A Pilot Study on the Concentration, Distribution and Bioaccumulation of Polybrominated Diphenyl Ethers (PBDEs) in Tissues and Organs of Grassland Sheep
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Chemical Analysis
2.3. Quality Control and Assurance
2.4. Statistical Analyses
3. Results and Discussion
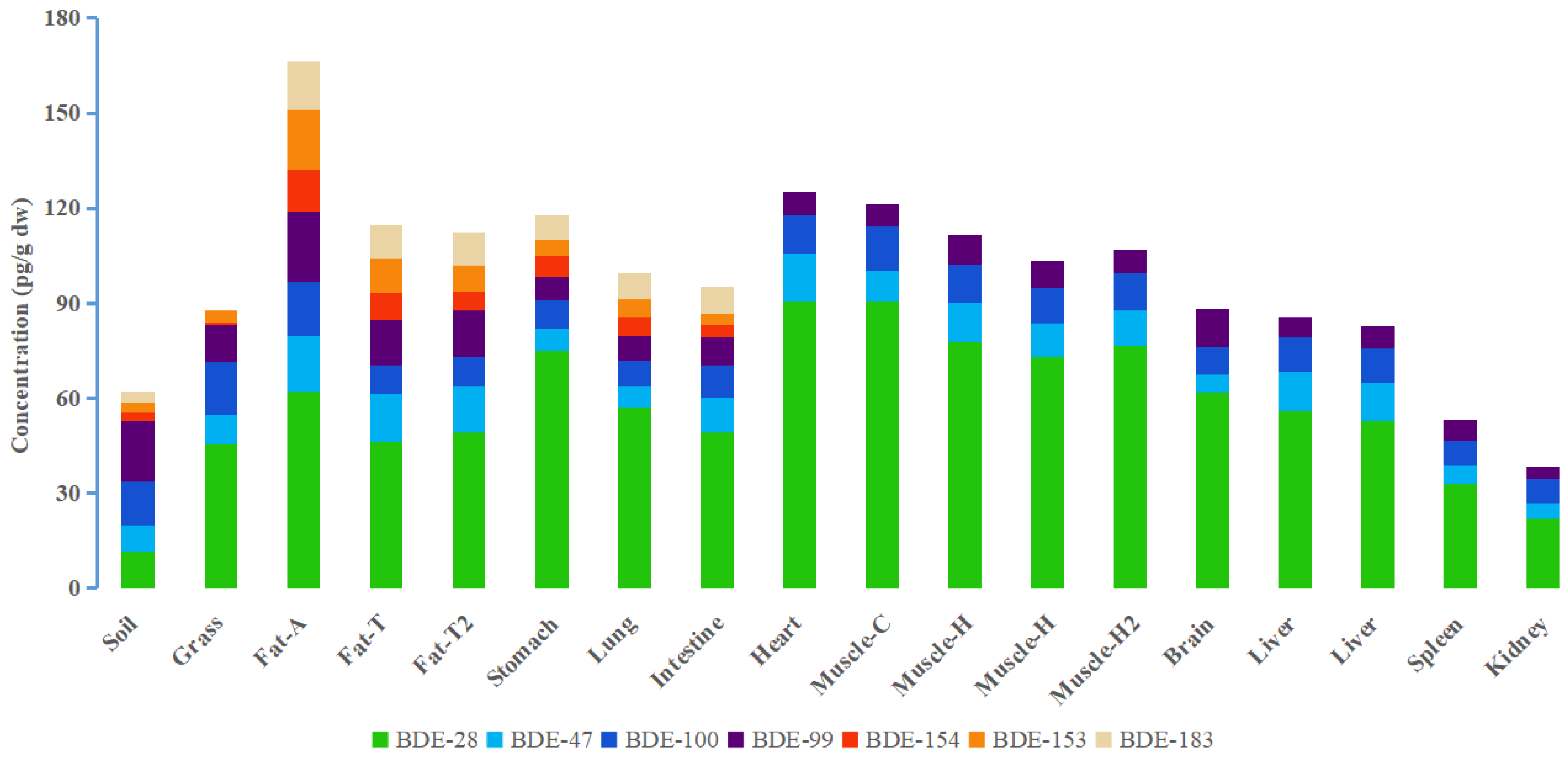
3.1. PBDE Concentrations in Grassland Sheep Organs and Tissues
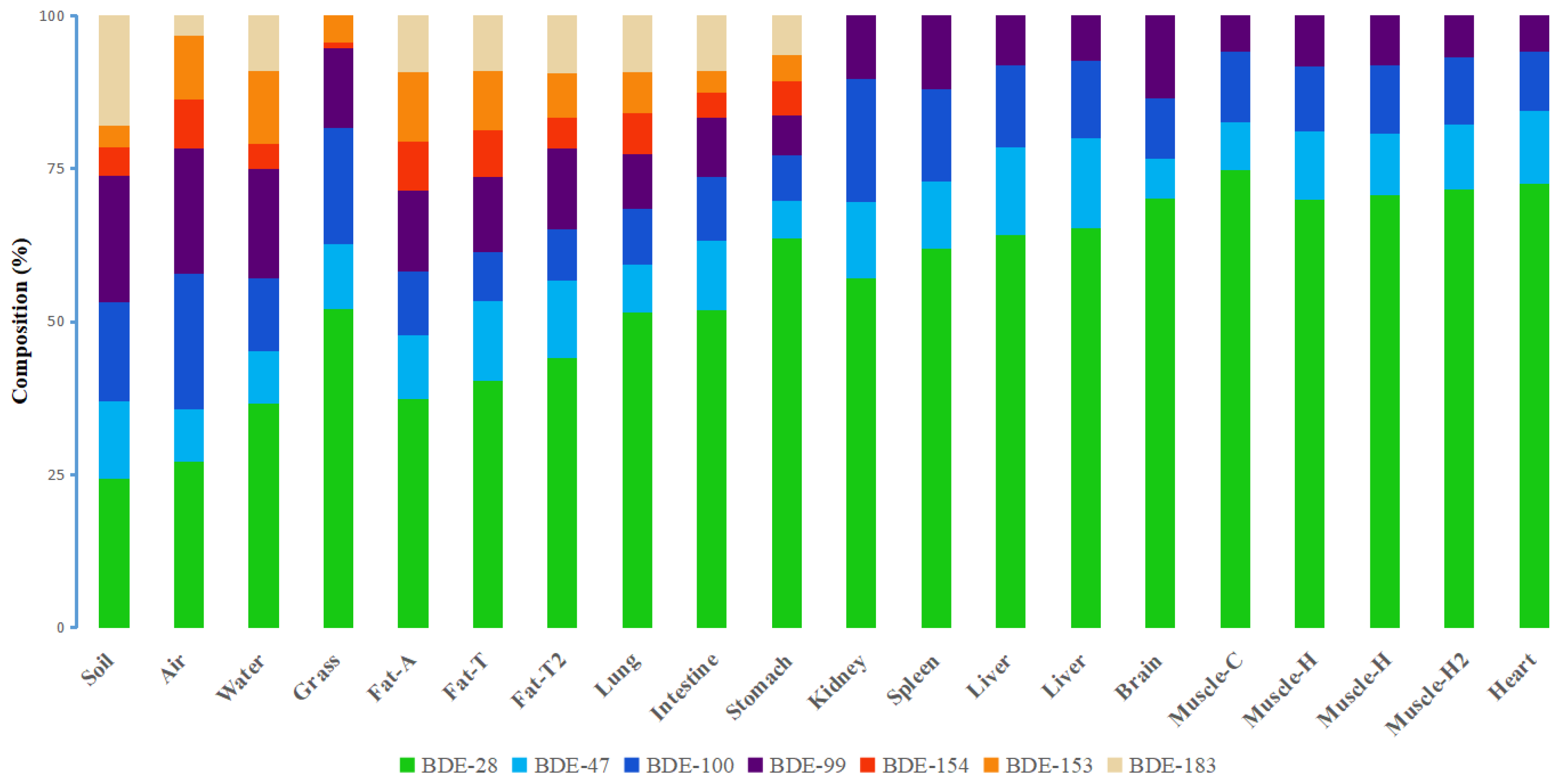
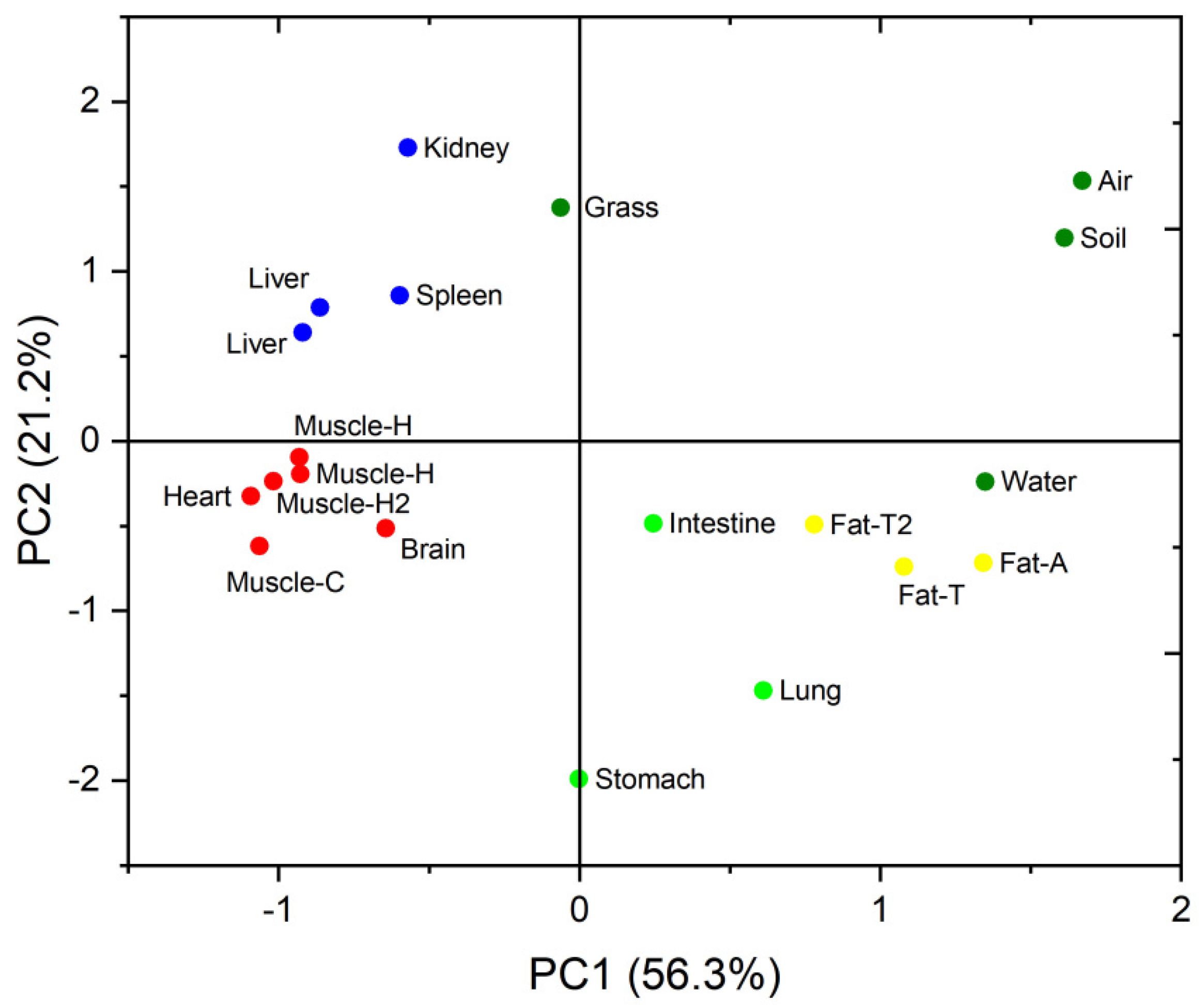
3.2. PBDE Congener Patterns in Different Organs and Tissues
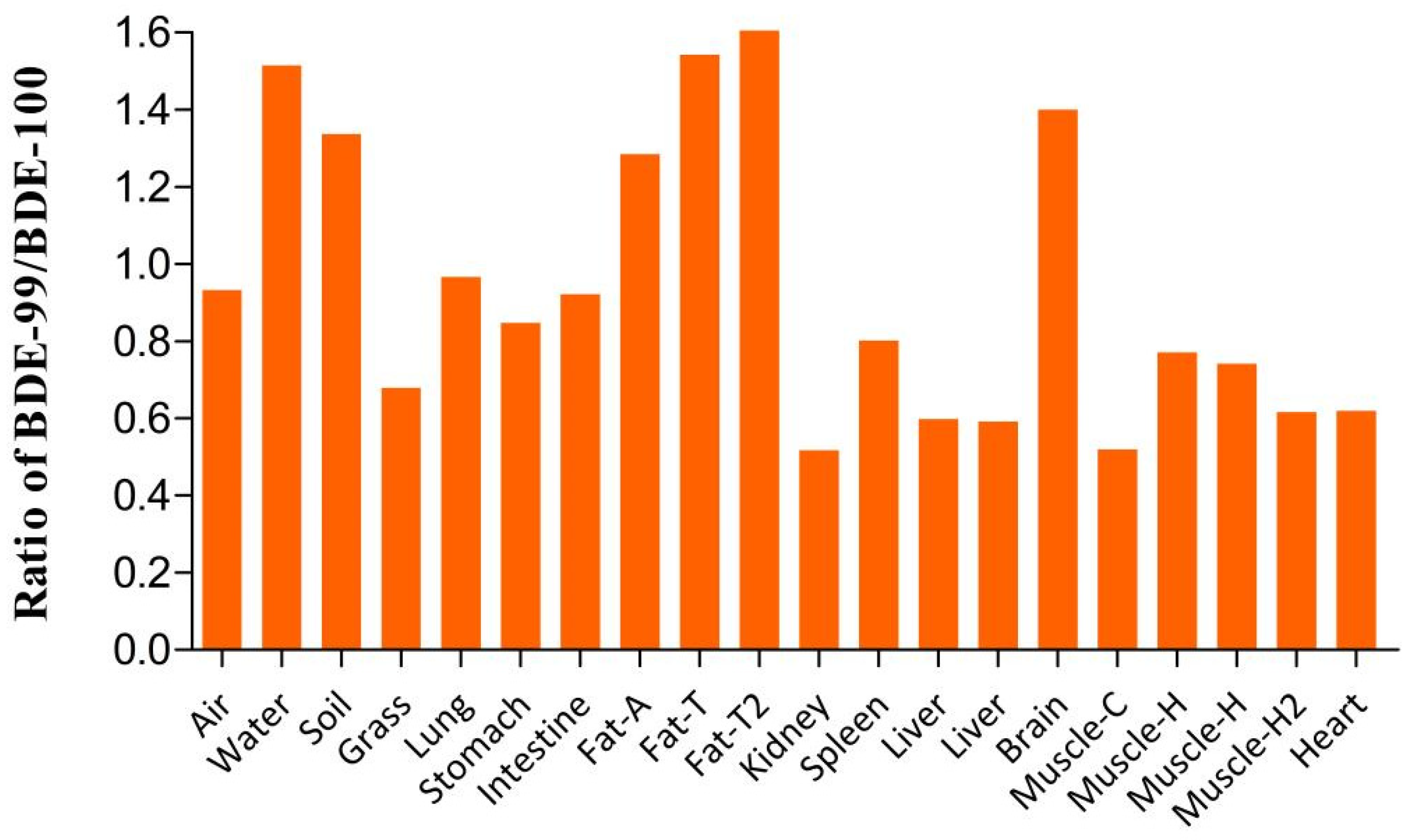
3.3. Selective Bioaccumulation of PBDE Isomers in the Sheep Tissues and Organs
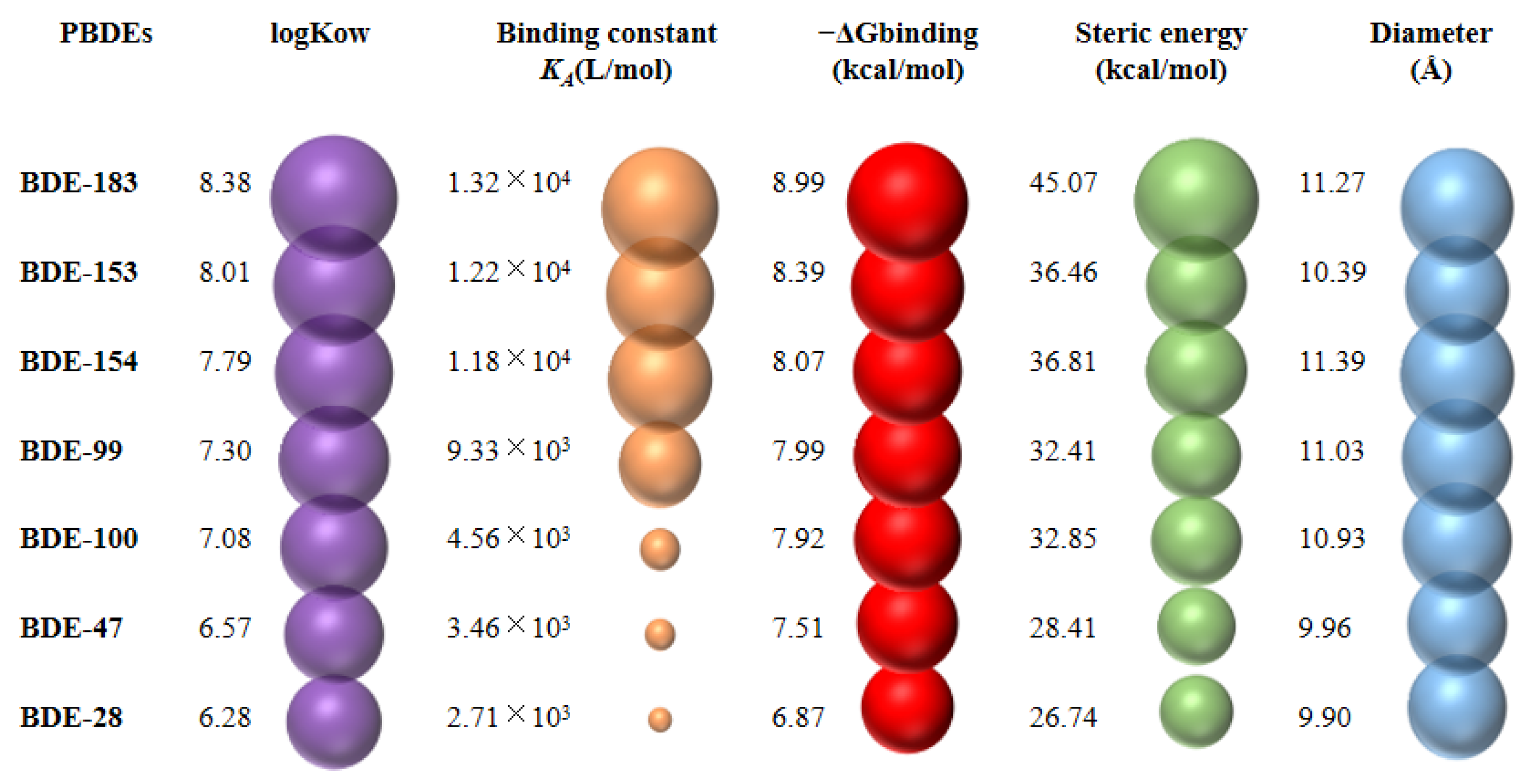
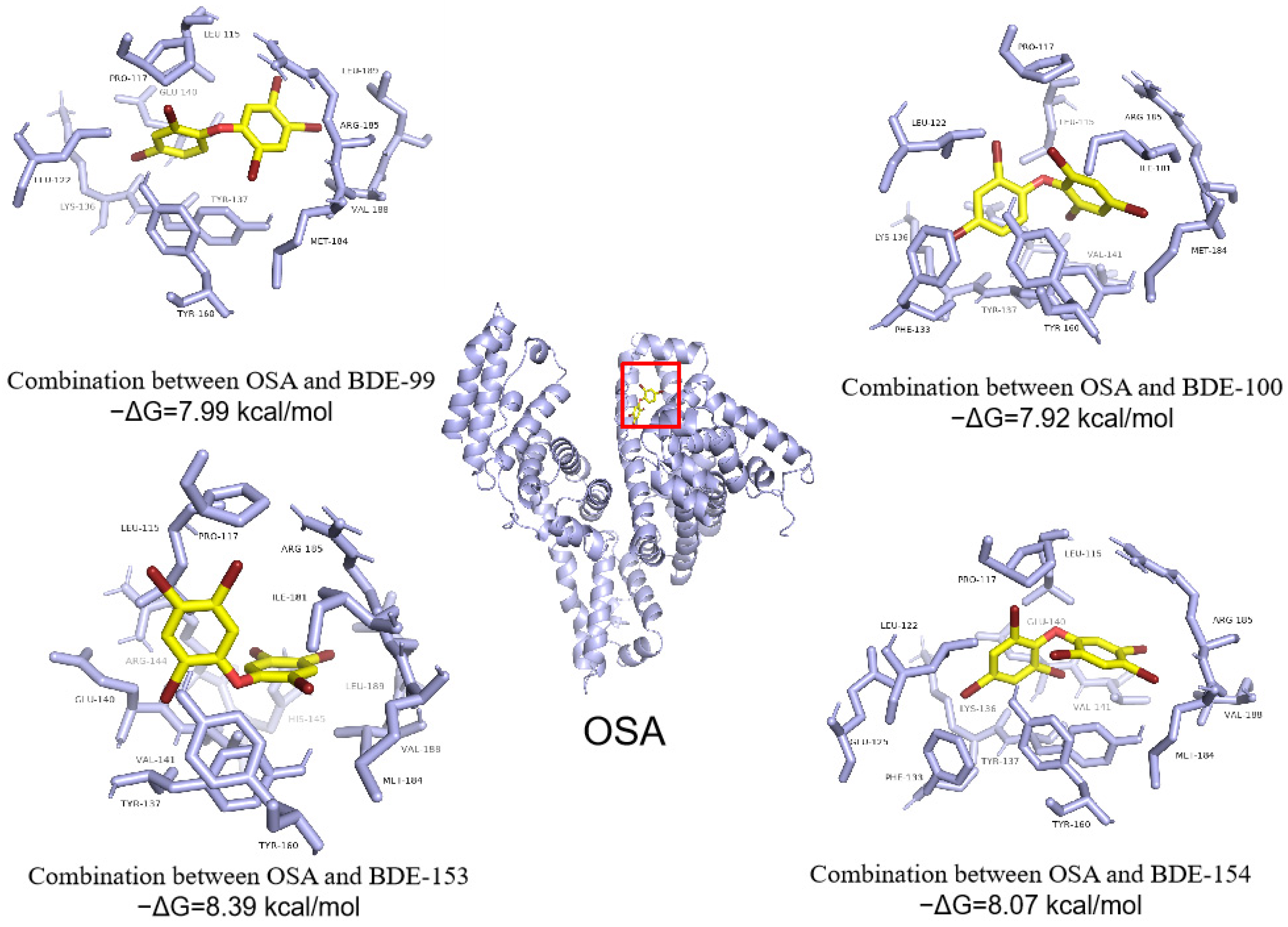
3.4. Abilities of PBDEs to Bind to Ovine Serum Albumin (OSA) and Cytochrome P450 Enzyme (CYP3A24)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Y.M.; Jiang, G.B.; Wang, Y.W.; Wang, P.; Zhang, Q.H. Concentrations, profiles and gas-particle partitioning of PCDD/Fs, PCBs and PBDEs in the ambient air of an E-waste dismantling area, southeast China. Chin. Sci. Bul. 2008, 53, 521–528. [Google Scholar] [CrossRef]
- Hale, R.C.; Alaee, M.; Manchester-Neesvig, J.B.; Stapleton, H.M.; Ikonomou, M.G. Polybrominated diphenyl ether flame retardants in the North American environment. Environ. Int. 2003, 29, 771–779. [Google Scholar] [CrossRef]
- Wang, D.L.; Cai, Z.W.; Jiang, G.B.; Leung, A.; Wong, M.H.; Wong, W.K. Determination of polybrominated diphenyl ethers in soil and sediment from an electronic waste recycling facility. Chemosphere 2005, 60, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Wania, F.; Westgate, J.N. On the mechanism of mountain cold-trapping of organic chemicals. Environ. Sci. Technol. 2008, 42, 9092–9098. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.L.; Luo, X.J.; Liu, H.Y.; Yu, L.H.; Chen, S.J.; Mai, B.X. Bioaccumulation of several brominated flame retardants and dechlorane plus in waterbirds from an e-waste recycling region in South China, associated with trophic level and diet sources. Environ. Sci. Technol. 2011, 45, 400. [Google Scholar] [CrossRef]
- Zhao, Y.X.; Li, Y.Y.; Qin, X.F.; Lou, Q.Q.; Qin, Z.F. Accumulation of polybrominated diphenyl ethers in the brain compared with the levels in other tissues among different vertebrates from an e-waste recycling site. Environ. Pollut. 2016, 218, 1334–1341. [Google Scholar] [CrossRef]
- Yu, L.H.; Luo, X.J.; Wu, J.P.; Song, J.; Sun, Q.H.; Zhang, X.L.; Chen, D.; Mai, B.X. Biomagnification of higher brominated PBDE congeners in an urban terrestrial food web in North China based on field observation of prey deliveries. Environ. Sci. Technol. 2011, 45, 5125–5131. [Google Scholar] [CrossRef]
- Voorspoels, S.; Covaci, A.; Lepom, P.; Jaspers, V.L.B.; Schepens, P. Levels and distribution of polybrominated diphenyl ethers in various tissues of birds of prey. Environ. Pollut. 2006, 144, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Staskal, D.F.; Dilibertom, J.J.; Devito, M.J.; Birnbaum, L.S. Toxicokinetics of BDE 47 in female mice, effect of dose, route of exposure, and time. Toxicol. Sci. 2005, 2, 215–223. [Google Scholar] [CrossRef]
- Sanders, J.M.; Lebetkin, E.H.; Chen, L.J.; Burka, L.T. Disposition of 2,2′,4,4′,5,5′-hexabromodiphenyl ether (BDE-153) and its interaction with other PBDEs in rodents. Xenobiotica 2006, 36, 824–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, K.; Lin, K.; Guo, J.; Zhou, X.; Wang, J.; Zhou, P.; Xu, F.; Liu, L.; Zhang, W.; Zhao, J. Polybrominated diphenyl ethers in birds from Chongming Island, Yangtze estuary, China, insight into migratory behavior. Chemosphere 2013, 91, 1416–1425. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.Q.; Ma, Q.M.; Huang, C.; He, F.; Yao, P. Preparation, characterization, and in vivo evaluation of doxorubicin loaded BSA nanoparticles with folic acid modified dextran surface. Int. J. Pharmaceut. 2013, 444, 77–84. [Google Scholar] [CrossRef]
- Furge, L.L.; Guengerich, F.P. Cytochrome P450 enzymes in drug metabolism and chemical toxicology, An introduction. Biochem. Mol. Biol. Edu. 2010, 34, 66–74. [Google Scholar] [CrossRef]
- Wilkens, M.R.; Maté, L.M.; Schnepel, N. Influence of 25-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 on expression of P-glycoprotein and cytochrome P450 3A in sheep. J. Steroid Biochem. Mol. Biol. 2016, 164, 271–276. [Google Scholar] [CrossRef]
- Stuchlikova, L.; Matouskova, P.; Bartikova, H. Monepantel induces hepatic cytochromes p450 in sheep in vitro and in vivo. Chem.-Biol. Interac. 2015, 227, 63–68. [Google Scholar] [CrossRef]
- Zhu, M.Q.; Pang, X.H.; Wan, J.; Xu, X.P.; Wei, X.Y.; Hua, R.M.; Zhang, X.Y.; Wang, Y.; Yang, X.F. Potential toxic effects of sulfonamides antibiotics: Molecular modeling, multiple-spectroscopy techniques and density functional theory calculations. Ecotox. Environ. Safe. 2022, 243, 113979. [Google Scholar] [CrossRef]
- Zhu, M.Q.; Xu, Y.M.; Sang, L.F.; Zhao, Z.Y.; Wang, L.J.; Wu, X.Q.; Fan, F.G.; Wang, Y.; Li, H. An ICT-based fluorescent probe with a large Stokes shift for measuring hydrazine in biological and water samples, Environ. Pollu. 2020, 256, 113427. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.Q.; Tang, J.; Shi, T.Z.; Ma, X.; Wang, Y.; Wu, X.W.; Li, H.; Hua, R.M. Uptake, translocation and metabolism of imidacloprid loaded within fluorescent mesoporous silica nanoparticles in tomato (Solanum lycopersicum). Ecotox. Environ. Saf. 2022, 232, 113243. [Google Scholar] [CrossRef]
- Zhu, M.Q.; Zhao, Z.Y.; Liu, X.N.; Chen, P.P.; Fan, F.G.; Wu, X.W.; Hua, R.M.; Wang, Y. A novel near-infrared fluorimetric method for point-of-care monitoring of Fe2+ and its application in bioimaging. J. Hazard. Mater. 2020, 406, 124767. [Google Scholar] [CrossRef]
- Zhu, M.Q.; Zhao, Z.Y.; Huang, Y.; Fan, F.G.; Wang, F.; Li, W.L.; Wu, X.W.; Hua, R.M.; Wang, Y. Hydrazine exposure: A near-infrared ICT-based fluorescent probe and its application in bioimaging and sewage analysis. Sci. Total. Environ. 2021, 759, 143102. [Google Scholar] [CrossRef]
- Zhu, M.Q.; Ou, X.; Tang, J.; Shi, T.Z.; Ma, X.; Wang, Y.; Wu, X.W.; Li, Q.X.; Hua, R.M. Uptake, distribution and translocation of imidacloprid-loaded fluorescence double hollow shell mesoporous silica nanoparticles and metabolism of imidacloprid in pakchoi. Sci. Total. Environ. 2021, 787, 147578. [Google Scholar] [CrossRef]
- Tayyab, S.; Sam, S.E.; Kabir, M.Z. Molecular interaction study of an anticancer drug, ponatinib with human serum albumin using spectroscopic and molecular docking methods. Spectrochim. Acta A 2019, 214, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Mohammadzadeh, A.H.; Akbari, N.; Esazadeh, K.; Dolatabadi, J.E.N. Molecular and technical aspects on the interaction of serum albumin with multifunctional food preservatives. Food Chem. 2019, 293, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Faletrov, Y.V.; Gilep, K.A.; Falchevskaya, A.S.; Shkumatov, V.M. In silico modeling of isoniazid-steroid conjugates interactions with mycobacterial Cytochromes P450 and their bioconversion in vitro by the cells. Biochem. Mosc. Suppl. Ser. B Biomed. Chem. 2021, 15, 111–118. [Google Scholar] [CrossRef]
- Shang, Y.; Hua, L. Studies of the interaction between hesperidin and its aglycone hesperetin with bovine serum albumin by spectroscopic methods. Asian J. Chem. 2011, 23, 3033–3039. [Google Scholar]
- Tan, S.W.; Chi, Z.X.; Shan, Y.; Wen, Z.Z.; Li, W.G. Interaction studies of polybrominated diphenyl ethers (PBDEs) with human serum albumin (HSA): Molecular docking investigations. Environ. Toxicol. Phar. 2017, 54, 34–39. [Google Scholar] [CrossRef]
- Cao, Y.M.; Xu, L.; Jia, L.Y. Analysis of PCBs degradation abilities of biphenyl dioxygenase derived from Enterobacter sp. LY402 by molecular simulation. New Biotechnol. 2011, 29, 90–98. [Google Scholar]
- Chen, W.M.; Liu, C.; Wei, B.K.; Bao, J.S.; Wang, Y.; Hu, J.C.; Jin, J.; Zeng, F.G. Uptake and translocation of polybrominated diphenyl ethers in the rhizosphere soil–crop–atmosphere system in e-waste dismantling areas in Taizhou, China. Chemosphere 2021, 280, 130586. [Google Scholar] [CrossRef]
- Bu, T.; Chen, W.M.; Li, T.W.; Liu, Y.M.; Wang, H.T.; Zhao, P.Y.; Hu, J.C.; Jin, J. Distributions and biomagnification of polybrominated diphenyl ethers in a grassland ecosystem food chain. Sci. Total Environ. 2020, 747, 141141. [Google Scholar]
- Bi, S.Y.; Song, D.Q.; Yuan, T.; Zhou, X.; Liu, Z.Y.; Zhang, H.Q. Molecular spectroscopic study on the interaction of tetracyclines with serum albumins. Spectrochim. Acta A 2005, 61, 629–636. [Google Scholar] [CrossRef]
- Zheng, X.B. Organohalogenated Pollutants in Bioaccumulation, Maternal Transfer, and Embryo Development Processes of Chicken and In Vitro Metabolism. Ph.D. Thesis, University of Chinese Academy of Sciences, Beijing, China, 2015. (In Chinese). [Google Scholar]
- Matthews, H.B.; Dedrick, R.L. Pharmacokinetics of PCBs. Annu. Rev. Pharmacol. 1984, 24, 85–103. [Google Scholar] [CrossRef]
- Nomiyama, K.; Takaguchi, K.; Mizukawa, H.; Nagona, Y.; Oshihoi, T.; Nakatsu, S.; Kunisue, T.; Tanabe, S. Species- and tissue-specific profiles of polybrominated diphenyl ethers and their hydroxylated and methoxylated derivatives in cats and dogs. Environ. Sci. Technol. 2017, 51, 5811–5819. [Google Scholar] [CrossRef]
- Li, Y.F.; Yang, Z.Z.; Wang, C.H.; Yang, Z.J.; Qin, Z.F.; Fu, S. Tissue distribution of polybrominated diphenyl ethers (PBDEs) in captive domestic pigs, sus scrofa, from a village near an electronic waste recycling site in South China. Bull. Environ. Contam. Toxicol. 2010, 84, 208–211. [Google Scholar] [CrossRef] [Green Version]
- Gerecke, A.C.; Hartmann, P.C.; Heeb, N.V.; Kohler, H.P.E.; Giger, W.; Schmid, P.; Zennegg, M.; Kohler, M. Anaerobic degradation of decabromodiphenyl ether. Environ. Sci. Technol. 2005, 39, 1078. [Google Scholar] [CrossRef]
- Jiang, C.L.; Qian, H.; Luo, S.H.; Lin, J.; Yu, J.; Li, Y.J.; An, Q.; Luo, N.F.; Du, L. Vasopressors induce passive pulmonary hypertension by blood redistribution from systemic to pulmonary circulation. Basic Res. Cardiol. 2017, 112, 21–30. [Google Scholar] [CrossRef]
- Shi, J.R. Application of diagram method to resolve “the way of material transport”. Biol. Teach. 2010, 35, 16–18. (In Chinese) [Google Scholar]
- Suresh, K.; Shimoda, L.A. Lung Circulation. Compr. Physiol. 2016, 6, 897–943. [Google Scholar]
- Letcher, R.J.; Marteinson, S.C.; Fernie, K.J. Dietary exposure of American kestrels (Falco sparverius) to decabromodiphenyl ether (BDE-209) flame retardant, Uptake, distribution, debromination and cytochrome P450 enzyme induction. Environ. Int. 2014, 63, 182–190. [Google Scholar] [CrossRef]
- Zheng, X.B.; Luo, X.J.; Zheng, J.; Zeng, Y.H.; Mai, B.X. Contaminant sources, gastrointestinal absorption, and tissue distribution of organohalogenated pollutants in chicken from an e-waste site. Sci. Total Environ. 2015, 505, 1003–1010. [Google Scholar] [CrossRef]
- Gebbink, W.A.; Sonne, C.; Dietz, R.; Kirkegaard, M.; Riget, F.F.; Born, E.W.; Muir, D.C.G.; Letcher, R.J. Tissue-specific congener composition of organohalogen and metabolite contaminants in East Greenland polar bears (Ursus maritimus). Environ. Pollut. 2008, 152, 621–629. [Google Scholar] [CrossRef]
- Sahoo, S.; Kariya, T.; Ishikawa, K. Targeted delivery of therapeutic agents to the heart. Nat. Rev. Cardiol. 2021, 18, 389–399. [Google Scholar] [CrossRef]
- Sissung, T.M.; Gardner, E.R.; Piekarz, R.L.; Howden, R.; Che, X.; Woo, S.; Franke, R.; Clark, J.A.; Miller-Degraff, L.; Steinberg, S.M. Impact of ABCB1 allelic variants on QTc interval prolongation. Clin. Cancer Res. 2011, 17, 937–946. [Google Scholar] [CrossRef]
- Liu, P.Y.; Chen, X.R.; Zhao, Y.X.; Li, Y.Y.; Qin, X.F.; Qin, Z.F. Changes of polybrominated diphenyl ether concentrations in ducks with background exposure level and time. Chemosphere 2015, 118, 253–260. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Y.; Luo, C.; Yin, H.; Zhang, G. Plant selective uptake of halogenated flame retardants at an e-waste recycling site in southern China. Environ. Pollut. 2016, 214, 705–712. [Google Scholar] [CrossRef]
- Yang, Z.Z.; Li, Y.F.; Fu, S.; Zhao, X.R. Special distribution of polybrominated diphenyl ethers in brain tissues of free-range domestic hens and ducks from a village near an electronic waste recycling site in South China. Bull. Environ. Contam. Toxicol. 2011, 86, 283–288. [Google Scholar] [CrossRef]
- Yu, Y.X.; Wang, M.M.; Zhang, K.Q.; Yang, D.; Zhong, Y.F.; An, J.; Lei, B.L.; Zhang, X.Y. The transepithelial transport mechanism of polybrominated diphenyl ethers in human intestine determined using a Caco-2 cell monolayer. Environ. Res. 2017, 154, 93–100. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; Yang, X.; Bao, J.; Lin, Z.; Li, T.; Wang, Y.; Zhang, A.; Hu, J.; Jin, J. A Pilot Study on the Concentration, Distribution and Bioaccumulation of Polybrominated Diphenyl Ethers (PBDEs) in Tissues and Organs of Grassland Sheep. Int. J. Environ. Res. Public Health 2022, 19, 12170. https://doi.org/10.3390/ijerph191912170
Chen W, Yang X, Bao J, Lin Z, Li T, Wang Y, Zhang A, Hu J, Jin J. A Pilot Study on the Concentration, Distribution and Bioaccumulation of Polybrominated Diphenyl Ethers (PBDEs) in Tissues and Organs of Grassland Sheep. International Journal of Environmental Research and Public Health. 2022; 19(19):12170. https://doi.org/10.3390/ijerph191912170
Chicago/Turabian StyleChen, Wenming, Xinrui Yang, Junsong Bao, Ziyi Lin, Tianwei Li, Ying Wang, Aiqin Zhang, Jicheng Hu, and Jun Jin. 2022. "A Pilot Study on the Concentration, Distribution and Bioaccumulation of Polybrominated Diphenyl Ethers (PBDEs) in Tissues and Organs of Grassland Sheep" International Journal of Environmental Research and Public Health 19, no. 19: 12170. https://doi.org/10.3390/ijerph191912170
APA StyleChen, W., Yang, X., Bao, J., Lin, Z., Li, T., Wang, Y., Zhang, A., Hu, J., & Jin, J. (2022). A Pilot Study on the Concentration, Distribution and Bioaccumulation of Polybrominated Diphenyl Ethers (PBDEs) in Tissues and Organs of Grassland Sheep. International Journal of Environmental Research and Public Health, 19(19), 12170. https://doi.org/10.3390/ijerph191912170






