Applying ACE-III, M-ACE and MMSE to Diagnostic Screening Assessment of Cognitive Functions within the Polish Population
Abstract
:1. Introduction
2. Methods
2.1. Patients
2.2. Measurements
2.2.1. Mini-Mental State Examination (MMSE)
2.2.2. ACE-III
- Attention: score 0–18,
- Memory: score 0–26,
- Verbal Fluency: score 0–14,
- Language: score 0–26,
- Visuospatial Abilities: score 0–16.
2.2.3. M-ACE
2.3. Procedure
- A partly structured interview was conducted, comprising the educational and professional background, selected aspects of social functioning, health and conditions, which could impact cognitive functions, such as hypothyroidism, cardiovascular diseases, mental disorders and others. Participants with the following conditions were excluded from the study: sense organ disorders (unless participants had the recommended prescriptive glasses or a hearing aid), intractable non-nociceptive pain, suspected but untreated cases of severe depression, psychiatric disorders severe enough to prevent assessment. Participants who displayed any of the symptoms above were referred to a specialist for diagnosis and treatment. People over 60 years of age, capable of linguistic, visual and auditory communication in a stable somatic state, were included in the study. Persons declaring severe somatic complaints or pain, as well as those whose mental state required medical intervention in the first place, were excluded.
- MMSE and ACE-III screening tests were conducted, and the results were used to calculate the M-ACE results.
- In accordance with the standards of psychological assessment, each participant was presented with the results of the assessment and further recommendations.
2.4. Data Analysis
2.4.1. Data Analysis for a Single Assessment to Establish Group Characteristics and Test Results Analysis
- raw MMSE results and age, sex or years of schooling;
- ACE-III results and age, sex or years of schooling;
- results of M-ACE and age, sex or years of schooling.
2.4.2. Comparing the Sensitivity of the Screening Tests
- r = 0—no correlation,
- 0 < r < 0.2—remote correlation,
- 0.2 ≤ r < 0.4—weak correlation,
- 0.4 ≤ r < 0.6—average (medium) correlation,
- 0.6 ≤ r < 0.8—high (strong) correlation,
- 0.8 ≤ r < 1—very high (very strong) correlation,
- r = 1—perfect correlation (complete).
- MMSE and ACE-III,
- MMSE and M-ACE,
- ACE-III and M-ACE.
2.4.3. Analysing Cross-Category Migration—Norms, MCI and Dementia
2.4.4. Analysing Lower Cut-Off Domains for ACE-III against the MMSE Norm Range
3. Results
3.1. Test Group Profile
3.2. Results for Each Scale
3.2.1. MMSE
Age and Raw MMSE Data
Years of Schooling and MMSE Results
3.2.2. ACE-III
- Attention (pts)—15.5 ± 2.9 (17.0; 5.0–18.0), range 0–18 pts;
- Fluency (pts)—9.0 ± 3.5 (9.0; 0.0–25.0), range 0–14 pts;
- Memory (pts)—18.1 ± 6.9 (21.0; 25.0–26.0), range 0–26 pts;
- Language (pts)—22.7 ± 4.0 (24.0; 2.0–26.0), range 0–26 pts;
- Visuospatial Abilities (pts)—13.2 ± 2.6 (14.0; 5.0–16.0), range 0–16 pts.
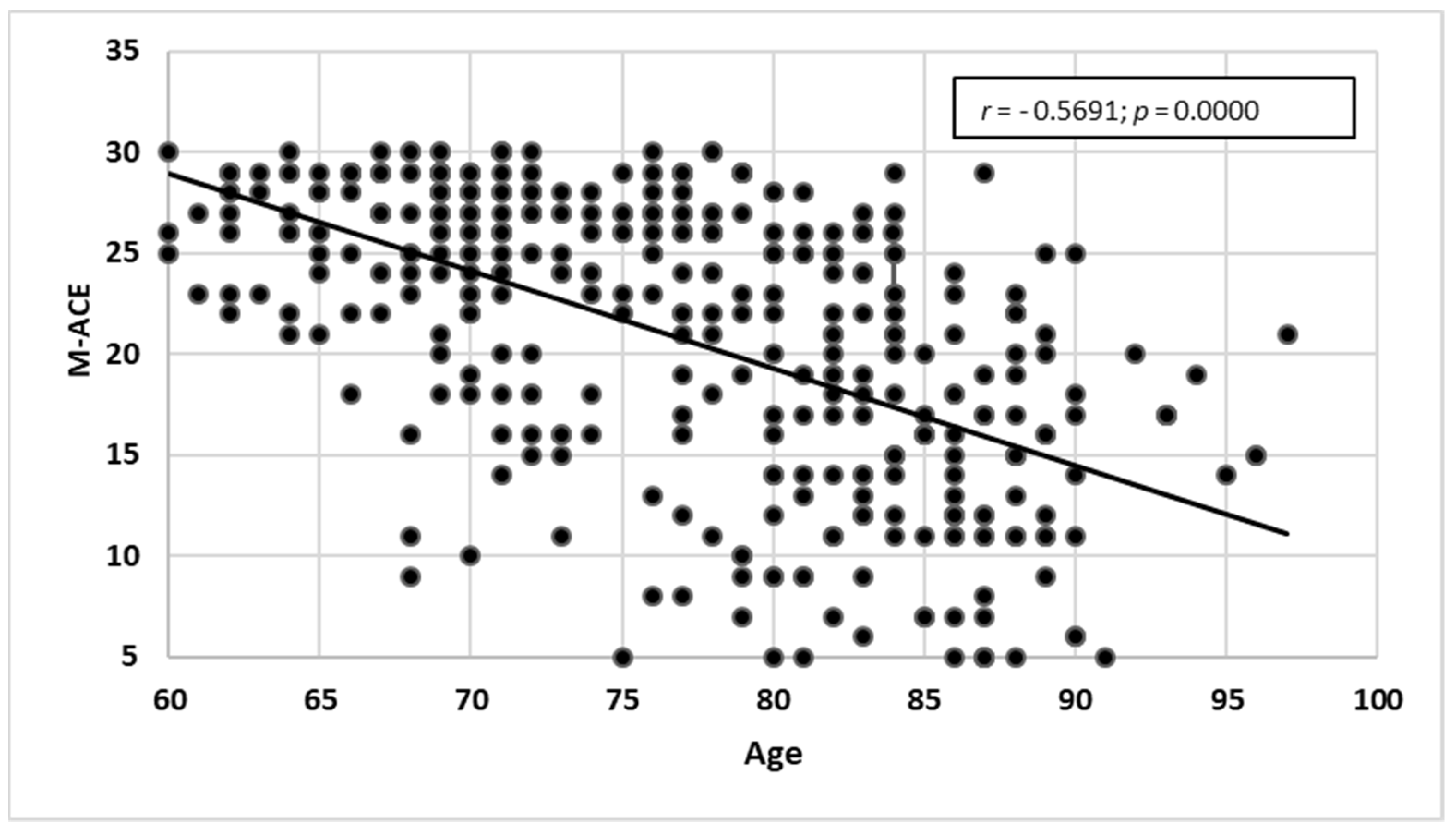
3.2.3. M-ACE
3.2.4. Comparing the Scales against MMSE
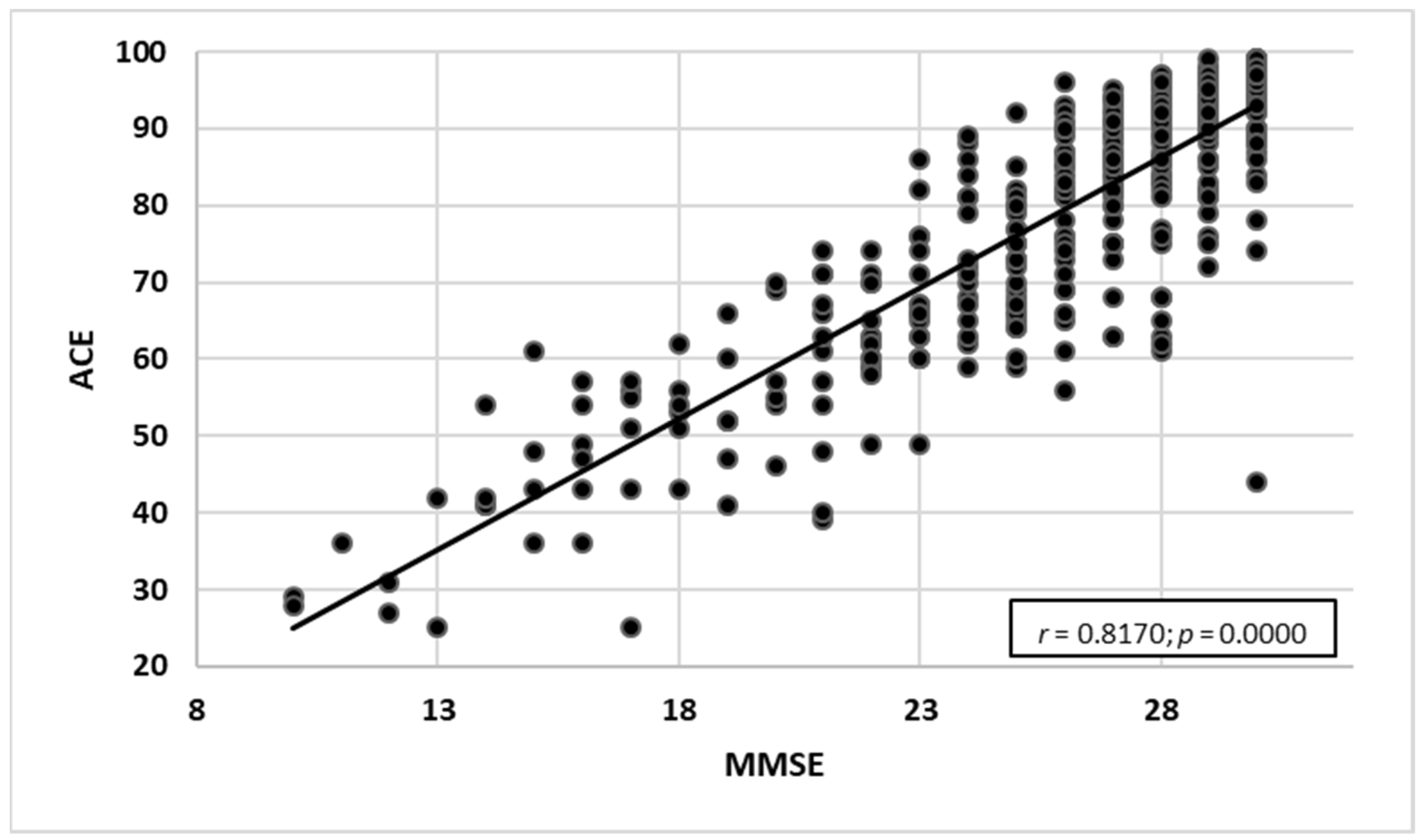
- MMSE and ACE-III—a very high correlation with the strength of r = 0.817. As the ACE-III test results grew, the MMSE test results increased as well (Figure 7).
- MMSE and M-ACE—a very high correlation with the strength of r = 0.753. As the M-ACE test results grew, the MMSE scores increased as well (Figure 8).
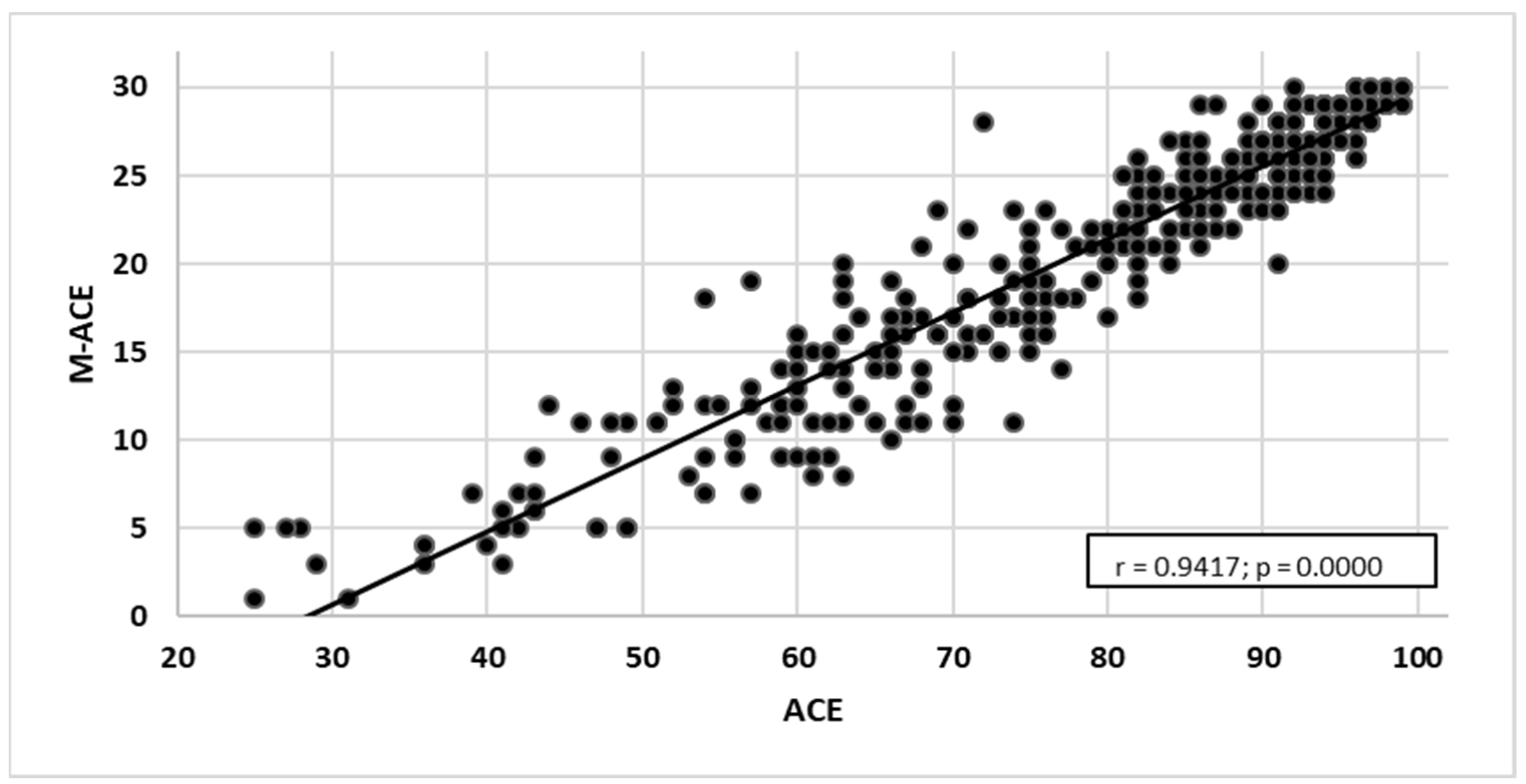
- ACE-III and M-ACE—a very high correlation with the strength of r = 0.942. As the ACE-III test results grew, the M-ACE test results also increased (Figure 9).
3.2.5. ACE-III and MMSE
ACE-III and MMSE—Two Categories (below the Norm and Norm)
- true positive—159,
- false positive—77,
- false negative—10,
- true negative—140.
ACE-III—ROC Curve
ACE-III and MMSE—Three Categories (Dementia, MCI, Norm)
- Two people (0.5%) whose MMSE results allocated them to the category of dementia received the status of MCI in ACE-III.
- Ten people (2.6%) whose scores assigned them to the category of MCI in MMSE were classified within the norm in ACE-III.
- For 132 respondents (34.1%), the category obtained in ACE-III was lower as compared with MMSE.
- Fifty-five people (14.2%) who were categorised as MCI in MMSE obtained results classified as dementia in ACE-III.
- Thirty-one people (8.0%) whose scores classified them as within the norm in MMSE obtained results classified as dementia in ACE-III.
- Forty-six people (11.9%) whose scores allocated them within the norm in MMSE obtained the status of MCI in ACE-III.
Analysing Undercut Areas for ACE-III against the MMSE Norm Range
- Attention 1 pt = 5.6%,
- Fluency 1 pt = 7.1%,
- Memory/Language 1 pt = 3.8%,
- Visuospatial Abilities 1 pt = 6.3%.
3.2.6. M-ACE and MMSE
M-ACE (Cut-Off Point of 25) and MMSE—Two Categories (below Norm and within Norm)
- true positive—158,
- false positive—90,
- false negative—11,
- true negative—127.
M-ACE—ROC Curve
- Three people (0.8%) who were assigned to the category of dementia in MMSE received the category of MCI in M-ACE;
- One person (0.3%) whom the MMSE test results placed in the category of dementia obtained the status of MCI in M-ACE;
- Ten people (2.6%) whose scores categorised them as MCI in MMSE were allocated within the norm in M-ACE.
- Forty-four people (11.4%) who were assigned MCI in MMSE obtained the category of dementia in M-ACE;
- Twenty-six people (6.7%) whose scores allocated them to within the norm in MMSE were classified as dementia in M-ACE;
- Sixty-four people (16.6%) who scored within the norm in MMSE received the status of MCI in M-ACE.
4. Discussion
4.1. Notes on the Test Group Profile
4.2. Assessment with the Use of Three Screening Tests
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Foster, N.L.; Bondi, M.W.; Das, R.; Foss, M.; Hershey, L.A.; Koh, S.; Logan, R.; Poole, C.; Shega, J.W.; Sood, A.; et al. Quality improvement in neurology: Mild cognitive impairment quality measurement set. Neurology 2019, 93, 705–713. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). “Zero Draft” WHO Global Action Plan on the Public Health Response to Dementia 2017–2025. Available online: https://www.euro.who.int/__data/assets/pdf_file/0020/316145/66wd06e_Add.1_Dementia_160630.pdf (accessed on 26 June 2022).
- Barczak, A.; Gorzkowska, A.; Klimkowicz-Mrowiec, A. Ocena zaburzeń funkcjonowania poznawczego. In Diagnostyka i Leczenie Otępień. Rekomendacje Ekspertów Polskiego Towarzystwa Alzheimerowskiego; Medisfera: Otwock, Poland, 2012; pp. 11–29. (In Polish) [Google Scholar]
- Beishon, L.C.; Batterham, A.P.; Quinn, T.J.; Nelson, C.P.; Panerai, R.B.; Robinson, T.; Haunton, V.J. Addenbrooke’s Cognitive Examination III (ACE-III) and mini-ACE for the detection of dementia and mild cognitive impairment. Cochrane Database Syst. Rev. 2019, 3, CD013282. [Google Scholar]
- Barczak, A. Jak rozpoznać otępienie? Wskazówki neuropsychologa. Med. Dypl. 2013, 2, 4–7. (In Polish) [Google Scholar]
- Barczak, A. Wczesne rozpoznawanie choroby Alzheimera. Pediatr. Med. Rodz. 2018, 14, 157–166. (In Polish) [Google Scholar] [CrossRef]
- Hsieh, S.; Schubert, S.; Hoon, C.; Mioshi, E.; Hodges, J.R. Validation of the Addenbrooke’s Cognitive Examination III in frontotemporal dementia and Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 2013, 36, 242–250. [Google Scholar] [CrossRef]
- Hsieh, S.; McGrory, S.; Leslie, F.; Dawson, K.; Ahmed, S.; Butler, C.R.; Rowe, J.B.; Mioshi, E.; Hodges, J.R. The Mini-Addenbrooke’s Cognitive Examination: A new assessment tool for dementia. Dement. Geriatr. Cogn. Disord. 2015, 39, 1–11. [Google Scholar] [CrossRef]
- Sitek, E.J.; Barczak, A.; Senderecka, M. Zastosowanie jakościowej analizy profilu wykonania skali ACE-III w diagnostyce różnicowej chorób otępiennych. Aktualn. Neurolog. 2017, 17, 34–41. (In Polish) [Google Scholar] [CrossRef]
- Galton, C.; Erzinçlioglu, S.; Sahakian, B.; Antoun, N.; Hodges, J. A comparison of the Addenbrooke’s Cognitive Examination (ACE), conventional neuropsychological assessment, and simple MRI-based medial temporal lobe evaluation in the early diagnosis of Alzheimer’s disease. Cogn. Behav. Neurol. 2005, 18, 144–150. [Google Scholar] [CrossRef]
- Matias-Guiu, J.A.; Fernandez-Bobadilla, R. Validation of the Spanish-language version of Mini-Addenbrooke’s Cognitive Examination as a dementia screening tool. Neurología 2016, 31, 646–648. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Larner, A.J. M-ACE versus MoCA: Equivalence or superiority? Pragmatic diagnostic test accuracy study. Int. Psychogeriatr. 2017, 29, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Larner, A.J. Evaluating cognitive screening instruments with the “likelihood to be diagnosed or misdiagnosed” measure. Int. J. Clin. Pract. 2019, 73, e13265. [Google Scholar] [CrossRef] [PubMed]
- Larner, A.J. New unitary metrics for dementia test accuracy studies. Prog. Neurol. Psychiatry 2019, 23, 21–25. [Google Scholar] [CrossRef]
- Charernboon, T. Diagnostic accuracy of the Thai version of the Mini-Addenbrooke’s Cognitive Examination as a mild cognitive impairment and dementia screening test. Psychogeriatrics 2019, 19, 340–344. [Google Scholar] [CrossRef]
- Senda, M.; Terada, S.; Takenoshita, S.; Hayashi, S.; Yabe, M.; Imai, N.; Horiuchi, M.; Yamada, N. Diagnostic utility of the Addenbrooke’s Cognitive Examination-III (ACE-III), Mini-ACE, Mini-Mental State Examination, Montreal Cognitive Assessment, and Hasegawa Dementia Scale-Revised for detecting mild cognitive impairment and dementia. Psychogeriatrics 2020, 20, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Larner, A.J. Mini-Addenbrooke’s Cognitive Examination: A pragmatic diagnostic accuracy study. Int. J. Geriatr. Psychiatry 2015, 30, 547–548. [Google Scholar] [CrossRef] [PubMed]
- Williamson, J.C.; Larner, A.J. M-ACE for diagnosis of dementia and MCI: 3-year pragmatic diagnostic test accuracy study. Dement. Geriatr. Cogn. Disord. 2018, 45, 300–307. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Stańczak, J. MINIMENTAL (MMSE)—Normalizacja Polska; Pracownia Testów Psychologicznych PTP: Warsaw, Poland, 2013. (In Polish) [Google Scholar]
- Senderecka, M.; Zabawa, J.; Kluj-Kozłowska, K.; Greń, M.; Konkel, A.; Kuklińska, M.; Paprot, E.; Sikorski, R.; Barczak, A.; Sitek, E. Addenbrooke’s Cognitive Examination—ACE-III. Wersja A-PL (2014)—Copyright Prof. John Hodges. 2014. Available online: https://www.sydney.edu.au/brain-mind/resources-for-clinicians/dementia-test.html (accessed on 13 June 2022).
- Mackenzie, C.S.; Visperas, A.; Ogrodniczuk, J.S.; Oliffe, J.L.; Nurmi, M.A. Age and sex differences in self-stigma and public stigma concerning depression and suicide in men. Stigma Health 2019, 4, 233–241. [Google Scholar] [CrossRef]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia prevention, intervention, and care: 2020 report of the Lancet commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef]
- Evans, I.; Martyr, A.; Collins, R.; Brayne, C.; Clare, L. Social isolation and cognitive function in later life: A systematic review and meta-analysis. J. Alzheimer’s Dis. 2019, 70, S119–S144. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Murata, C.; Saito, M.; Takeda, T.; Kondo, K. Influence of social relationship domains and their combinations on incident dementia: A prospective cohort study. J. Epidemiol. Community Health 2018, 72, 7–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Legdeur, N.; Heymans, M.W.; Comijs, H.C.; Huisman, M.; Maier, A.B.; Visser, P.J. Age dependency of risk factors for cognitive decline. BMC Geriatr. 2018, 18, 187. [Google Scholar] [CrossRef] [PubMed]
- Stephan, B.; Muniz-Terrera, G.; Granic, A.; Collerton, J.; Davies, K.; Saxby, B.K.; Wesnes, K.A.; Kirkwood, T.B.L.; Jagger, C. Longitudinal changes in global and domain specific cognitive function in the very-old: Findings from the Newcastle 85+ study. Int. J. Geriatr. Psychiatry 2018, 33, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Arenaza-Urquijo, E.M.; Przybelski, S.A.; Lesnick, T.L.; Graff-Radford, J.; Machulda, M.M.; Knopman, D.S.; Schwarz, C.G.; Lowe, V.J.; Mielke, M.M.; Petersen, R.C.; et al. The metabolic brain signature of cognitive resilience in the 80+: Beyond Alzheimer pathologies. Brain J. Neurol. 2019, 142, 1134–1147. [Google Scholar] [CrossRef]
- Groot, C.; van Loenhoud, A.C.; Barkhof, F.; van Berckel, B.N.M.; Koene, T.; Teunissen, C.C.; Scheltens, P.; van der Flier, W.M.; Ossenkoppele, R. Differential effects of cognitive reserve and brain reserve on cognition in Alzheimer disease. Neurology 2018, 90, e149–e156. [Google Scholar] [CrossRef]
- Barczak, A. Wykształcenie, aktywność umysłowa i socjalna jako czynniki protekcyjne otępienia. Aktualn. Neurol. 2014, 14, 161–166. (In Polish) [Google Scholar] [CrossRef]
- Wang, B.-R.; Zheng, H.-F.; Xu, C.; Sun, Y.; Zhang, Y.-D.; Shi, J.-Q. Comparative diagnostic accuracy of ACE-III and MoCA for detecting mild cognitive impairment. Neuropsychiatr. Dis. Treat. 2019, 15, 2647–2653. [Google Scholar] [CrossRef]
- Peixoto, B.; Machado, M.; Rocha, P.; Macedo, C. Validation of the Portuguese version of Addenbrooke’s Cognitive Examination III in mild cognitive impairment and dementia. Adv. Clin. Exp. Med. 2018, 27, 781–786. [Google Scholar] [CrossRef]
- Larner, A.J. MACE for diagnosis of dementia and MCI: Examining cut-offs and predictive values. Diagnostics 2019, 9, 51. [Google Scholar] [CrossRef]
- Miranda, D.C.; Brucki, S.M.D.; Yassuda, M.S. The Mini-Addenbrooke’s Cognitive Examination (M-ACE) as a brief cognitive screening instrument in mild cognitive impairment and mild Alzheimer’s disease. Dement. Neuropsychol. 2018, 12, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Chasles, M.J.; Tremblay, A.; Escudier, F.; Lajeunesse, A.; Benoit, S.; Langlois, R.; Joubert, S.; Rouleau, I. An examination of semantic impairment in amnestic MCI and AD: What can we learn from verbal fluency? Arch. Clin. Neuropsychol. 2019, 35, 22–30. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, M.; Dill, L.; Panos, S.; Amano, S. Verbal fluency as a screening tool for mild cognitive impairment. Int. Psychogeriatr. 2020, 32, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Sudo, F.K.; de Souza, A.S.; Drummond, C.; Assuncao, N.; Teldeschi, A.; Oliveira, N.; Rodrigues, F.; Santiago-Bravo, G.; Calil, V.; Lima, G.; et al. Inter-method and anatomical correlates of episodic memory tests in the Alzheimer’s disease spectrum. PLoS ONE 2019, 14, e0223731. [Google Scholar]
- Moscoso, A.; Silva-Rodríguez, J.; Aldrey, J.M.; Cortés, J.; Fernández-Ferreiro, A.; Gómez-Lado, N.; Ruibal, Á.; Aguiar, P.; Alzheimer’s Disease Neuroimaging Initiative. Staging the cognitive continuum in prodromal Alzheimer’s disease with episodic memory. Neurobiol. Aging 2019, 84, 1–8. [Google Scholar] [CrossRef]
- Eikelboom, W.; Janssen, N.; Jiskoot, L.C.; van der Berg, E.; Roelofs, A.; Kessels, R.P.C. Episodic and working memory function in primary progressive aphasia: A meta-analysis. Neurosci. Biobehav. Rev. 2018, 92, 243–254. [Google Scholar] [CrossRef]
- Van den Berg, E.; Jiskoot, L.C.; Grosveld, M.J.H.; van Swieten, J.C.; Papma, J.M. Qualitative assessment of verbal fluency performance in frontotemporal dementia. Dement. Geriatr. Cogn. Dis. 2017, 44, 35–44. [Google Scholar] [CrossRef] [Green Version]










| Age | n | MMSE [pts] | H | p | |
|---|---|---|---|---|---|
| up to 70 years old [G1] | 108 | 28.0 ± 2.7 (29.0; 11–30) | 97.6 | 0.0000 | |
| 71–75 years old [G2] | 60 | 27.0 ± 3.7 (28.0; 10–30) | |||
| 76–80 years old [G3] | 73 | 26.2 ± 3.6 (27.0; 14–30) | [G1] p = 0.0009 | ||
| over 80 years old [G4] | 145 | 23.3 ± 4.6 (24.0; 10–30) | [G1] and [G2] p < 0.0001 [G3] p = 0.0001 |
| Scale | ACE-III | n | Mean, SD, Median, Range | Mann–Whitney U-Test | |
|---|---|---|---|---|---|
| Z | p | ||||
| Attention (pts) | below | 77 | 16.9 ± 1.4 (17.0; 11–18) | −4.46 | 0.0000 |
| norm | 140 | 17.6 ± 0.7 (18.0; 15–18) | |||
| Verbal Fluency (pts) | below | 77 | 8.3 ± 2.3 (9.0; 2–12) | −9.58 | 0.0000 |
| norm | 140 | 12.0 ± 2.2 (12.0; 6–25) | |||
| Memory (pts) | below | 77 | 18.6 ± 4.6 (20.0; 7–25) | −9.53 | 0.0000 |
| norm | 140 | 23.9 ± 2.3 (24.0; 9–29) | |||
| Language (pts) | below | 77 | 22.9 ± 2.5 (24.0; 15–26) | −8.55 | 0.0000 |
| norm | 140 | 25.3 ± 1.0 (26.0; 21–26) | |||
| Visuospatial Abilities (pts) | below | 77 | 13.2 ± 2.2 (13.0; 5–16) | −6.92 | 0.0000 |
| norm | 140 | 15.0 ± 1.2 (15.0; 10–16) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaczmarek, B.; Ilkowska, Z.; Kropinska, S.; Tobis, S.; Krzyminska-Siemaszko, R.; Kaluzniak-Szymanowska, A.; Wieczorowska-Tobis, K. Applying ACE-III, M-ACE and MMSE to Diagnostic Screening Assessment of Cognitive Functions within the Polish Population. Int. J. Environ. Res. Public Health 2022, 19, 12257. https://doi.org/10.3390/ijerph191912257
Kaczmarek B, Ilkowska Z, Kropinska S, Tobis S, Krzyminska-Siemaszko R, Kaluzniak-Szymanowska A, Wieczorowska-Tobis K. Applying ACE-III, M-ACE and MMSE to Diagnostic Screening Assessment of Cognitive Functions within the Polish Population. International Journal of Environmental Research and Public Health. 2022; 19(19):12257. https://doi.org/10.3390/ijerph191912257
Chicago/Turabian StyleKaczmarek, Beata, Zofia Ilkowska, Sylwia Kropinska, Sławomir Tobis, Roma Krzyminska-Siemaszko, Aleksandra Kaluzniak-Szymanowska, and Katarzyna Wieczorowska-Tobis. 2022. "Applying ACE-III, M-ACE and MMSE to Diagnostic Screening Assessment of Cognitive Functions within the Polish Population" International Journal of Environmental Research and Public Health 19, no. 19: 12257. https://doi.org/10.3390/ijerph191912257
APA StyleKaczmarek, B., Ilkowska, Z., Kropinska, S., Tobis, S., Krzyminska-Siemaszko, R., Kaluzniak-Szymanowska, A., & Wieczorowska-Tobis, K. (2022). Applying ACE-III, M-ACE and MMSE to Diagnostic Screening Assessment of Cognitive Functions within the Polish Population. International Journal of Environmental Research and Public Health, 19(19), 12257. https://doi.org/10.3390/ijerph191912257







