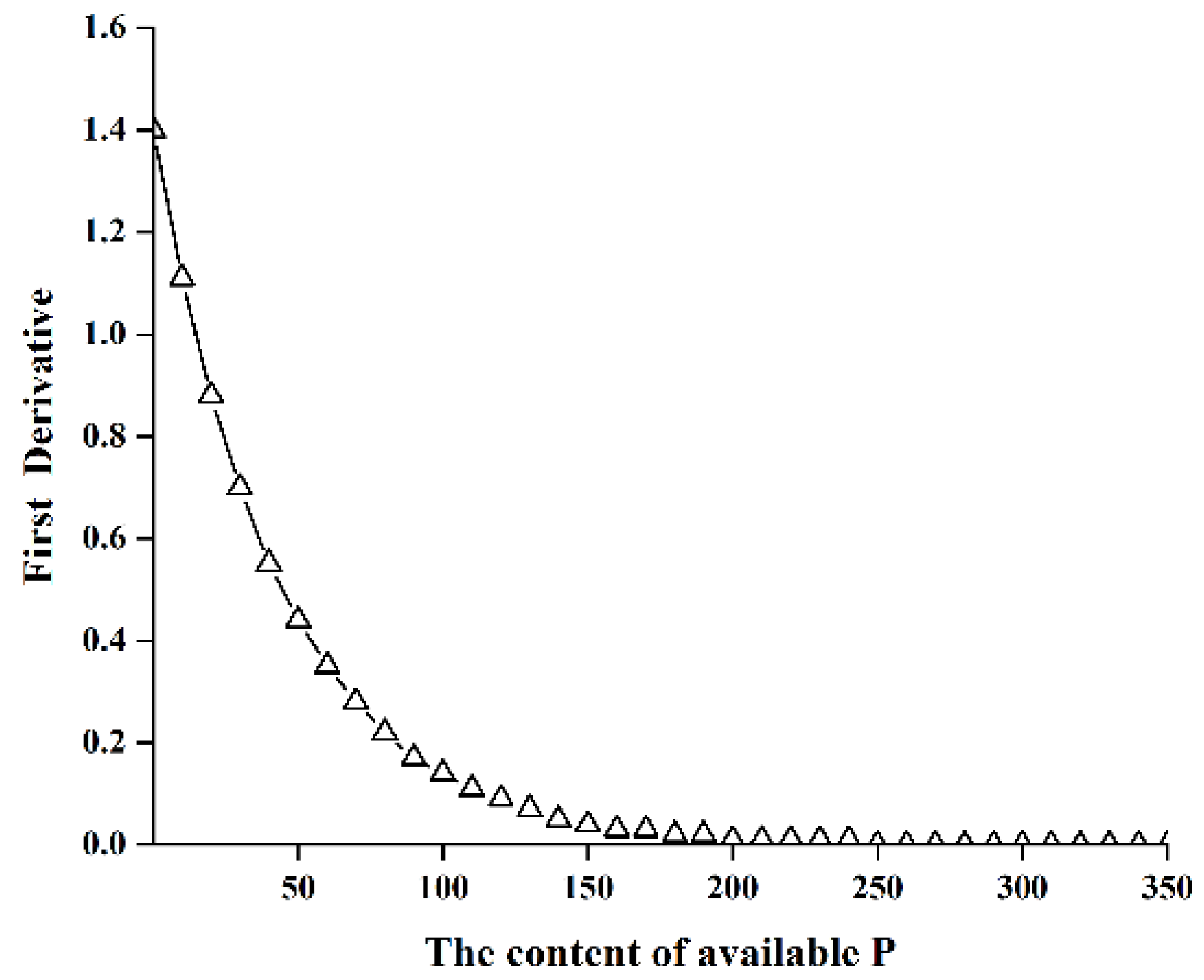
| No. | Texture | pH | Available P | Hydrolyzed N | Available K | Total P | Total N | Total K | Organic Carbon |
|---|---|---|---|---|---|---|---|---|---|
| mg kg−1 | g kg−1 | ||||||||
| A | sandy clay | 5.25 | 1133.51 | 96.51 | 126.30 | 3.18 | 2.50 | 35.05 | 13.81 |
| B | sandy clay | 5.50 | 1048.16 | 143.44 | 138.95 | 5.55 | 2.01 | 37.22 | 18.49 |
| C | sandy clay | 5.54 | 733.36 | 86.35 | 136.96 | 3.25 | 2.31 | 34.97 | 11.42 |
| D | clay | 5.36 | 587.95 | 223.34 | 221.28 | 2.21 | 1.74 | 39.72 | 11.27 |
| E | sandy clay | 5.19 | 463.16 | 96.56 | 103.31 | 2.12 | 1.80 | 27.18 | 12.50 |
| F | loamy clay | 5.96 | 29.34 | 71.91 | 167.29 | 0.42 | 1.27 | 37.98 | 11.21 |
| No. | Type | Total P (TP) (g kg−1) 1 | Inorganic P (Pi) (g kg−1) 1 | Pi/TP % 2 | Organophosphorus (Po) (g kg−1) 1 | Po/TP % 2 | Available P (Pav) (g kg−1) 1 | Pav/TP % 2 |
|---|---|---|---|---|---|---|---|---|
| 1 | Protected Field | 2.45 ± 0.35 | 1.24 ± 0.49 | 50.5 | 1.21 ± 0.34 | 49.5 | 0.22 ± 0.04 | 9 |
| 2 | 2.03 ± 0.08 | 1.76 ± 0.35 | 86.9 | 0.27 ± 0.05 | 13.1 | 0.11 ± 0.02 | 5.6 | |
| 3 | 2.49 ± 0.26 | 1.75 ± 0.17 | 70.4 | 0.74 ± 0.22 | 29.6 | 0.26 ± 0.01 | 10.6 | |
| 4 | 0.80 ± 0.11 | 0.63 ± 0.20 | 78.5 | 0.17 ± 0.05 | 21.5 | 0.13 ± 0.05 | 16.7 | |
| 5 | 0.80 ± 0.06 | 0.64 ± 0.12 | 80 | 0.16 ± 0.06 | 20 | 0.04 ± 0.01 | 5.1 | |
| 6 | 1.28 ± 0.18 | 0.92 ± 0.13 | 71.7 | 0.36 ± 0.03 | 28.3 | 0.18 ± 0.02 | 14.3 | |
| 7 | 2.43 ± 0.06 | 1.61 ± 0.29 | 66.1 | 0.82 ± 0.16 | 33.9 | 0.53 ± 0.12 | 21.8 | |
| 8 | 1.77 ± 0.15 | 1.37 ± 0.46 | 77.5 | 0.40 ± 0.11 | 22.5 | 0.22 ± 0.02 | 12.2 | |
| 9 | Farmland | 0.36 ± 0.02 | 0.27 ± 0.08 | 75.1 | 0.09 ± 0.02 | 24.9 | 0.03 ± 0.01 | 9.6 |
| No. | Type | Total P (TP) (g kg−1) 1 | Inorganic P (Pi) (g kg−1) 1 | Pi/TP % 2 | Organophosphorus (Po) (g kg−1) 1 | Po/TP % 2 | Available P (Pav) (g kg−1) 1 | Pav/TP % 2 |
|---|---|---|---|---|---|---|---|---|
| 1 | Protected Field | 0.70 ± 0.31 | 0.44 ± 0.15 | 62.4 | 0.26 ± 0.08 | 37.6 | 0.23 ± 0.02 | 32.8 |
| 2 | 0.61 ± 0.18 | 0.29 ± 0.14 | 48.5 | 0.32 ± 0.09 | 51.5 | 0.15 ± 0.02 | 24.5 | |
| 3 | 1.02 ± 0.64 | 0.77 ± 0.20 | 75.4 | 0.25 ± 0.03 | 24.6 | 0.57 ± 0.04 | 55.7 | |
| 4 | 0.74 ± 0.25 | 0.48 ± 0.15 | 65.5 | 0.26 ± 0.03 | 34.5 | 0.16 ± 0.03 | 21.3 | |
| 5 | 0.65 ± 0.22 | 0.36 ± 0.19 | 54.6 | 0.29 ± 0.04 | 45.4 | 0.09 ± 0.02 | 13.4 | |
| 6 | 1.03 ± 0.45 | 0.79 ± 0.21 | 76.1 | 0.24 ± 0.02 | 23.9 | 0.23 ± 0.04 | 22 | |
| 7 | 2.02 ± 0.67 | 1.24 ± 0.32 | 61.5 | 0.78 ± 0.14 | 38.5 | 0.48 ± 0.07 | 23.9 | |
| 8 | 1.25 ± 0.43 | 0.91 ± 0.41 | 72.5 | 0.34 ± 0.02 | 27.5 | 0.21 ± 0.09 | 17 | |
| 9 | Farmland | 0.34 ± 0.24 | 0.16 ± 0.10 | 46.2 | 0.18 ± 0.09 | 53.8 | 0.04 ± 0.01 | 11.1 |
| No. | Type | WSLC-P | Al-P | Fe-P | O-P | Ca-P | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| mg kg−1 | % 2 | mg kg−1 | %2 | mg kg−1 | % 2 | mg kg−1 | % 2 | mg kg−1 | % 2 | ||
| 1 | Protected Field | 173.02 ± 7.56 | 14.0 | 260.27 ± 8.93 | 21.0 | 264.81 ± 1.26 | 21.4 | 179.51 ± 9.88 | 14.5 | 361.22 ± 4.02 | 29.2 |
| 2 | 227.84 ± 8.66 | 12.9 | 427.81 ± 2.54 | 24.3 | 368.85 ± 7.62 | 20.9 | 171.83 ± 7.87 | 9.7 | 567.84 ± 3.39 | 32.2 | |
| 3 | 42.96 ± 2.83 | 2.5 | 534.94 ± 4.40 | 30.6 | 362.63 ± 4.46 | 20.7 | 450.90 ± 2.65 | 25.8 | 357.87 ± 6.34 | 20.5 | |
| 4 | 25.00 ± 2.98 | 4.0 | 99.64 ± 1.39 | 15.9 | 247.54 ± 1.61 | 39.6 | 142.78 ± 9.09 | 22.8 | 110.06 ± 3.28 | 17.6 | |
| 5 | 20.62 ± 3.67 | 3.2 | 161.43 ± 7.41 | 25.1 | 356.12 ± 3.87 | 55.5 | 56.19 ± 2.20 | 8.8 | 47.69 ± 2.36 | 7.4 | |
| 6 | 72.04 ± 8.93 | 7.9 | 256.32 ± 3.85 | 27.9 | 277.49 ± 4.43 | 30.2 | 50.75 ± 1.57 | 5.5 | 260.83 ± 9.48 | 28.4 | |
| 7 | 40.13 ± 4.33 | 2.5 | 420.18 ± 5.33 | 26.1 | 650.46 ± 5.01 | 40.5 | 182.97 ± 9.83 | 11.4 | 313.07 ± 1.21 | 19.5 | |
| 8 | 35.76 ± 3.47 | 2.6 | 161.34 ± 5.45 | 11.8 | 655.42 ± 4.74 | 47.9 | 177.25 ± 9.19 | 13.0 | 338.93 ± 6.12 | 24.8 | |
| 9 | Farmland | 34.10 ± 2.44 | 12.6 | 45.99 ± 8.09 | 17.0 | 33.05 ± 3.14 | 12.2 | 62.66 ± 2.64 | 23.2 | 94.85 ± 3.54 | 35.0 |
| No. | Type | WSLC-P | Al-P | Fe-P | O-P | Ca-P | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| mg kg−1 | %2 | mg kg−1 | % 2 | mg kg−1 | % 2 | mg kg−1 | % 2 | mg kg−1 | % 2 | ||
| 1 | Protected Field | 13.57 ± 0.66 | 3.1 | 97.55 ± 10.1 | 22.3 | 108.05 ± 22.69 | 24.7 | 110.08 ± 17.38 | 25.2 | 108.23 ± 19.41 | 24.7 |
| 2 | 17.00 ± 4.03 | 5.8 | 32.58 ± 2.0 | 11.1 | 95.06 ± 15.19 | 32.4 | 113.27 ± 10.45 | 38.6 | 35.91 ± 1.77 | 12.2 | |
| 3 | 21.23 ± 1.55 | 2.7 | 227.92 ± 29.7 | 29.5 | 311.77 ± 6.08 | 40.3 | 180.31 ± 4.58 | 23.3 | 31.57 ± 2.77 | 4.1 | |
| 4 | 19.17 ± 1.41 | 4.0 | 31.48 ± 10.8 | 6.5 | 123.06 ± 11.77 | 25.6 | 227.46 ± 9.62 | 47.2 | 80.33 ± 3.66 | 16.7 | |
| 5 | 6.06 ± 0.92 | 1.7 | 29.35 ± 3.7 | 8.2 | 126.13 ± 21.40 | 35.4 | 151.54 ± 5.36 | 42.5 | 43.53 ± 1.55 | 12.2 | |
| 6 | 20.69 ± 0.93 | 2.6 | 81.20 ± 7.4 | 10.3 | 283.61 ± 51.50 | 36.1 | 243.37 ± 22.76 | 31.0 | 156.96 ± 23.43 | 20.0 | |
| 7 | 18.27 ± 1.47 | 1.5 | 281.09 ± 12.0 | 22.6 | 471.89 ± 58.59 | 38.0 | 305.52 ± 34.92 | 24.6 | 164.83 ± 21.87 | 13.3 | |
| 8 | 21.74 ± 2.00 | 2.4 | 204.36 ± 10.6 | 22.5 | 460.36 ± 46.47 | 50.6 | 131.11 ± 11.38 | 14.4 | 91.98 ± 14.51 | 10.1 | |
| 9 | Farmland | 6.43 ± 1.56 | 4.1 | 13.37 ± 8.5 | 8.4 | 33.93 ± 3.69 | 21.4 | 59.57 ± 11.49 | 37.6 | 45.29 ± 1.67 | 28.6 |
| No. | Fitted Model | R | Number of Planting Stubbles | Initial Available P (mg kg−1) |
|---|---|---|---|---|
| A | y = 44.670 − 0.038x | 0.996 | 37 | 1133.51 |
| B | y = 44.746 − 0.0420x | 0.986 | 36 | 1048.16 |
| C | y = 34.250 − 0.045x | 0.996 | 25 | 733.36 |
| D | y = 118.159 − 18.463 × ln(x) | 0.841 | 20 | 587.95 |
| E | y = 34.087 − 0.0711x | 0.957 | 19 | 463.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, H.; Wang, C.; Lu, X.; Sai, C.; Liang, Y. Dynamic Changes in Soil Phosphorus Accumulation and Bioavailability in Phosphorus-Contaminated Protected Fields. Int. J. Environ. Res. Public Health 2022, 19, 12262. https://doi.org/10.3390/ijerph191912262
Liang H, Wang C, Lu X, Sai C, Liang Y. Dynamic Changes in Soil Phosphorus Accumulation and Bioavailability in Phosphorus-Contaminated Protected Fields. International Journal of Environmental Research and Public Health. 2022; 19(19):12262. https://doi.org/10.3390/ijerph191912262
Chicago/Turabian StyleLiang, Hongyue, Chen Wang, Xinrui Lu, Chunmei Sai, and Yunjiang Liang. 2022. "Dynamic Changes in Soil Phosphorus Accumulation and Bioavailability in Phosphorus-Contaminated Protected Fields" International Journal of Environmental Research and Public Health 19, no. 19: 12262. https://doi.org/10.3390/ijerph191912262
APA StyleLiang, H., Wang, C., Lu, X., Sai, C., & Liang, Y. (2022). Dynamic Changes in Soil Phosphorus Accumulation and Bioavailability in Phosphorus-Contaminated Protected Fields. International Journal of Environmental Research and Public Health, 19(19), 12262. https://doi.org/10.3390/ijerph191912262





