Limited Stress Response to Transplantation in the Mediterranean Macroalga Ericaria amentacea, a Key Species for Marine Forest Restoration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Experimental Setup
2.2. Biochemical Analysis
2.2.1. Total Phenolic Compounds
2.2.2. Lipids and Fatty Acids
2.2.3. Data Elaboration and Statistical Analysis
3. Results
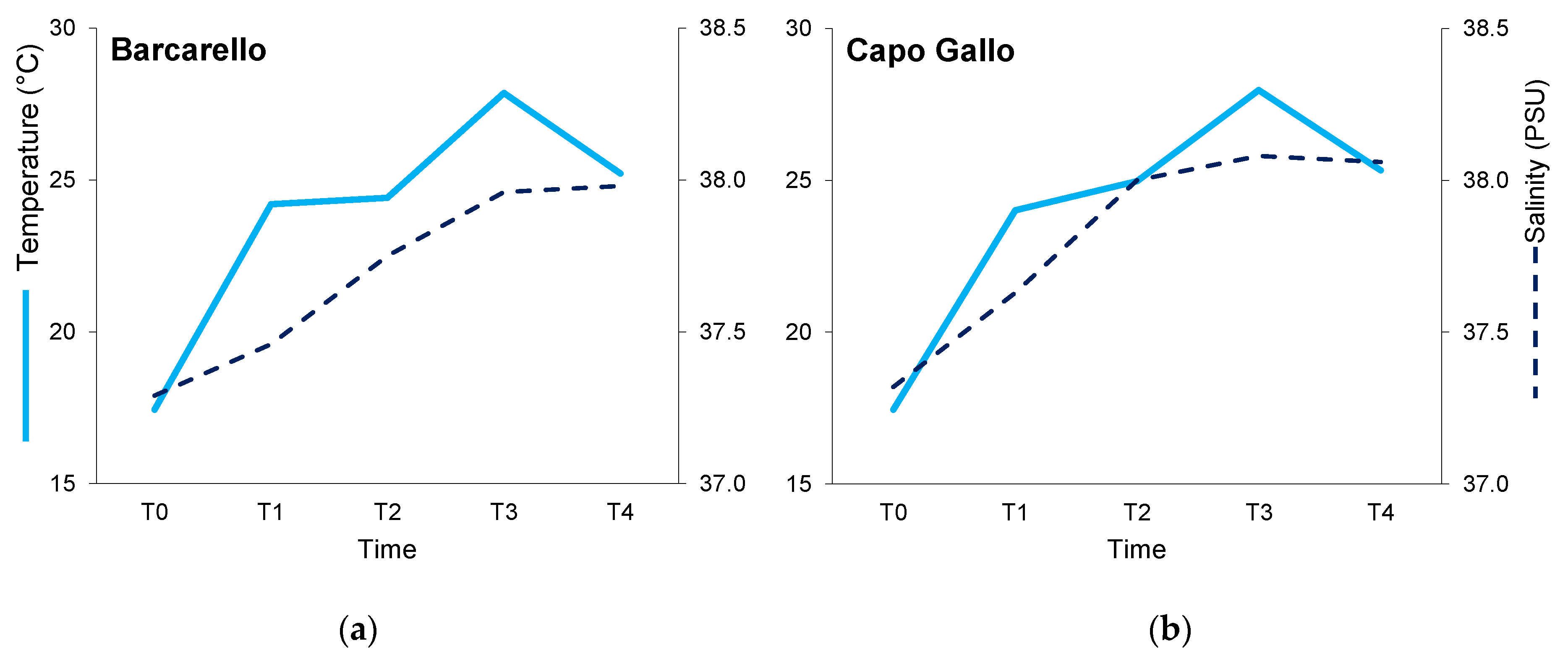
3.1. Environmental Variables
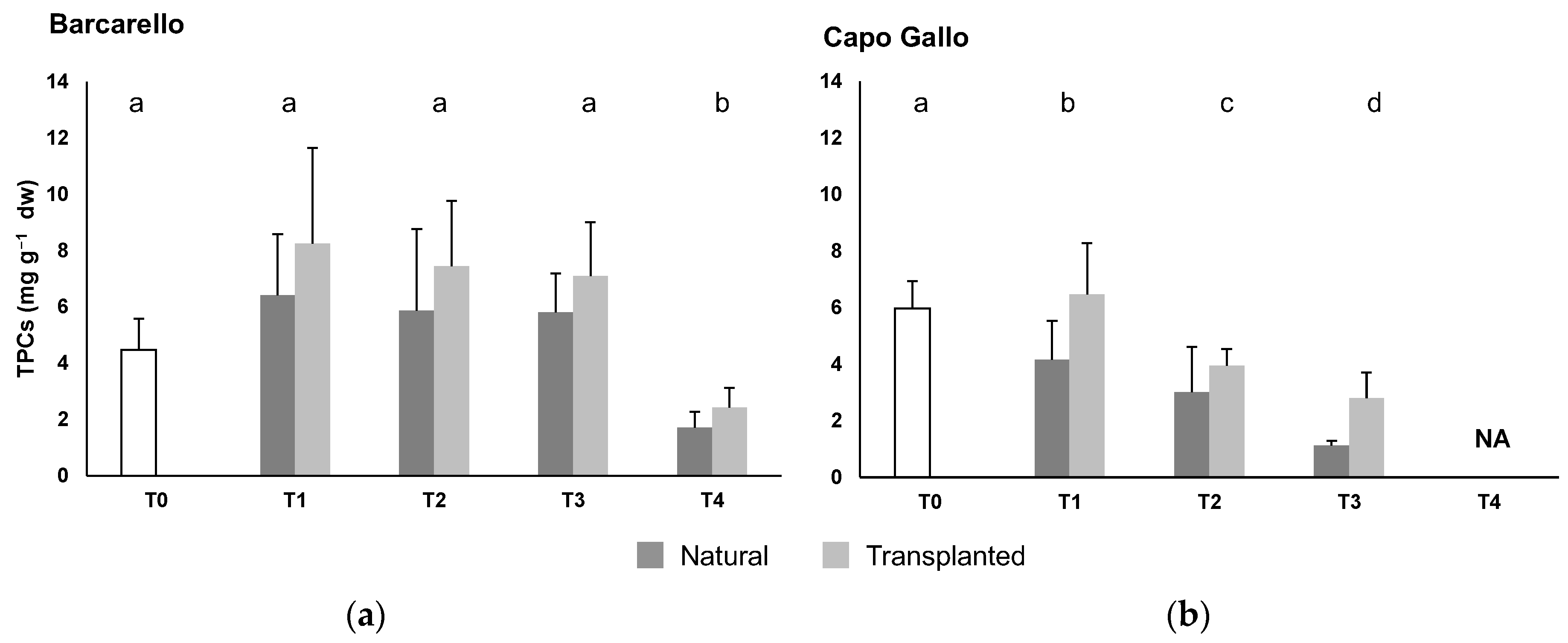
3.2. Total Phenolic Compounds
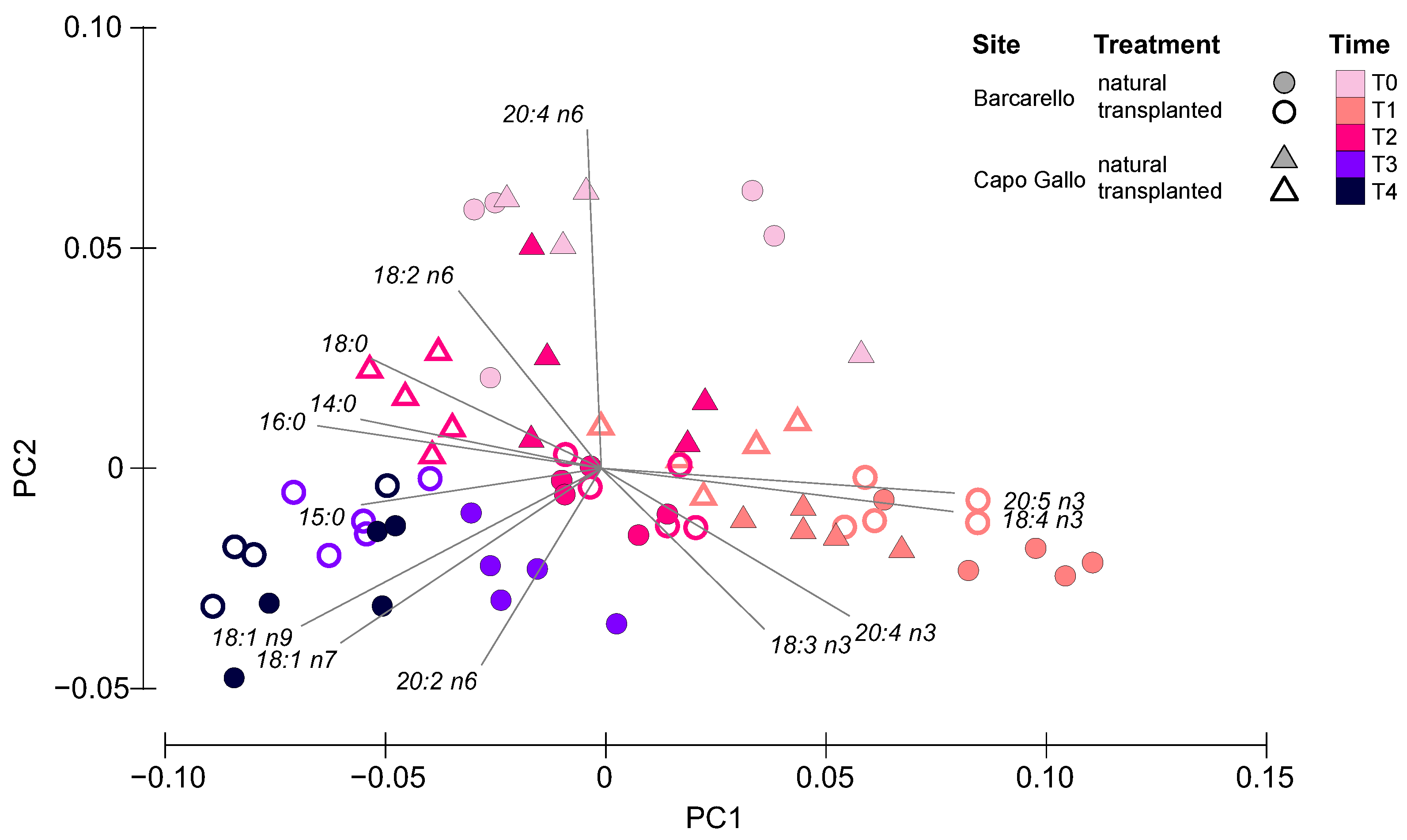
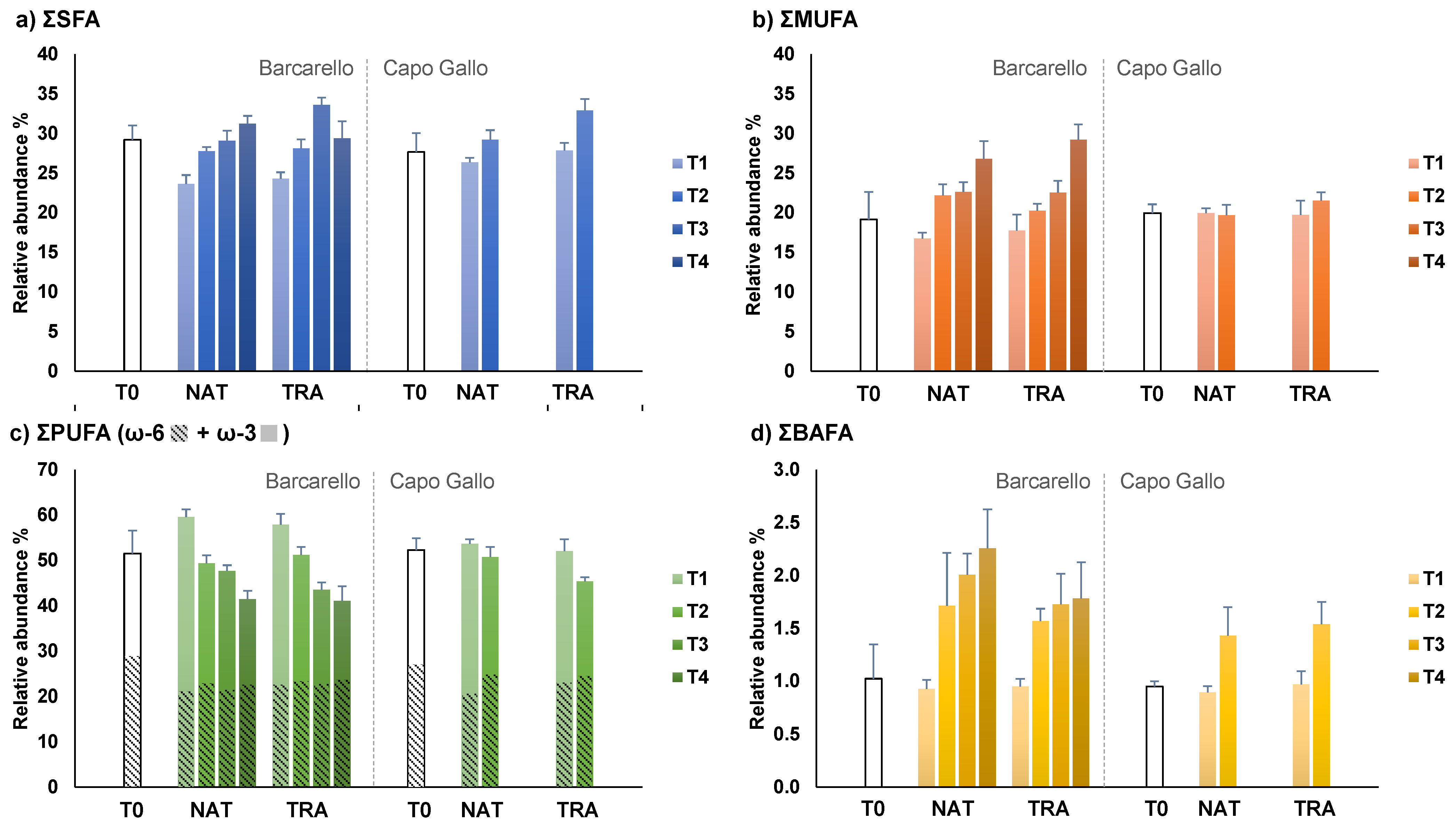
3.3. Lipids and Fatty Acids
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bellgrove, A.; McKenzie, P.F.; Cameron, H.; Pocklington, J.B. Restoring rocky intertidal communities: Lessons from a benthic macroalgal ecosystem engineer. Mar. Pollut. Bull. 2017, 117, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Cheminée, A.; Sala, E.; Pastor, J.; Bodilis, P.; Thiriet, P.; Mangialajo, L.; Cottalorda, J.M.; Francour, P. Nursery value of Cystoseira forests for Mediterranean rocky reef fishes. J. Exp. Mar. Biol. Ecol. 2013, 442, 70–79. [Google Scholar] [CrossRef]
- Mineur, F.; Arenas, F.; Assis, J.; Davies, A.J.; Engelen, A.H.; Fernandes, F.; Malta, E.j.; Thibaut, T.; Van Nguyen, T.; Vaz-Pinto, F.; et al. European seaweeds under pressure: Consequences for communities and ecosystem functioning. J. Sea Res. 2015, 98, 91–108. [Google Scholar] [CrossRef]
- Piazzi, L.; Bonaviri, C.; Castelli, A.; Ceccherelli, G.; Costa, G.; Curini-Galletti, M.; Langeneck, J.; Manconi, R.; Montefalcone, M.; Pipitone, C.; et al. Biodiversity in canopy-forming algae: Structure and spatial variability of the Mediterranean Cystoseira assemblages. Estuar. Coast. Shelf Sci. 2018, 207, 132–141. [Google Scholar] [CrossRef]
- Molinari, E.A.; Guiry, M.D. Reinstatement of the genera Gongolaria Boehmer and Ericaria Stackhouse (Sargassaceae, Phaeophyceae). Not. Algarum 2020, 172, 1–10. [Google Scholar]
- Bernal-Ibáñez, A.; Gestoso, I.; Wirtz, P.; Kaufmann, M.; Serrão, E.A.; Canning-Clode, J.; Cacabelos, E. The collapse of marine forests: Drastic reduction in populations of the family Sargassaceae in Madeira Island (NE Atlantic). Reg. Environ. Chang. 2021, 21, 71. [Google Scholar] [CrossRef]
- Thibaut, T.; Pinedo, S.; Torras, X.; Ballesteros, E. Long-term decline of the populations of Fucales (Cystoseira spp. and Sargassum spp.) in the Albères coast (France, North-western Mediterranean). Mar. Pollut. Bull. 2005, 50, 1472–1489. [Google Scholar] [CrossRef]
- De La Fuente, G.; Asnaghi, V.; Chiantore, M.; Thrush, S.; Povero, P.; Vassallo, P.; Petrillo, M.; Paoli, C. The effect of Cystoseira canopy on the value of midlittoral habitats in NW Mediterranean, an emergy assessment. Ecol. Modell. 2019, 404, 1–11. [Google Scholar] [CrossRef]
- Benedetti-Cecchi, L.; Pannacciulli, F.; Bulleri, F.; Moschella, P.S.; Airoldi, L.; Relini, G.; Cinelli, F. Predicting the consequences of anthropogenic disturbance: Large-scale effects of loss of canopy algae on rocky shores. Mar. Ecol. Prog. Ser. 2001, 214, 137–150. [Google Scholar] [CrossRef]
- Capdevila, P.; Hereu, B.; Salguero-Gómez, R.; Rovira, G.; Medrano, A.; Cebrian, E.; Garrabou, J.; Kersting, D.K.; Linares, C. Warming impacts on early life stages increase the vulnerability and delay the population recovery of a long-lived habitat-forming macroalga. J. Ecol. 2019, 107, 1129–1140. [Google Scholar] [CrossRef]
- Mangialajo, L.; Chiantore, M.; Cattaneo-Vietti, R. Loss of fucoid algae along a gradient of urbanisation, and structure of benthic assemblages. Mar. Ecol. Prog. Ser. 2008, 358, 63–74. [Google Scholar] [CrossRef] [Green Version]
- Riquet, F.; De Kuyper, C.A.; Fauvelot, C.; Airoldi, L.; Planes, S.; Fraschetti, S.; Mačić, V.; Milchakova, N.; Mangialajo, L.; Bottin, L. Highly restricted dispersal in habitat-forming seaweed may impede natural recovery of disturbed populations. Sci. Rep. 2021, 11, 16792. [Google Scholar] [CrossRef]
- Cebrian, E.; Tamburello, L.; Verdura, J.; Guarnieri, G.; Medrano, A.; Linares, C.; Hereu, B.; Garrabou, J.; Cerrano, C.; Galobart, C.; et al. A Roadmap for the Restoration of Mediterranean Macroalgal Forests. Front. Mar. Sci. 2021, 8, 709219. [Google Scholar] [CrossRef]
- Jacob, C.; Buffard, A.; Pioch, S.; Thorin, S. Marine ecosystem restoration and biodiversity offset. Ecol. Eng. 2018, 120, 585–594. [Google Scholar] [CrossRef]
- Tamburello, L.; Papa, L.; Guarnieri, G.; Basconi, L.; Zampardi, S.; Scipione, M.B.; Terlizzi, A.; Zupo, V.; Fraschetti, S. Are we ready for scaling up restoration actions? An insight from Mediterranean macroalgal canopies. PLoS ONE 2019, 14, e0224477. [Google Scholar] [CrossRef]
- Falace, A.; Kaleb, S.; De La Fuente, G.; Asnaghi, V.; Chiantore, M. Ex situ cultivation protocol for Cystoseira amentacea var. stricta (Fucales, Phaeophyceae) from a restoration perspective. PLoS ONE 2018, 13, e0193011. [Google Scholar] [CrossRef]
- Falace, A.; Zanelli, E.; Bressan, G. Algal transplantation as a potential tool for artificial reef management and environmental mitigation. Bull. Mar. Sci. 2006, 78, 161–166. [Google Scholar]
- Kumari, P.; Kumar, M.; Reddy, C.R.K.; Jha, B. Algal lipids, fatty acids and sterols. In Functional Ingredients from Algae for Foods and Nutraceuticals; Elsevier Science: Amsterdam, The Netherlands, 2013; pp. 87–133. ISBN 9780857095121. [Google Scholar]
- Amsler, C.D.; Fairhead, V.A. Defensive and Sensory Chemical Ecology of Brown Algae. Adv. Bot. Res. 2005, 43, 1–91. [Google Scholar] [CrossRef]
- Schnitzler, I.; Pohnert, G.; Hay, M.; Boland, W. Chemical defense of brown algae (Dictyopteris spp.) against the herbivorous amphipod Ampithoe longimana. Oecologia 2001, 126, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Karsten, U.; Bischof, K.; Wiencke, C. Photosynthetic performance of arctic macroalgae after transplantation from deep to shallow waters. Oecologia 2001, 127, 11–20. [Google Scholar] [CrossRef]
- Figueroa, F.L.; Domínguez-González, B.; Korbee, N. Vulnerability and acclimation to increased UVB radiation in three intertidal macroalgae of different morpho-functional groups. Mar. Environ. Res. 2014, 97, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Contreras, L.; Mella, D.; Moenne, A.; Correa, J.A. Differential responses to copper-induced oxidative stress in the marine macroalgae Lessonia nigrescens and Scytosiphon lomentaria (Phaeophyceae). Aquat. Toxicol. 2009, 94, 94–102. [Google Scholar] [CrossRef]
- Kováčik, J.; Micalizzi, G.; Dresler, S.; Babula, P.; Hladký, J.; Chemodanov, A.; Mondello, L. Metabolic responses of Ulva compressa to single and combined heavy metals. Chemosphere 2018, 213, 384–394. [Google Scholar] [CrossRef]
- Bischof, K.; Rautenberger, R. Seaweed Responses to Environmental Stress: Reactive Oxygen and Antioxidative Strategies. In Seaweed Biology; Springer: Berlin/Heidelberg, Germany, 2012; pp. 109–132. [Google Scholar]
- Dring, M.J. Stress Resistance and Disease Resistance in Seaweeds: The Role of Reactive Oxygen Metabolism. Adv. Bot. Res. 2005, 43, 175–207. [Google Scholar]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Mannino, A.M.; Micheli, C. Ecological function of phenolic compounds from mediterranean fucoid algae and seagrasses: An overview on the genus Cystoseira sensu lato and Posidonia oceanica (L.) Delile. J. Mar. Sci. Eng. 2020, 8, 19. [Google Scholar] [CrossRef]
- Cruces, E.; Flores-Molina, M.R.; Díaz, M.J.; Huovinen, P.; Gómez, I. Phenolics as photoprotective mechanism against combined action of UV radiation and temperature in the red alga Gracilaria chilensis? J. Appl. Phycol. 2018, 30, 1247–1257. [Google Scholar] [CrossRef]
- Celis-Plá, P.S.M.; Martínez, B.; Korbee, N.; Hall-Spencer, J.M.; Figueroa, F.L. Photoprotective responses in a brown macroalgae Cystoseira tamariscifolia to increases in CO2 and temperature. Mar. Environ. Res. 2017, 130, 157–165. [Google Scholar] [CrossRef]
- Čagalj, M.; Skroza, D.; Razola-Díaz, M.D.C.; Verardo, V.; Bassi, D.; Frleta, R.; Mekinić, I.G.; Tabanelli, G.; Šimat, V. Variations in the Composition, Antioxidant and Antimicrobial Activities of Cystoseira compressa during Seasonal Growth. Mar. Drugs 2022, 20, 64. [Google Scholar] [CrossRef]
- Figueroa, F.L.; Bonomi Barufi, J.; Malta, E.J.; Conde-Álvarez, R.; Nitschke, U.; Arenas, F.; Mata, M.; Connan, S.; Abreu, M.H.; Marquardt, R.; et al. Short-term effects of increasing CO2, nitrate and temperature on three mediterranean macroalgae: Biochemical composition. Aquat. Biol. 2014, 22, 177–193. [Google Scholar] [CrossRef]
- Kumar, A.; Abdelgawad, H.; Castellano, I.; Selim, S.; Beemster, G.T.S.; Asard, H.; Buia, M.C.; Palumbo, A. Effects of ocean acidification on the levels of primary and secondary metabolites in the brown macroalga Sargassum vulgare at different time scales. Sci. Total Environ. 2018, 643, 946–956. [Google Scholar] [CrossRef] [PubMed]
- Van Alstyne, K.L.; McCarthy, J.J.; Hustead, C.L.; Duggins, D.O. Geographic variation in polyphenolic levels of northeastern Pacific kelps and rockweeds. Mar. Biol. 1999, 133, 371–379. [Google Scholar] [CrossRef]
- Xu, P.; Tan, H.; Jin, W.; Li, Y.; Santhoshkumar, C.; Li, P.; Liu, W. Antioxidative and antimicrobial activities of intertidal seaweeds and possible effects of abiotic factors on these bioactivities. J. Oceanol. Limnol. 2018, 36, 2243–2256. [Google Scholar] [CrossRef]
- Barkina, M.Y.; Pomazenkova, L.A.; Chopenko, N.S.; Velansky, P.V.; Kostetsky, E.Y.; Sanina, N.M. Influence of Warm-Acclimation Rate on Polar Lipids of Ulva lactuca. Russ. J. Plant Physiol. 2020, 67, 111–121. [Google Scholar] [CrossRef]
- Britton, D.; Schmid, M.; Noisette, F.; Havenhand, J.N.; Paine, E.R.; McGraw, C.M.; Revill, A.T.; Virtue, P.; Nichols, P.D.; Mundy, C.N.; et al. Adjustments in fatty acid composition is a mechanism that can explain resilience to marine heatwaves and future ocean conditions in the habitat-forming seaweed Phyllospora comosa (Labillardière) C. Agardh. Glob. Chang. Biol. 2020, 26, 3512–3524. [Google Scholar] [CrossRef]
- Koch, K.; Thiel, M.; Hagen, W.; Graeve, M.; Gómez, I.; Jofre, D.; Hofmann, L.C.; Tala, F.; Bischof, K. Short- and long-term acclimation patterns of the giant kelp Macrocystis pyrifera (Laminariales, Phaeophyceae) along a depth gradient. J. Phycol. 2016, 52, 260–273. [Google Scholar] [CrossRef]
- Da Costa, E.; Domingues, P.; Melo, T.; Coelho, E.; Pereira, R.; Calado, R.; Abreu, M.H.; Domingues, M.R. Lipidomic signatures reveal seasonal shifts on the relative abundance of high-valued lipids from the brown algae Fucus vesiculosus. Mar. Drugs 2019, 17, 335. [Google Scholar] [CrossRef]
- Los, D.A.; Murata, N. Membrane fluidity and its roles in the perception of environmental signals. Biochim. Biophys. Acta 2004, 1666, 142–157. [Google Scholar] [CrossRef]
- Seemann, J.; Sawall, Y.; Auel, H.; Richter, C. The use of lipids and fatty acids to measure the trophic plasticity of the coral Stylophora subseriata. Lipids 2013, 48, 275–286. [Google Scholar] [CrossRef]
- Davis, K.M.; Mazel, F.; Parfrey, L.W. The microbiota of intertidal macroalgae Fucus distichus is site-specific and resistant to change following transplant. Environ. Microbiol. 2021, 23, 2617–2631. [Google Scholar] [CrossRef]
- Donnarumma, L.; D’Argenio, A.; Sandulli, R.; Russo, G.F.; Chemello, R. Unmanned aerial vehicle technology to assess the state of threatened biogenic formations: The vermetid reefs of mediterranean intertidal rocky coasts. Estuar. Coast. Shelf Sci. 2021, 251, 107228. [Google Scholar] [CrossRef]
- Vizzini, S.; Colombo, F.; Costa, V.; Mazzola, A. Contribution of planktonic and benthic food sources to the diet of the reef-forming vermetid gastropod Dendropoma petraeum in the western Mediterranean. Estuar. Coast. Shelf Sci. 2011, 96, 262–267. [Google Scholar] [CrossRef]
- Mangialajo, L.; Chiantore, M.; Susini, M.L.; Meinesz, A.; Cattaneo-Vietti, R.; Thibaut, T. Zonation patterns and interspecific relationships of fucoids in microtidal environments. J. Exp. Mar. Biol. Ecol. 2012, 412, 72–80. [Google Scholar] [CrossRef]
- Susini, M.L.; Mangialajo, L.; Thibaut, T.; Meinesz, A. Development of a transplantation technique of Cystoseira amentacea var. stricta and Cystoseira compressa. Hydrobiologia 2007, 580, 241–244. [Google Scholar] [CrossRef]
- Harrison, P.G.; Durance, C. Seasonal variation in phenolic content of eelgrass shoots. Aquat. Bot. 1989, 35, 409–413. [Google Scholar] [CrossRef]
- Bolser, R.C.; Hay, M.E.; Lindquist, N.; Fenical, W.; Wilson, D. Chemical defenses of freshwater macrophytes against crayfish herbivory. J. Chem. Ecol. 1998, 24, 1639–1658. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Mannino, A.M.; Vaglica, V.; Cammarata, M.; Oddo, E. Effects of temperature on total phenolic compounds in Cystoseira amentacea (C. Agardh) Bory (Fucales, Phaeophyceae) from southern Mediterranean Sea. Plant Biosyst. 2016, 150, 152–160. [Google Scholar] [CrossRef]
- Polo, L.K.; Chow, F. Physiological performance by growth rate, pigment and protein content of the brown seaweed Sargassum filipendula (Ochrophyta: Fucales) induced by moderate UV radiation exposure in the laboratory. Sci. Mar. 2020, 84, 59–70. [Google Scholar] [CrossRef]
- Gaubert, J.; Payri, C.E.; Vieira, C.; Solanki, H.; Thomas, O.P. High metabolic variation for seaweeds in response to environmental changes: A case study of the brown algae Lobophora in coral reefs. Sci. Rep. 2019, 9, 993. [Google Scholar] [CrossRef]
- Connan, S.; Stengel, D.B. Impacts of ambient salinity and copper on brown algae: 2. Interactive effects on phenolic pool and assessment of metal binding capacity of phlorotannin. Aquat. Toxicol. 2011, 104, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, M.; Nishio, T.; Yokoyama, A.; Yatsuya, K.; Nishigaki, T.; Yoshikawa, S.; Ohki, K. Seasonal variation of phlorotannin in sargassacean species from the coast of the Sea of Japan. Phycol. Res. 2010, 58, 53–61. [Google Scholar] [CrossRef]
- Khotimchenko, S.V.; Vaskovsky, V.E.; Titlyanova, T.V. Fatty acids of marine algae from the pacific coast of North California. Bot. Mar. 2002, 45, 17–22. [Google Scholar] [CrossRef]
- Schmid, M.; Guihéneuf, F.; Stengel, D.B. Fatty acid contents and profiles of 16 macroalgae collected from the Irish Coast at two seasons. J. Appl. Phycol. 2014, 26, 451–463. [Google Scholar] [CrossRef]
- Graeve, M.; Kattner, G.; Wiencke, C.; Karsten, U. Fatty acid composition of Arctic and Antarctic macroalgae: Indicator of phylogenetic and trophic relationships. Mar. Ecol. Prog. Ser. 2002, 231, 67–74. [Google Scholar] [CrossRef]
- Nomura, M.; Kamogawa, H.; Susanto, E.; Kawagoe, C.; Yasui, H.; Saga, N.; Hosokawa, M.; Miyashita, K. Seasonal variations of total lipids, fatty acid composition, and fucoxanthin contents of Sargassum horneri (Turner) and Cystoseira hakodatensis (Yendo) from the northern seashore of Japan. J. Appl. Phycol. 2012, 25, 1159–1169. [Google Scholar] [CrossRef]
- Schmid, M.; Fernández, P.A.; Gaitán-Espitia, J.D.; Virtue, P.; Leal, P.P.; Revill, A.T.; Nichols, P.D.; Hurd, C.L. Stress due to low nitrate availability reduces the biochemical acclimation potential of the giant kelp Macrocystis pyrifera to high temperature. Algal Res. 2020, 47, 101895. [Google Scholar] [CrossRef]
- Quigley, C.T.C.; Capistrant-Fossa, K.A.; Morrison, H.G.; Johnson, L.E.; Morozov, A.; Hertzberg, V.S.; Brawley, S.H. Bacterial Communities Show Algal Host (Fucus spp.)/Zone Differentiation Across the Stress Gradient of the Intertidal Zone. Front. Microbiol. 2020, 11, 563118. [Google Scholar] [CrossRef]
- Ghaderiardakani, F.; Quartino, M.L.; Wichard, T. Microbiome-Dependent Adaptation of Seaweeds Under Environmental Stresses: A Perspective. Front. Mar. Sci. 2020, 7, 575228. [Google Scholar] [CrossRef]
- McDowell, R.E.; Amsler, C.D.; Dickinson, D.A.; Mcclintock, J.B.; Baker, B.J. Reactive oxygen species and the antarctic macroalgal wound response. J. Phycol. 2014, 50, 71–80. [Google Scholar] [CrossRef]
- Kumari, P.; Reddy, R.; Jha, B. Quantification of Selected Endogenous Hydroxy-oxylipins from Tropical Marine Macroalgae. Mar. Biotechnol. 2014, 16, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Howe, G.A.; Schilmiller, A.L. Oxylipin metabolism in response to stress. Curr. Opin. Plant Biol. 2002, 5, 230–236. [Google Scholar] [CrossRef]
- Flukes, E.B.; Wright, J.T.; Johnson, C.R. Phenotypic plasticity and biogeographic variation in physiology of habitat-forming seaweed: Response to temperature and nitrate. J. Phycol. 2015, 51, 896–909. [Google Scholar] [CrossRef] [PubMed]
- Hays, C.G. Adaptive phenotypic differentiation across the intertidal gradient in the alga Silvetia compressa. Ecology 2007, 88, 149–157. [Google Scholar] [CrossRef]
- Dubois, K.; Pollard, K.N.; Kauffman, B.J.; Williams, S.L.; Stachowicz, J.J. Local adaptation in a marine foundation species: Implications for resilience to future global change. Glob. Chang. Biol. 2022, 28, 2596–2610. [Google Scholar] [CrossRef] [PubMed]
- Sales, M.; Cebrian, E.; Tomas, F.; Ballesteros, E. Pollution impacts and recovery potential in three species of the genus Cystoseira (Fucales, Heterokontophyta). Estuar. Coast. Shelf Sci. 2011, 92, 347–357. [Google Scholar] [CrossRef]
- Hernández-Carmona, G.; García, O.; Robledo, D.; Foster, M. Restoration techniques for Macrocystis pyrifera (Phaeophyceae) populations at the southern limit of their distribution in Mexico. Bot. Mar. 2000, 43, 273–284. [Google Scholar] [CrossRef]
- Carney, L.T.; Waaland, J.R.; Klinger, T.; Ewing, K. Restoration of the bull kelp Nereocystis luetkeana in nearshore rocky habitats. Mar. Ecol. Prog. Ser. 2005, 302, 49–61. [Google Scholar] [CrossRef]
- Graham, T.D.J.; Morris, R.L.; Strain, E.M.A.; Swearer, S.E. Identifying key factors for transplantation success in the restoration of kelp (Ecklonia radiata) beds. Restor. Ecol. 2021, 30, e13536. [Google Scholar] [CrossRef]
- Hong, S.; Kim, J.; Ko, Y.W.; Yang, K.M.; Macias, D.; Kim, J.H. Effects of sea urchin and herbivorous gastropod removal, coupled with transplantation, on seaweed forest restoration. Bot. Mar. 2021, 64, 427–438. [Google Scholar] [CrossRef]

| Site Barcarello | Natural | Transplanted | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time | T0 | T1 | T2 | T3 | T4 | T1 | T2 | T3 | T4 | |||||||||
| FAs | mean | sd | mean | sd | mean | sd | mean | sd | mean | sd | mean | sd | mean | sd | mean | sd | mean | sd |
| 14:0 | 4.0 | 0.4 | 3.0 | 0.3 | 3.5 | 0.7 | 3.6 | 0.2 | 4.2 | 0.4 | 3.0 | 0.2 | 3.4 | 0.4 | 4.0 | 0.3 | 4.1 | 0.2 |
| 15:0 | 0.2 | 0.0 | 0.2 | 0.0 | 0.2 | 0.1 | 0.2 | 0.1 | 0.3 | 0.0 | 0.1 | 0.0 | 0.2 | 0.0 | 0.2 | 0.0 | 0.3 | 0.0 |
| 16:0 (PALM) | 23.4 | 1.8 | 19.6 | 1.1 | 22.9 | 0.9 | 24.1 | 1.4 | 25.4 | 1.0 | 20.3 | 0.9 | 23.0 | 0.9 | 27.8 | 0.6 | 23.9 | 2.1 |
| 17:0 | 0.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 18:0 | 0.5 | 0.1 | 0.3 | 0.1 | 0.4 | 0.1 | 0.5 | 0.1 | 0.4 | 0.0 | 0.3 | 0.1 | 0.5 | 0.0 | 0.8 | 0.1 | 0.4 | 0.0 |
| 20:0 | 0.1 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.1 | 0.1 | 0.1 | 0.1 | 0.0 | 0.0 | 0.1 | 0.0 | 0.1 | 0.1 | 0.1 | 0.0 |
| LCFAs (>22:0) | 0.5 | 0.1 | 0.4 | 0.1 | 0.6 | 0.2 | 0.6 | 0.1 | 0.6 | 0.1 | 0.4 | 0.1 | 0.5 | 0.1 | 0.5 | 0.1 | 0.5 | 0.1 |
| ƩSFA | 29.2 | 1.8 | 23.7 | 1.1 | 27.8 | 0.5 | 29.1 | 1.3 | 31.2 | 1.0 | 24.3 | 0.8 | 28.1 | 1.1 | 33.6 | 0.9 | 29.4 | 2.1 |
| 16:1 n7 (PALMo) | 5.0 | 1.2 | 4.2 | 0.5 | 5.6 | 1.4 | 4.4 | 0.5 | 6.3 | 0.5 | 4.0 | 0.5 | 4.5 | 0.3 | 4.5 | 0.5 | 6.2 | 0.6 |
| 18:1 n7 | 0.9 | 0.3 | 0.9 | 0.1 | 1.1 | 0.1 | 1.6 | 0.1 | 1.8 | 0.2 | 0.9 | 0.1 | 1.2 | 0.1 | 1.4 | 0.2 | 1.6 | 0.3 |
| 18:1 n9 (OLE) | 13.2 | 2.1 | 11.5 | 0.6 | 15.5 | 0.4 | 16.6 | 1.1 | 18.5 | 2.5 | 12.8 | 1.6 | 14.5 | 0.7 | 16.6 | 1.5 | 21.4 | 2.2 |
| ƩMUFA | 19.2 | 3.5 | 16.7 | 0.7 | 22.2 | 1.4 | 22.6 | 1.2 | 26.8 | 2.2 | 17.8 | 2.0 | 20.3 | 0.9 | 22.5 | 1.5 | 29.2 | 1.9 |
| 18:2 n6 (LA) | 3.9 | 0.9 | 2.7 | 0.3 | 3.0 | 0.5 | 3.7 | 0.5 | 3.6 | 0.6 | 2.7 | 0.5 | 3.3 | 0.4 | 4.6 | 0.3 | 2.5 | 0.4 |
| 18:3 n3 (ALA) | 9.9 | 0.8 | 13.2 | 1.3 | 12.1 | 1.3 | 12.6 | 1.0 | 10.8 | 0.6 | 12.8 | 1.8 | 12.8 | 0.8 | 11.4 | 0.5 | 10.3 | 0.5 |
| 18:3 n6 | 0.4 | 0.1 | 0.4 | 0.1 | 0.3 | 0.1 | 0.2 | 0.0 | 0.3 | 0.0 | 0.3 | 0.1 | 0.3 | 0.0 | 0.3 | 0.2 | 0.2 | 0.0 |
| 18:4 n3 (SDA) | 4.5 | 1.9 | 10.8 | 1.7 | 5.1 | 0.7 | 3.0 | 1.1 | 2.1 | 0.6 | 9.3 | 0.5 | 5.0 | 0.6 | 2.4 | 0.2 | 1.6 | 0.7 |
| 20:2 n6 | 0.5 | 0.2 | 0.6 | 0.0 | 0.8 | 0.3 | 0.8 | 0.2 | 1.2 | 0.2 | 0.7 | 0.2 | 0.6 | 0.2 | 0.4 | 0.1 | 1.3 | 0.2 |
| 20:3 n3 | 0.2 | 0.1 | 0.3 | 0.1 | 0.3 | 0.1 | 0.3 | 0.1 | 0.3 | 0.1 | 0.4 | 0.1 | 0.4 | 0.1 | 0.1 | 0.1 | 0.3 | 0.1 |
| 20:3 n6 | 1.4 | 0.3 | 1.0 | 0.2 | 0.9 | 0.2 | 0.6 | 0.2 | 1.8 | 0.7 | 1.0 | 0.2 | 1.1 | 0.2 | 1.2 | 0.2 | 2.0 | 0.5 |
| 20:4 n3 | 1.1 | 0.1 | 2.0 | 0.2 | 1.7 | 0.1 | 1.7 | 0.1 | 1.3 | 0.2 | 2.0 | 0.2 | 1.8 | 0.2 | 1.5 | 0.2 | 1.4 | 0.1 |
| 20:4 n6 (ARA) | 22.2 | 1.4 | 16.1 | 0.7 | 17.7 | 0.9 | 15.8 | 0.8 | 15.3 | 0.5 | 17.5 | 0.6 | 17.5 | 0.6 | 15.9 | 0.8 | 17.2 | 2.5 |
| 20:5 n3 (EPA) | 7.0 | 1.6 | 12.1 | 0.9 | 7.5 | 0.8 | 7.9 | 0.6 | 4.3 | 0.7 | 11.1 | 0.7 | 8.0 | 0.8 | 4.6 | 0.8 | 3.8 | 0.9 |
| 22:4 n6 | 0.1 | 0.0 | 0.2 | 0.0 | 0.2 | 0.1 | 0.2 | 0.1 | 0.3 | 0.1 | 0.1 | 0.0 | 0.1 | 0.0 | 0.1 | 0.1 | 0.2 | 0.0 |
| 22:6 n3 | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.9 | 0.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.1 | 0.8 | 0.9 | 0.0 | 0.0 |
| Ʃω-3 | 22.8 | 4.0 | 38.6 | 2.3 | 26.6 | 1.9 | 26.4 | 1.5 | 18.9 | 1.8 | 35.5 | 2.1 | 28.1 | 1.9 | 20.9 | 1.8 | 17.4 | 1.8 |
| Ʃω-6 | 28.7 | 2.5 | 21.0 | 1.0 | 22.8 | 0.6 | 21.3 | 0.6 | 22.5 | 0.2 | 22.3 | 0.5 | 23.1 | 0.9 | 22.6 | 0.4 | 23.6 | 3.1 |
| ƩPUFA | 51.5 | 5.1 | 59.6 | 1.6 | 49.4 | 1.7 | 47.7 | 1.2 | 41.4 | 1.9 | 57.9 | 2.4 | 51.2 | 1.7 | 43.5 | 1.6 | 41.1 | 3.2 |
| ƩPUFA/ƩSFA | 1.8 | 0.3 | 2.5 | 0.2 | 1.8 | 0.1 | 1.6 | 0.1 | 1.3 | 0.1 | 2.4 | 0.2 | 1.8 | 0.1 | 1.3 | 0.1 | 1.4 | 0.2 |
| Anteiso | 0.1 | 0.0 | 0.0 | 0.0 | 0.3 | 0.2 | 0.2 | 0.0 | 0.3 | 0.1 | 0.0 | 0.0 | 0.2 | 0.0 | 0.1 | 0.1 | 0.2 | 0.0 |
| -OH | 0.0 | 0.0 | 0.0 | 0.0 | 0.2 | 0.2 | 0.2 | 0.1 | 0.1 | 0.1 | 0.0 | 0.0 | 0.2 | 0.1 | 0.1 | 0.1 | 0.0 | 0.1 |
| ƩBAFA * | 1.0 | 0.3 | 0.9 | 0.1 | 1.7 | 0.5 | 2.0 | 0.2 | 2.3 | 0.4 | 0.9 | 0.1 | 1.6 | 0.1 | 1.7 | 0.3 | 1.8 | 0.3 |
| TL (mg g−1) | 38.4 | 4.2 | 33.2 | 5.2 | 30.2 | 1.7 | 28.4 | 2.6 | 26.7 | 2.5 | 29.2 | 3.2 | 27.6 | 2.3 | 28.2 | 3.2 | 24.3 | 5.4 |
| Site Capo Gallo | Natural | Transplanted | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Time | T0 | T1 | T2 | T1 | T2 | |||||
| FAs | mean | sd | mean | sd | mean | sd | mean | sd | mean | sd |
| 14:00 | 3.6 | 0.4 | 2.1 | 0.1 | 3.4 | 0.2 | 3.1 | 0.3 | 3.7 | 0.7 |
| 15:00 | 0.2 | 0.0 | 0.1 | 0.0 | 0.2 | 0.0 | 0.2 | 0.0 | 0.2 | 0.1 |
| 16:0 (PALM) | 22.7 | 2.0 | 23.5 | 0.6 | 24.4 | 1.0 | 23.5 | 0.8 | 27.6 | 1.6 |
| 18:00 | 0.4 | 0.1 | 0.1 | 0.0 | 0.5 | 0.1 | 0.3 | 0.0 | 0.6 | 0.1 |
| 20:00 | 0.2 | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | 0.1 | 0.0 | 0.1 | 0.1 |
| LCFAs (>22:0) | 0.4 | 0.1 | 0.3 | 0.1 | 0.5 | 0.1 | 0.5 | 0.1 | 0.5 | 0.2 |
| ƩSFA | 27.7 | 2.4 | 26.3 | 0.6 | 29.2 | 1.2 | 27.8 | 1.0 | 32.9 | 1.4 |
| 16:1 n7 (PALMo) | 6.0 | 0.6 | 5.4 | 0.6 | 5.0 | 0.5 | 5.1 | 0.5 | 4.9 | 0.2 |
| 18:1 n7 | 0.9 | 0.0 | 0.9 | 0.1 | 1.1 | 0.2 | 1.0 | 0.1 | 1.3 | 0.2 |
| 18:1 n9 (OLE) | 13.1 | 1.2 | 13.6 | 0.6 | 13.6 | 1.4 | 13.6 | 1.2 | 15.2 | 1.0 |
| ƩMUFA | 20.0 | 1.1 | 20.0 | 0.6 | 19.7 | 1.3 | 19.7 | 1.8 | 21.5 | 1.0 |
| 18:2 n6 (LA) | 4.1 | 0.5 | 2.5 | 0.2 | 3.8 | 0.5 | 3.1 | 0.4 | 4.4 | 0.9 |
| 18:3 n3 (ALA) | 9.3 | 0.6 | 10.7 | 0.6 | 11.8 | 1.2 | 11.0 | 1.0 | 10.1 | 0.7 |
| 18:3 n6 | 0.4 | 0.1 | 0.1 | 0.1 | 0.3 | 0.1 | 0.3 | 0.1 | 0.3 | 0.1 |
| 18:4 n3 (SDA) | 5.8 | 3.3 | 9.1 | 1.2 | 4.2 | 1.1 | 6.5 | 1.0 | 2.9 | 0.5 |
| 20:2 n6 | 0.5 | 0.2 | 0.6 | 0.1 | 0.4 | 0.2 | 0.6 | 0.2 | 0.3 | 0.2 |
| 20:3 n3 | 0.3 | 0.1 | 0.2 | 0.1 | 0.2 | 0.0 | 0.3 | 0.1 | 0.1 | 0.1 |
| 20:3 n6 | 1.2 | 0.3 | 0.9 | 0.1 | 1.0 | 0.2 | 1.0 | 0.3 | 1.1 | 0.1 |
| 20:4 n3 | 1.4 | 0.1 | 2.1 | 0.2 | 1.7 | 0.1 | 1.9 | 0.2 | 1.5 | 0.1 |
| 20:4 n6 (ARA) | 20.6 | 3.0 | 16.3 | 0.5 | 19.3 | 2.0 | 17.8 | 0.7 | 18.1 | 0.9 |
| 20:5 n3 (EPA) | 8.4 | 1.9 | 11.0 | 0.9 | 7.8 | 1.0 | 9.3 | 0.8 | 5.9 | 0.9 |
| 22:4 n6 | 0.0 | 0.0 | 0.1 | 0.0 | 0.1 | 0.1 | 0.2 | 0.1 | 0.2 | 0.0 |
| 22:6 n3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.2 | 0.2 |
| Ʃω-3 | 25.2 | 5.7 | 33.2 | 1.4 | 25.8 | 3.1 | 29.1 | 2.4 | 20.8 | 1.1 |
| Ʃω-6 | 27.0 | 3.5 | 20.5 | 0.5 | 24.9 | 2.4 | 22.9 | 1.0 | 24.5 | 0.9 |
| ƩPUFA | 52.2 | 2.6 | 53.6 | 1.0 | 50.7 | 2.2 | 52.0 | 2.6 | 45.3 | 1.0 |
| ƩPUFA/ƩSFA | 1.9 | 0.3 | 2.0 | 0.1 | 1.7 | 0.1 | 1.9 | 0.2 | 1.4 | 0.1 |
| Anteiso | 0.1 | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.2 | 0.0 |
| -OH | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 |
| ƩBAFA * | 0.9 | 0.1 | 0.9 | 0.1 | 1.4 | 0.3 | 1.0 | 0.1 | 1.5 | 0.2 |
| TL (mg g−1) | 37.9 | 2.6 | 38.1 | 4.2 | 39.2 | 12.4 | 27.7 | 5.7 | 29.2 | 8.0 |
| Comparison between Time | Site | Natural | Transplanted | ||||
|---|---|---|---|---|---|---|---|
| FA | Contrib% | Cum% | FA | Contrib% | Cum% | ||
| T0 vs. T1 | Barcarello | SDA | 18.6 | 18.6 | SDA | 16.5 | 16.5 |
| ARA | 18.2 | 36.8 | ARA | 16.5 | 33.0 | ||
| EPA | 15.0 | 51.7 | EPA | 13.7 | 46.7 | ||
| Capo Gallo | ARA | 20.2 | 20.2 | ARA | 19.4 | 19.4 | |
| SDA | 16.2 | 36.4 | SDA | 17.3 | 36.7 | ||
| EPA | 10.9 | 47.3 | PALM | 10.7 | 47.3 | ||
| T1 vs. T2 | Barcarello | SDA | 22.1 | 22.1 | SDA | 23.5 | 23.5 |
| EPA | 17.9 | 40.0 | EPA | 16.6 | 40.0 | ||
| OLE | 15.1 | 55.1 | PALM | 14.8 | 54.8 | ||
| Capo Gallo | SDA | 24.2 | 24.2 | PALM | 20.3 | 20.3 | |
| EPA | 15.7 | 39.9 | SDA | 17.7 | 38.0 | ||
| ARA | 14.7 | 54.6 | EPA | 16.7 | 54.7 | ||
| T2 vs. T3 | Barcarello | SDA | 14.6 | 14.6 | PALM | 22.1 | 22.1 |
| ARA | 12.8 | 27.4 | EPA | 15.6 | 37.7 | ||
| PALM | 10.1 | 37.5 | SDA | 12.0 | 49.7 | ||
| T3 vs. T4 | Barcarello | EPA | 19.3 | 19.3 | OLE | 20.1 | 20.1 |
| OLE | 12.7 | 32.0 | PALM | 19.4 | 39.5 | ||
| PALMo | 9.9 | 41.9 | ARA | 10.6 | 50.1 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chemello, S.; Signa, G.; Mazzola, A.; Ribeiro Pereira, T.; Sousa Pinto, I.; Vizzini, S. Limited Stress Response to Transplantation in the Mediterranean Macroalga Ericaria amentacea, a Key Species for Marine Forest Restoration. Int. J. Environ. Res. Public Health 2022, 19, 12253. https://doi.org/10.3390/ijerph191912253
Chemello S, Signa G, Mazzola A, Ribeiro Pereira T, Sousa Pinto I, Vizzini S. Limited Stress Response to Transplantation in the Mediterranean Macroalga Ericaria amentacea, a Key Species for Marine Forest Restoration. International Journal of Environmental Research and Public Health. 2022; 19(19):12253. https://doi.org/10.3390/ijerph191912253
Chicago/Turabian StyleChemello, Silvia, Geraldina Signa, Antonio Mazzola, Tania Ribeiro Pereira, Isabel Sousa Pinto, and Salvatrice Vizzini. 2022. "Limited Stress Response to Transplantation in the Mediterranean Macroalga Ericaria amentacea, a Key Species for Marine Forest Restoration" International Journal of Environmental Research and Public Health 19, no. 19: 12253. https://doi.org/10.3390/ijerph191912253
APA StyleChemello, S., Signa, G., Mazzola, A., Ribeiro Pereira, T., Sousa Pinto, I., & Vizzini, S. (2022). Limited Stress Response to Transplantation in the Mediterranean Macroalga Ericaria amentacea, a Key Species for Marine Forest Restoration. International Journal of Environmental Research and Public Health, 19(19), 12253. https://doi.org/10.3390/ijerph191912253







