Brain Functional Connectivity in Middle-Aged Hong Chuan Tai Chi Players in Resting State
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Design
2.2. Procedure and Measurements
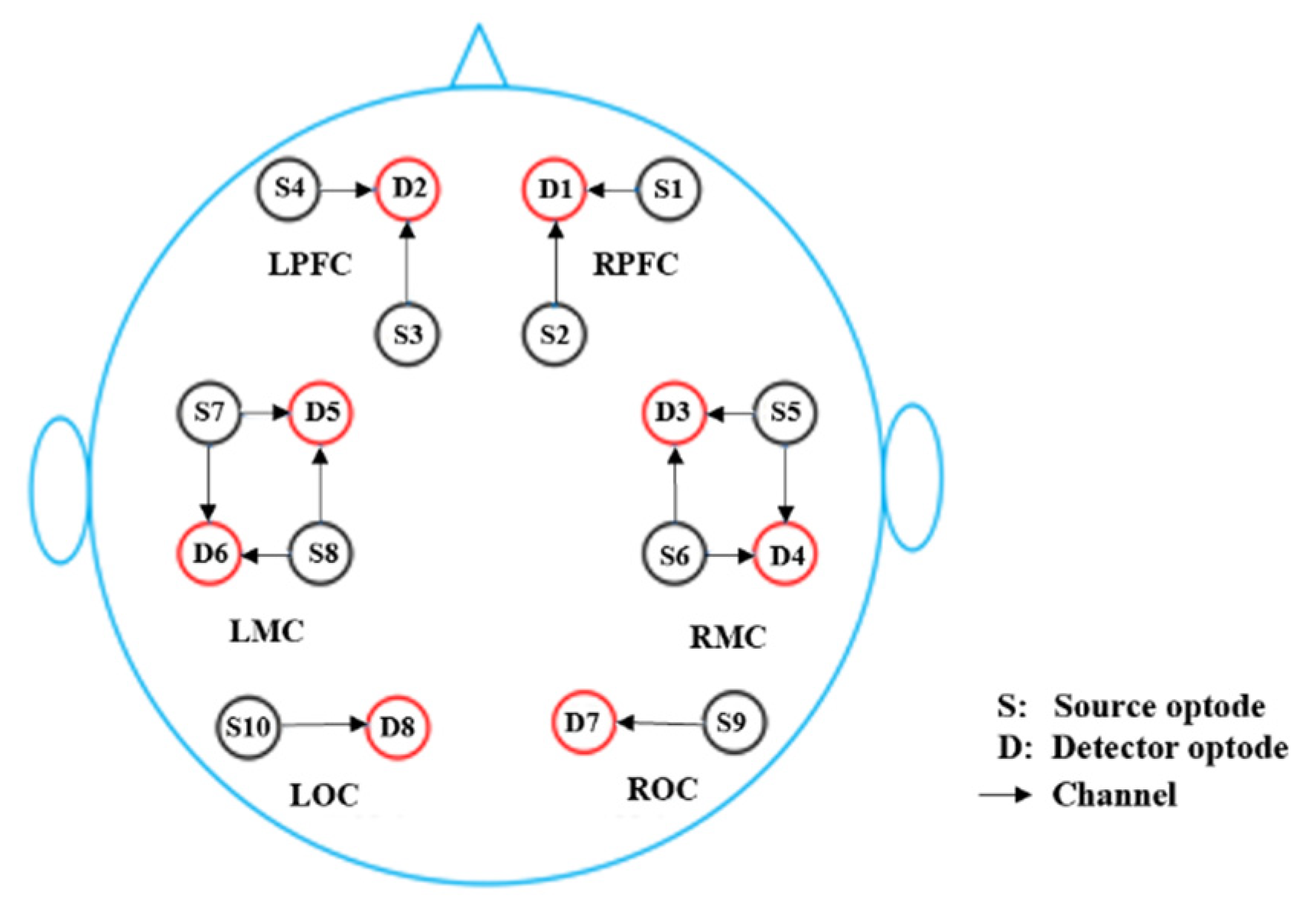
2.3. Functional Near-Infrared Spectroscopy
2.4. Data Preprocessing
2.5. Functional Connectivity Analysis
2.6. Statistical Analysis
3. Results
Wavelet Phase Coherence Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jia, L.; Du, Y.; Chu, L.; Zhang, Z.; Li, F.; Lyu, D.; Li, Y.; Li, Y.; Zhu, M.; Jiao, H.; et al. Prevalence, risk factors, and management of dementia and mild cognitive impairment in adults aged 60 years or older in China: A cross-sectional study. Lancet Public Health 2020, 5, e661–e671. [Google Scholar] [CrossRef]
- Soldan, A.; Pettigrew, C.; Zhu, Y.; Wang, M.C.; Bilgel, M.; Hou, X.; Lu, H.; Miller, M.I.; Albert, M. Association of Lifestyle Activities with Functional Brain Connectivity and Relationship to Cognitive Decline among Older Adults. Cereb. Cortex. 2021, 31, 5637–5651. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Chen, X.; Nie, P.; Lin, S.; Guo, J.; Chen, J.; Yu, L. Can Tai Chi Improve Cognitive Function? A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Altern. Complement Med. 2021, 27, 1070–1083. [Google Scholar] [CrossRef] [PubMed]
- Wayne, P.M.; Walsh, J.N.; Taylor-Piliae, R.E.; Wells, R.E.; Papp, K.V.; Donovan, N.J.; Yeh, G.Y. Effect of tai chi on cognitive performance in older adults: Systematic review and meta-analysis. J. Am. Geriatr. Soc. 2014, 62, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Liu, J.; Egorova, N.; Chen, X.; Sun, S.; Xue, X.; Huang, J.; Zheng, G.; Wang, Q.; Chen, L.; et al. Increased Hippocampus-Medial Prefrontal Cortex Resting-State Functional Connectivity and Memory Function after Tai Chi Chuan Practice in Elder Adults. Front. Aging Neurosci. 2016, 8, 25. [Google Scholar] [CrossRef]
- Wei, G.X.; Dong, H.M.; Yang, Z.; Luo, J.; Zuo, X.N. Tai Chi Chuan optimizes the functional organization of the intrinsic human brain architecture in older adults. Front. Aging Neurosci. 2014, 6, 74. [Google Scholar] [CrossRef]
- Park, H.J.; Friston, K. Structural and functional brain networks: From connections to cognition. Science 2013, 342, 1238411. [Google Scholar] [CrossRef] [PubMed]
- Sasai, S.; Homae, F.; Watanabe, H.; Taga, G. Frequency-specific functional connectivity in the brain during resting state revealed by NIRS. Neuroimage 2011, 56, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.M.; Zhang, Y.J.; Biswal, B.B.; Zang, Y.F.; Peng, D.L.; Zhu, C.Z. Use of fNIRS to assess resting state functional connectivity. J. Neurosci. Methods 2010, 186, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, Y.J.; Lu, C.M.; Ma, S.Y.; Zang, Y.F.; Zhu, C.Z. Functional connectivity as revealed by independent component analysis of resting-state fNIRS measurements. Neuroimage 2010, 51, 1150–1161. [Google Scholar] [CrossRef]
- Cui, R.; Zhang, M.; Li, Z.; Xin, Q.; Lu, L.; Zhou, W.; Han, Q.; Gao, Y. Wavelet coherence analysis of spontaneous oscillations in cerebral tissue oxyhemoglobin concentrations and arterial blood pressure in elderly subjects. Microvasc. Res. 2014, 93, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Shang, Y.; Hayes, D.J.; Saha, S.P.; Yu, G. Noninvasive optical evaluation of spontaneous low frequency oscillations in cerebral hemodynamics. Neuroimage 2012, 62, 1445–1454. [Google Scholar] [CrossRef] [PubMed]
- Bu, L.; Li, J.; Li, F.; Liu, H.; Li, Z. Wavelet coherence analysis of cerebral oxygenation signals measured by near-infrared spectroscopy in sailors: An exploratory, experimental study. BMJ Open 2016, 6, e13357. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Zhang, M.; Wang, Y.; Zhang, M.; Wang, B.; Xin, Q.; Li, Z. Age-related alterations in phase synchronization of oxyhemoglobin concentration changes in prefrontal tissues as measured by near-infrared spectroscopy signals. Microvasc. Res. 2016, 103, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Cao, N.; Pi, Y.; Liu, K.; Meng, H.; Wang, Y.; Zhang, J.; Wu, Y.; Tan, X. Inhibitory and facilitatory connections from dorsolateral prefrontal to primary motor cortex in healthy humans at rest-An rTMS study. Neurosci. Lett. 2018, 687, 82–87. [Google Scholar] [CrossRef]
- Svoboda, K.; Li, N. Neural mechanisms of movement planning: Motor cortex and beyond. Curr. Opin Neurobiol. 2018, 49, 33–41. [Google Scholar] [CrossRef]
- Xie, H.; Zhang, M.; Huo, C.; Xu, G.; Li, Z.; Fan, Y. Tai Chi Chuan exercise related change in brain function as assessed by functional near-infrared spectroscopy. Sci. Rep. 2019, 9, 13198. [Google Scholar] [CrossRef] [PubMed]
- Strigaro, G.; Ruge, D.; Chen, J.C.; Marshall, L.; Desikan, M.; Cantello, R.; Rothwell, J.C. Interaction between visual and motor cortex: A transcranial magnetic stimulation study. J. Physiol. 2015, 593, 2365–2377. [Google Scholar] [CrossRef]
- Chen, L.Z.; Yuan, X.; Zhang, Y.; Zhang, S.; Zou, L.; Yang, L.; Chang, Y.K.; Xia, Q.; Wang, Y.; Wei, G.X. Brain Functional Specialization Is Enhanced Among Tai Chi Chuan Practitioners. Arch Phys. Med. Rehabil. 2020, 101, 1176–1182. [Google Scholar] [CrossRef] [PubMed]
- Yue, C.; Zhang, Y.; Jian, M.; Herold, F.; Yu, Q.; Mueller, P.; Lin, J.; Wang, G.; Tao, Y.; Zhang, Z.; et al. Differential Effects of Tai Chi Chuan (Motor-Cognitive Training) and Walking on Brain Networks: A Resting-State fMRI Study in Chinese Women Aged 60. Healthcare 2020, 8, 67. [Google Scholar] [CrossRef] [Green Version]
- Zou, L.; Loprinzi, P.D.; Yu, J.J.; Yang, L.; Li, C.; Yeung, A.S.; Kong, Z.; Chiou, S.Y.; Xiao, T. Superior Effects of Modified Chen-Style Tai Chi versus 24-Style Tai Chi on Cognitive Function, Fitness, and Balance Performance in Adults over 55. Brain Sci. 2019, 9, 102. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Chen, X.; Egorova, N.; Liu, J.; Xue, X.; Wang, Q.; Zheng, G.; Li, M.; Hong, W.; Sun, S.; et al. Tai Chi Chuan and Baduanjin practice modulates functional connectivity of the cognitive control network in older adults. Sci. Rep. 2017, 7, 41581. [Google Scholar] [CrossRef] [PubMed]
- Tsai, P.S.; Wang, S.Y.; Wang, M.Y.; Su, C.T.; Yang, T.T.; Huang, C.J.; Fang, S.C. Psychometric evaluation of the Chinese version of the Pittsburgh Sleep Quality Index (CPSQI) in primary insomnia and control subjects. Qual. Life Res. 2005, 14, 1943–1952. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.R.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Xu, L.; Wang, B.; Xu, G.; Wang, W.; Liu, Z.; Li, Z. Functional connectivity analysis using fNIRS in healthy subjects during prolonged simulated driving. Neurosci. Lett. 2017, 640, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.S.; Ho, Y.C.; Cheung, M.C.; Albert, M.S.; Chiu, H.F.; Lam, L.C. Association between mind-body and cardiovascular exercises and memory in older adults. J. Am. Geriatr. Soc. 2005, 53, 1754–1760. [Google Scholar] [CrossRef] [PubMed]
- Man, D.W.; Tsang, W.W.; Hui-Chan, C.W. Do older t’ai chi practitioners have better attention and memory function? J. Altern. Complement Med. 2010, 16, 1259–1264. [Google Scholar] [CrossRef] [PubMed]
- Lam, L.C.; Tam, C.W.; Lui, V.W.; Chan, W.C.; Chan, S.S.; Chiu, H.F.; Wong, A.; Tham, M.K.; Ho, K.S.; Chan, W.M. Modality of physical exercise and cognitive function in Hong Kong older Chinese community. Int. J. Geriatr. Psychiatry 2009, 24, 48–53. [Google Scholar] [CrossRef]
- Huang, N.; Li, W.; Rong, X.; Champ, M.; Wei, L.; Li, M.; Mu, H.; Hu, Y.; Ma, Z.; Lyu, J. Effects of a Modified Tai Chi Program on Older People with Mild Dementia: A Randomized Controlled Trial. J. Alzheimers Dis. 2019, 72, 947–956. [Google Scholar] [CrossRef]
- Ji, Z.; Li, A.; Feng, T.; Liu, X.; You, Y.; Meng, F.; Wang, R.; Lu, J.; Zhang, C. The benefits of Tai Chi and brisk walking for cognitive function and fitness in older adults. PeerJ 2017, 5, e3943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damoiseaux, J.S. Effects of aging on functional and structural brain connectivity. Neuroimage 2017, 160, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Onoda, K.; Ishihara, M.; Yamaguchi, S. Decreased functional connectivity by aging is associated with cognitive decline. J. Cogn. Neurosci. 2012, 24, 2186–2198. [Google Scholar] [CrossRef] [PubMed]
- Farras-Permanyer, L.; Mancho-Fora, N.; Montala-Flaquer, M.; Bartres-Faz, D.; Vaque-Alcazar, L.; Pero-Cebollero, M.; Guardia-Olmos, J. Age-related changes in resting-state functional connectivity in older adults. Neural Regen. Res. 2019, 14, 1544–1555. [Google Scholar] [CrossRef] [PubMed]
- Blum, L.; Hofmann, A.; Rosenbaum, D.; Elshehabi, M.; Suenkel, U.; Fallgatter, A.J.; Ehlis, A.C.; Metzger, F.G. Effects of aging on functional connectivity in a neurodegenerative risk cohort: Resting state versus task measurement using near-infrared spectroscopy. Sci. Rep. 2022, 12, 11262. [Google Scholar] [CrossRef]
- Zheng, G.; Li, S.; Huang, M.; Liu, F.; Tao, J.; Chen, L. The effect of Tai Chi training on cardiorespiratory fitness in healthy adults: A systematic review and meta-analysis. PLoS ONE 2015, 10, e117360. [Google Scholar] [CrossRef]
- Hyodo, K.; Dan, I.; Kyutoku, Y.; Suwabe, K.; Byun, K.; Ochi, G.; Kato, M.; Soya, H. The association between aerobic fitness and cognitive function in older men mediated by frontal lateralization. Neuroimage 2016, 125, 291–300. [Google Scholar] [CrossRef]
- Song, Q.H.; Xu, R.M.; Shen, G.Q.; Zhang, Q.H.; Ma, M.; Zhao, X.P.; Guo, Y.H.; Wang, Y. Influence of Tai Chi exercise cycle on the senile respiratory and cardiovascular circulatory function. Int. J. Clin. Exp. Med. 2014, 7, 770–774. [Google Scholar]
- Quan, L. Study on physiological and psychological effects of long term 24-style tai chi chuan exercise on middle-aged people. China Sport Sci. Technol. 2016, 52, 68–74. [Google Scholar]
- Doll, A.; Holzel, B.K.; Mulej, B.S.; Boucard, C.C.; Xie, X.; Wohlschlager, A.M.; Sorg, C. Mindful attention to breath regulates emotions via increased amygdala-prefrontal cortex connectivity. Neuroimage 2016, 134, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Zhang, M.; Wang, Y.; Zhang, M.; Wang, Y.; Xin, Q.; Wang, B.; Li, Z. Frequency-specific functional connectivity revealed by wavelet-based coherence analysis in elderly subjects with cerebral infarction using NIRS method. Med. Phys. 2015, 42, 5391–5403. [Google Scholar] [CrossRef] [PubMed]
- Vermeij, A.; Meel-van, D.A.A.; Kessels, R.P.; van Beek, A.H.; Claassen, J.A. Very-low-frequency oscillations of cerebral hemodynamics and blood pressure are affected by aging and cognitive load. Neuroimage 2014, 85 Pt 1, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.A.; Kuo, C.D. The effect of Tai Chi Chuan on the autonomic nervous modulation in older persons. Med. Sci. Sports Exerc. 2003, 35, 1972–1976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willie, C.K.; Tzeng, Y.C.; Fisher, J.A.; Ainslie, P.N. Integrative regulation of human brain blood flow. J. Physiol. 2014, 592, 841–859. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H.; Lee, Y.; Kim, S.G.; Choi, B.Y.; Lee, H.S.; Bang, S.Y. The beneficial effects of Tai Chi exercise on endothelial function and arterial stiffness in elderly women with rheumatoid arthritis. Arthritis Res Ther. 2015, 17, 380. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| TCC (n = 18) | C (n = 22) | p | |
|---|---|---|---|
| Age (year) | 55.78 ± 2.64 | 54.69 ± 3.10 | 0.401 |
| Gender (men) | 10 | 13 | NA |
| Body mass (kg) | 60.67 ± 6.40 | 61.29 ± 6.49 | 0.764 |
| Height (m) | 1.65 ± 0.06 | 1.67 ± 0.10 | 0.475 |
| BMI (kg/m2) | 22.10 ± 0.61 | 22.27 ± 0.68 | 0.556 |
| Education (year) | 12.67 ± 2.00 | 12.00 ± 2.12 | 0.467 |
| Tai Chi experience (year) | 4.61 ± 0.89 | NA | NA |
| Duration (hour/w) | 13.89 ± 1.62 | NA | NA |
| Handedness (right) | 18 | 22 | NA |
| Systolic pressure (mmHg) | 116.00 ± 7.38 | 120.85 ± 6.79 | 0.128 |
| Diastolic pressure (mmHg) | 73.89 ± 3.48 | 76.61 ± 5.38 | 0.197 |
| Sleep quality (PQSI) | 3.61 ± 0.78 | 3.82 ± 0.85 | 0.432 |
| TCC (n = 18) | C (n = 22) | p | |
|---|---|---|---|
| Visual spatial/executive function | 4.39 ± 0.61 ** | 3.68 ± 0.72 | 0.002 |
| Naming | 2.94 ± 0.24 | 3.00 ± 0.00 | 0.274 |
| Memory | 4.44 ± 0.51 * | 3.91 ± 0.75 | 0.014 |
| Attention | 5.83 ± 0.38 | 5.45 ± 0.74 | 0.057 |
| Language | 2.67 ± 0.49 | 2.59 ± 0.5 | 0.633 |
| Abstraction | 1.89 ± 0.32 | 1.82 ± 0.39 | 0.545 |
| Orientation | 5.89 ± 0.32 | 5.82 ± 0.39 | 0.545 |
| Total score | 28.06 ± 0.64 *** | 26.27 ± 1.2 | 0.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; Zhang, X.; Xie, H.; He, Q.; Shi, Z. Brain Functional Connectivity in Middle-Aged Hong Chuan Tai Chi Players in Resting State. Int. J. Environ. Res. Public Health 2022, 19, 12232. https://doi.org/10.3390/ijerph191912232
Chen W, Zhang X, Xie H, He Q, Shi Z. Brain Functional Connectivity in Middle-Aged Hong Chuan Tai Chi Players in Resting State. International Journal of Environmental Research and Public Health. 2022; 19(19):12232. https://doi.org/10.3390/ijerph191912232
Chicago/Turabian StyleChen, Weiqi, Xianliang Zhang, Hui Xie, Qiang He, and Zhenguo Shi. 2022. "Brain Functional Connectivity in Middle-Aged Hong Chuan Tai Chi Players in Resting State" International Journal of Environmental Research and Public Health 19, no. 19: 12232. https://doi.org/10.3390/ijerph191912232
APA StyleChen, W., Zhang, X., Xie, H., He, Q., & Shi, Z. (2022). Brain Functional Connectivity in Middle-Aged Hong Chuan Tai Chi Players in Resting State. International Journal of Environmental Research and Public Health, 19(19), 12232. https://doi.org/10.3390/ijerph191912232






