Systematic Review of Survival Analysis in Leprosy Studies—Including the Following Outcomes: Relapse, Impairment of Nerve Function, Reactions and Physical Disability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Registration Protocol
2.2. Data Sources and Research Strategy
2.3. Study Selection and Data Extraction
2.4. Risk of Bias and Quality Assessment
3. Results
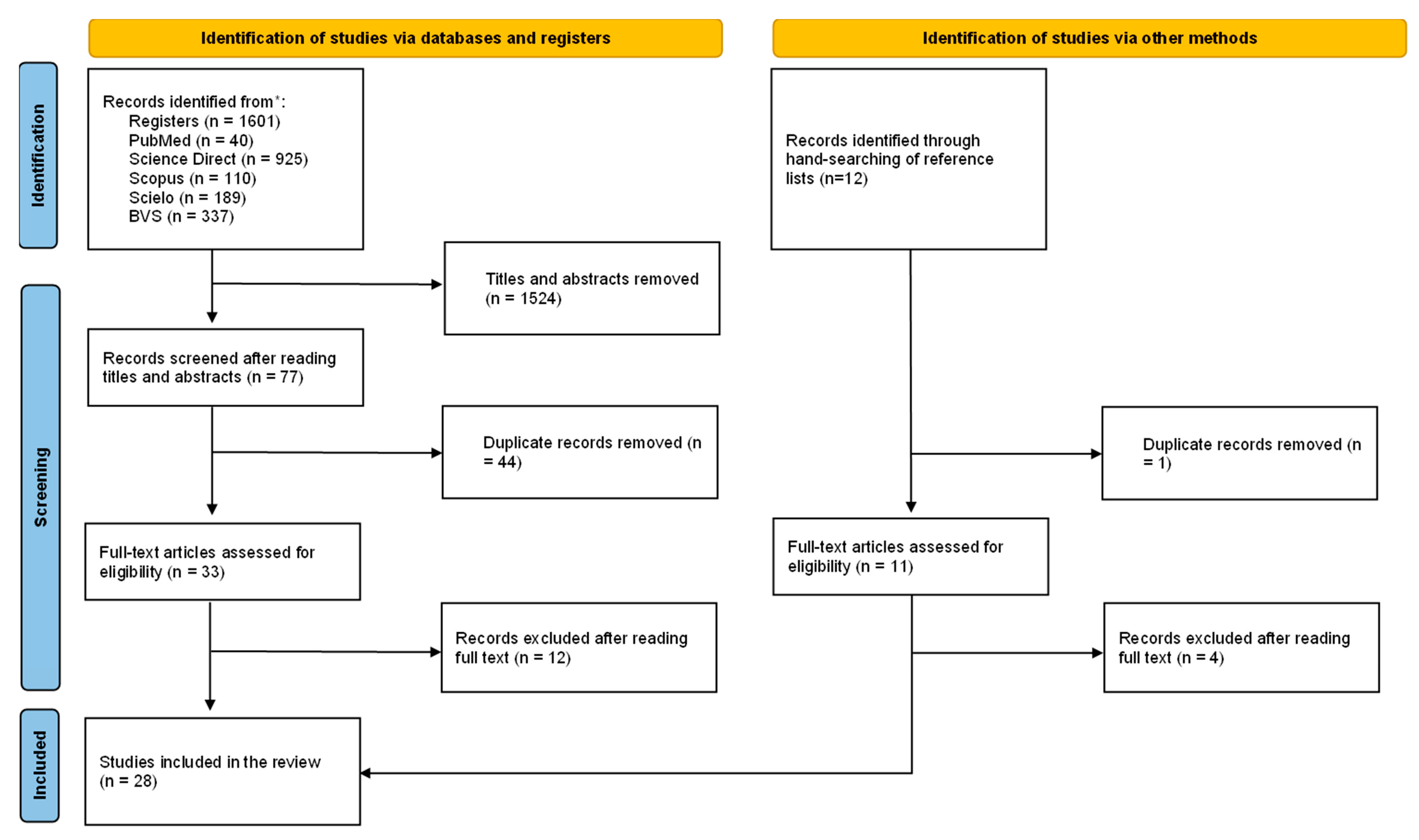
3.1. Flow Diagram of Included Studies
3.2. Study Description
3.2.1. Leprosy
3.2.2. Relapse
3.2.3. Clinical Manifestations before, during and after Treatment
3.3. Quality Assessment Criteria
4. Discussion
4.1. Leprosy
4.2. Relapse
4.3. Clinical Manifestations before, during and after Treatment
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leonardo, L.; Hernandez, L.; Magturo, T.C.; Palasi, W.; Rubite, J.M.; Cadiz, A.; Moendeg, K.; Fornillos, R.; Tabios, I.K.; Mistica, M.; et al. Current status of neglected tropical diseases (NTDs) in the Philippines. Acta Trop. 2020, 203, 105284. [Google Scholar] [CrossRef] [PubMed]
- Qian, M.B.; Chen, J.; Bergquist, R.; Li, Z.J.; Li, S.Z.; Xiao, N.; Utzinger, J.; Zhou, X.N. Neglected tropical diseases in the People’s Republic of China: Progress towards elimination. Infect. Dis. Poverty 2019, 8, 6–21. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M. Leprosy-an overview of clinical features, diagnosis, and treatment. J. Dtsch. Dermatol. Ges. 2017, 15, 801–827. [Google Scholar] [CrossRef]
- van Wijk, R.; van Selm, L.; Barbosa, M.C.; van Brakel, W.H.; Waltz, M.; Puchner, K.P. Psychosocial burden of neglected tropicaldiseases in eastern Colombia: An explorativequalitative study in persons affected by leprosy, cutaneous leishmaniasis and Chagas disease. Glob. Ment. Health 2021, 8, e21. [Google Scholar] [CrossRef]
- Vieira, C.; Lobato, M.; Figueira, M.; Amaral, M.; Vilel, M.; Silva, E. Life after Leprosy Treatment Discharge: Physical and Social limitations. Indian J. Lepr. 2018, 90, 177–188. [Google Scholar]
- World Health Organization. Available online: https://apps.who.int/neglected_diseases/ntddata/leprosy/leprosy.html (accessed on 10 May 2022).
- Santos, A.R.; Silva, P.R.D.S.; Steinmann, P.; Ignotti, E. Disability progression among leprosy patients released from treatment: A survival analysis. Infect. Dis. Poverty 2020, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Hacker, M.A.; Sales, A.M.; Duppre, N.C.; Sarno, E.N.; Moraes, M.O. Leprosy incidence and risk estimates in a 33-year contact cohort of leprosy patients. Sci. Rep. 2021, 11, 1947. [Google Scholar] [CrossRef]
- Dey, T.; Mukherjee, A.; Chakraborty, S. A Practical Overview and Reporting Strategies for Statistical Analysis of Survival Studies. Chest 2020, 158, S39–S48. [Google Scholar] [CrossRef]
- Koletsi, D.; Pandis, N. Survival analysis, part 3: Cox regression. Am. J. Orthod. Dentofacial. Orthop. 2017, 152, 722–723. [Google Scholar] [CrossRef]
- Pepito, V.C.F.; Amit, A.M.L.; Samontina, R.E.D.; Abdon, S.J.A.; Fuentes, D.N.L.; Saniel, O.P. Patterns and determinants of treatment completion and default among newly diagnosed multibacillary leprosy patients: A retrospective cohort study. Heliyon 2021, 7, e07279. [Google Scholar] [CrossRef]
- Glynn, J.R.; Dube, A.; Fielding, K.; Crampin, A.C.; Kanjala, C.; Fine, P.E.M.; Karonga Prevention Trial Group. The effect of BCG revaccination on all-cause mortality beyond infancy: 30-year follow-up of a population-based, double-blind, randomised placebo-controlled trial in Malawi. Lancet Infect Dis. 2021, 21, 1590–1597. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- PROSPERO, International Prospective Register of Systematic Reviews. Available online: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022296026 (accessed on 10 May 2022).
- Kmet, L.M.; Cook, L.S.; Lee, R.C. Standard Quality Assessment Criteria for Evaluating Primary Research Papers from a Variety of Fields; Alberta Heritage Foundation for Medical Research: Edmonton, AB, Canada, 2004; pp. 1–22. [Google Scholar]
- Pattyn, S.R.; Ghyst, P.; Janssenst, L.; Tshilumbat, K.; Kuykenst, L.; Karibushi, N. A randomized clinical trial of two single-dose treatments fo r paucibacillary leprosy. Lepr. Rev. 1994, 65, 45–57. [Google Scholar] [PubMed]
- Croft, R.P.; Nicholls, P.G.; Richardus, J.H.; Cairns, W.; Smith, S. Incidence rates of acute nerve function impairment in leprosy: A prospective cohort analysis after 24 months (The Bangladesh Acute Nerve Damage Study). Lepr. Rev. 2000, 71, 8–33. [Google Scholar] [CrossRef]
- Croft, R.P.; Nicholls, P.G.; Steyerberg, E.W.; Richardus, J.H.; Smith, W.C.S. A clinical prediction rule for nerve-function impairment in leprosy patients. Lancet 2000, 355, 1603–1606. [Google Scholar] [CrossRef]
- Girdhar, B.K.; Girdhar, A.; Kumar, A. Relapses in multibacillary leprosy patients: Effect of length of therapy. Lepr. Rev. 2000, 71, 144–153. [Google Scholar] [CrossRef]
- Cellona, R.V.; Balagon, M.F.V.; Cruz, E.C.; Burgos, J.A.; Abalos, R.M.; Walsh, G.P.; Topolski, R.; Gelber, R.H.; Walsh, D.S. Long-term efficacy of 2 year WHO multiple drug therapy (MDT) in multibacillary (MB) leprosy patients. Int. J. Lepr. Other Mycobact. Dis. 2003, 71, 308–319. [Google Scholar] [CrossRef]
- Richardus, J.H.; Nicholls, P.G.; Croft, R.P.; Withington, S.G.; Smith, W.C.S. Incidence of acute nerve function impairment and reactions in leprosy: A prospective cohort analysis after 5 years of follow-up. Int. J. Epidemiol. 2004, 33, 337–343. [Google Scholar] [CrossRef]
- Smith, W.C.S.; Anderson, A.M.; Withington, S.G.; van Brakel, W.H.; Croft, R.P.; Nicholls, P.G.; Richardus, J.H. Steroid prophylaxis for prevention of nerve function impairment in leprosy: Randomised placebo controlled trial (TRIPOD 1). BMJ 2004, 328, 1459. [Google Scholar] [CrossRef]
- Bakker, M.I.; Hatta, M.; Kwenang, A.; van Benthem, B.H.B.; van Beers, S.M.; Klatser, P.R.; Oskam, L. Prevention of leprosy using rifampicin as chemoprophylaxis. Am. J. Trop. Med. Hyg. 2005, 72, 443–448. [Google Scholar] [CrossRef]
- Bakker, M.I.; Hatta, M.; Kwenang, A.; van Mosseveld, P.; Faber, W.R.; Klatser, P.R.; Oskam, L. Risk factors for developing leprosy—A population-based cohort study in Indonesia. Lepr. Rev. 2006, 77, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Girdhar, A.; Girdhar, B.K. Incidence of leprosy in Agra District. Lepr. Rev. 2007, 78, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.K.; Stefani, M.; Sousa, A.L.O.M.; Rabello, P.F.B.; Pennini, S.; Narahashi, K.; Ueda, E.; Daxbacher, E.R.; Aslanian, F.M.N.P.; Sales, A.M.; et al. Single lesion leprosy pacients multicentric cohort treated with single dose drug therapy: Findings on three year follow up and public health perspective in Brazil. Cad Saude Colet 2008, 16, 363–376. [Google Scholar]
- Gonçalves, S.D.; Sampaio, R.F.; Antunes, C.M.F. Ocorrência de neurite em pacientes com hanseníase: Análise de sobrevida e fatores preditivos. Rev. Soc. Bras. Med. Trop. 2008, 41, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Schuring, R.P.; Richardus, J.H.; Steyerberg, E.W.; Pahan, D.; Faber, W.R.; Oskam, L. Preventing nerve function impairment in leprosy: Validation and updating of a prediction rule. PLoS Negl. Trop. Dis. 2008, 2, e283. [Google Scholar] [CrossRef] [PubMed]
- Smith, W.C.S.; Nicholls, P.G.L.; Barkataki, P.; Suneetha, S.; Suneetha, L.; Jadhav, R.; Rao, P.S.S.S.; Wilder-Smith, E.P.; Lockwood, D.N.J.; van Brakel, W.H. Predicting neuropathy and reactions in leprosy at diagnosis and before incident events—Results from the INFIR cohort study. PLoS Negl. Trop. Dis. 2009, 3, e500. [Google Scholar] [CrossRef] [PubMed]
- Girdhar, A.; Kumar, A.; Girdhar, B.K. A randomised controlled trial assessing the effect of adding clarithromycin to Rifampicin, ofloxacin and minocycline in the treatment of single lesion paucibacillary leprosy in Agra District, India. Lepr. Rev. 2011, 82, 46–54. [Google Scholar] [CrossRef]
- Guerrero-Guerrero, M.I.; Muvdi-Arenas, S.; León-Franco, C.I. Relapses in multibacillary leprosy patients: A retrospective cohort of 11 years in Colombia. Lepr. Rev. 2012, 83, 247–260. [Google Scholar] [CrossRef]
- Kumar, A.; Girdhar, A.; Girdhar, B.K. Risk of developing disability in pre and post-multidrug therapy treatment among multibacillary leprosy: Agra MB Cohort study. BMJ Open 2012, 2, e000361. [Google Scholar] [CrossRef]
- Penna, M.L.F.; Buhrer-Sékula, S.; Pontes, M.A.A.; Gonçalves, R.C.H.S.; Penna, G.O. Primary results of Clinical Trial for Uniform Multidrug Therapy for Leprosy Patients in Brazil (U-MDT/CT-BR): Reactions frequency in multibacillary patients. Lepr. Rev. 2012, 83, 308–319. [Google Scholar] [CrossRef]
- Sales, A.M.; Campos, D.P.; Hacker, M.A.; Nery, J.A.C.; Düppre, N.C.; Rangel, E.; Sarno, E.N.; Penna, M.L.F. Progression of leprosy disability after discharge: Is multidrug therapy enough? Trop. Med. Int. Health. 2013, 18, 1145–1153. [Google Scholar] [CrossRef] [PubMed]
- Penna, G.O.; Bührer-Sékula, S.; Kerr, L.R.S.; Stefani, M.M.A.; Rodrigues, L.C.; Araújo, M.G.; Ramos, A.M.C.; Andrade, A.R.C.; Costa, M.B.; Rosa, P.S.; et al. Uniform multidrug therapy for leprosy patients in Brazil (U-MDT/CT-BR): Results of an open label, randomized and controlled clinical trial, among multibacillary patients. PLoS Negl. Trop. Dis. 2017, 11, e0005725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes, R.R.; Antunes, D.E.; Santos, D.F.; Sabino, E.F.P.; Oliveira, D.B.; Goulart, I.M.B. BCG vaccine and leprosy household contacts: Protective effect and probability to becoming sick during follow-up. Vaccine 2019, 37, 6510–6517. [Google Scholar] [CrossRef] [PubMed]
- Manta, F.S.N.; Barbieri, R.R.; Moreira, S.J.M.; Santos, P.T.S.; Nery, J.A.C.; Duppre, N.C.; Sales, A.M.; Pacheco, A.G.; Hacker, M.A.; Machado, A.M.; et al. Quantitative PCR for leprosy diagnosis and monitoring in household contacts: A follow-up study, 2011–2018. Sci. Rep. 2019, 9, 16675. [Google Scholar] [CrossRef]
- Coriolano, C.R.F.; Freitas Neto, W.A.; Penna, G.O.; Sanchez, M.N. Factors associated with timing of lepra reactions in a cohort from 2008 to 2016 in Rondônia, Amazon Region, Brazil. Cad/Saude Publica 2021, 37, e00045321. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, P.; Purushothaman, G.K.C.; Ponnaiah, M.; Shanmugasundaram, D.; Padma, J.; Meena, R.L.; Vadivoo, S.; Mehendale, S.M. Low risk of relapse and deformity among leprosy patients who completed multi-drug therapy regimen from 2005 to 2010: A cohort study from four districts in south india. PLoS Negl. Trop. Dis. 2021, 15, e0009950. [Google Scholar] [CrossRef]
- Nascimento, A.C.M.; Santos, D.F.; Antunes, D.E.; Gonçalves, M.A.; Santana, M.A.O.; Dornelas, B.C.; Goulart, L.R.; Goulart, I.M.B. Leprosy Relapse: A Retrospective Study on Epidemiologic, Clinical, and Therapeutic Aspects at a Brazilian Referral Center. Int. J. Infect. Dis. 2022, 118, 44–51. [Google Scholar] [CrossRef]
- Sustainable Development Goal 3: Ensure Healthy Lives and Promote Well-Being for All at All Ages. Available online: https://sdgs.un.org/goals/goal3 (accessed on 10 May 2022).
- Global leprosy (Hansen’s Disease) Strategy 2021–2030. Available online: https://apps.who.int/iris/handle/10665/340774 (accessed on 10 May 2022).
- Shui, T.J.; Long, H.; Xiong, L.; Zhang, X.H.; He, J.; Chen, X. Towards the elimination of leprosy in Yunnan, China: A time-series analysis of surveillance data. PLoS Negl. Trop. Dis. 2021, 15, e0009201. [Google Scholar] [CrossRef]
- Quilter, E.E.V.; Butlin, C.R.; Singh, S.; Alam, K. Lockwood DNJ. Patients with skin smear positive leprosy in Bangladesh are the main risk factor for leprosy development: 21-year follow-up in the household contact study (COCOA). PLoS Negl. Trop. Dis. 2020, 14, e0008687. [Google Scholar] [CrossRef]
- Romanholo, H.S.B.; Souza, E.A.; Ramos Júnior, A.N.; Kaiser, A.C.G.C.B.; Silva, I.O.; Brito, A.L.; Vasconcellos, C. Surveillance of intradomiciliary contacts of leprosy cases: Perspective of the client in a hyperendemic municipality. Rev. Bras. Enferm. 2018, 71, 163–169. [Google Scholar] [CrossRef]
- Teixeira, C.S.S.; Pescarini, J.M.; Alves, F.J.O.; Nery, J.S.; Sanchez, M.N.; Teles, C.; Ichihara, M.Y.T.; Ramond, A.; Smeeth, L.; Penna, M.L.F.; et al. Incidence of and Factors Associated With Leprosy Among Household Contacts of Patients with Leprosy in Brazil. JAMA Dermatol. 2020, 156, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Chu, T.; Li, F.; Wang, Z.; Liu, D.; Chen, M.; Wang, H.; Niu, G.; Liu, D.; Zhang, M.; et al. The role of an active surveillance strategy of targeting household and neighborhood contacts related to leprosy cases released from treatment in a low-endemic area of China. PLoS Negl. Trop. Dis. 2020, 14, e0008563. [Google Scholar] [CrossRef] [PubMed]
- Richardus, J.H.; Tiwari, A.; Barth-Jaeggi, T.; Arif, M.A.; Banstola, N.L.; Baskota, R.; Blaney, D.; Blok, D.J.; Bonenberger, M.; Budiawan, T.; et al. Leprosy post-exposure prophylaxis with single-dose rifampicin (LPEP): An international feasibility programme. Lancet Glob. Health. 2021, 9, e81–e90. [Google Scholar] [CrossRef]
- Niitsuma, E.N.A.; Bueno, I.D.; Arantes, E.O.; Carvalho, A.P.M.; Xavier Junior, G.F.; Fernandes, G.R.; Lana, F.C.F. Factors associated with the development of leprosy in contacts: A systematic review and meta-analysis. Rev. Bras. Epidemiol. 2021, 24, e210039. [Google Scholar] [CrossRef] [PubMed]
- Global Leprosy Update, 2018: Moving towards a Leprosy Free World. Available online: https://apps.who.int/iris/bitstream/handle/10665/326776/WER9435-36-389-411-en-fr.pdf (accessed on 10 May 2022).
- Narang, T.; Kamat, D.; Thakur, V.; Lavania, M.; Singh, I.; Ahuja, M.; Dogra, S. Equal rates of drug resistance in leprosy cases with relapse and recurrent/chronic Type 2 reaction: Time to revise the guidelines for drug-resistance testing in leprosy? Clin. Exp. Dermatol. 2022, 47, 297–302. [Google Scholar] [CrossRef]
- Beltrame, A.; Barabino, G.; Wei, Y.; Clapasson, A.; Orza, P.; Perandin, F.; Piubelli, C.; Monteiro, G.B.; Longoni, S.S.; Rodari, P.; et al. Leprosy in Refugees and Migrants in Italy and a Literature Review of Cases Reported in Europe between 2009 and 2018. Microorganisms 2020, 8, 1113. [Google Scholar] [CrossRef]
- Sousa, P.P.; Sousa, A.L.M.; Turchi, M.D. Reviewing the therapeutic management of leprosy in primary care: Demand case series referred to a University Hospital in the Midwest region of Brazil. An. Bras. Dermatol. 2021, 96, 301–308. [Google Scholar] [CrossRef]
- Boigny, R.N.; Florêncio, C.M.G.D.; Cavalcante, K.K.S.; Moreno, J.O.; Almeida, P.J.; Almondes, J.G.S.; Nogueira, P.S.F.; Alencar, C.H. Magnitude and temporal trends of leprosy relapse in the state of Ceará, Brazil in the period 2001–2018. Rev. Soc. Bras. Med. Trop. 2021, 54, e03892020. [Google Scholar] [CrossRef]
- Chagas, D.F.; Diniz, L.M.; Lucas, E.A.; Moraes, M.O. Relapse in leprosy and drug resistance assessment in a tertiary hospital of the state of Espírito Santo, Brazil. Rev. Soc. Bras. Med. Trop. 2021, 54, e0375–e2020. [Google Scholar] [CrossRef]
- Gitte, S.V.; Nigam, C.; Chakraborty, A.B.; Kamble, K.; Soni, M.; Gahlot, R. Profile of Person Affected by Leprosy with Clinical Relapse among in High Endemic State of India. JMID 2018, 15, 103–107. [Google Scholar] [CrossRef]
- Sena, I.V.O.; Machado, R.S.; Brito, B.A.M.; Araújo, T.M.E.; Silva, G.R.F.; Andrade, E.M.L.R. Relapsed Cases of Leprosy In A Hyperendemic City In Northeast Brazil. Int. Arch. Med. 2017, 10, 1–8. [Google Scholar] [CrossRef]
- Stefani, M.M.A.; Avanzi, C.; Bührer-Sékula, S.; Benjak, A.; Loiseau, C.; Singh, P.; Pontes, M.A.A.; Gonçalves, H.S.; Hungria, E.M.; Busso, P.; et al. Whole genome sequencing distinguishes between relapse and reinfection in recurrent leprosy cases. PLoS Negl. Trop. Dis. 2017, 11, e0005598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diniz, L.M.; Moreira, M.V.; Puppin, M.A.; Oliveira, M.L.W.D.R. Estudo retrospectivo de recidiva da hanseníase no Estado do Espírito Santo. Rev. Soc. Bras. Med. Trop. 2009, 42, 420–424. [Google Scholar] [CrossRef]
- Gelber, R.; Balagon, V.; Cellona, R. The Relapse Rate in MB Leprosy Patients Treated with 2-Years of WHO-MDT is Not Low. Int. J. Lepr. Other Mycobact. Dis. 2004, 72, 493–500. [Google Scholar] [CrossRef]
- Kaimal, S.; Thappa, M. Relapse in leprosy. Indian J. Dermatol. Venereol. Leprol. 2009, 75, 126–135. [Google Scholar]
- Balagon, M.F.; Cellona, R.V.; Cruz, E.; Burgos, J.A.; Abalos, R.M.; Walsh, G.P.; Saunderson, P.R.; Walsh, D.S. Long-Term Relapse Risk of Multibacillary Leprosy after Completion of 2 Years of Multiple Drug Therapy (WHO-MDT) in Cebu, Philippines. Am. J. Trop. Med. Hyg. 2009, 81, 895–899. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, F.G.; Belone, A.D.F.F.; Rosa, P.S.; Laporta, G.Z. Underlying mechanisms of leprosy relapse in the Western Amazon: A retrospective cohort study. BMC Infect. Dis. 2019, 19, 460. [Google Scholar] [CrossRef] [PubMed]
- Lobo, D. Treatment failures with multidrug therapy. Lepr Rev. 1992, 63, 93s–98s. [Google Scholar] [CrossRef]
- Mowla, M.R.; Ara, S.; Mizanur Rahman, A.F.M.; Tripura, S.P.; Paul, S. Leprosy reactions in postelimination stage: The Bangladesh experience. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 705–711. [Google Scholar] [CrossRef]
- White, C.; Franco-Paredes, C. Leprosy in the 21st century. Clin. Microbiol. Rev. 2015, 28, 80–94. [Google Scholar] [CrossRef]
- Maymone, M.B.C.; Venkatesh, S.; Laughter, M.; Abdat, R.; Hugh, J.; Dacso, M.M.; Rao, P.N.; Stryjewska, B.M.; Dunnick, C.A.; Dellavalle, R.P. Leprosy: Treatment and management of complications. J. Am. Acad. Dermatol. 2020, 83, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Pitta, I.J.R.; Hacker, M.A.; Vital, R.T.; Andrade, L.R.; Spitz, C.N.; Sales, A.M.; Antunes, S.L.G.; Sarno, E.N.; Jardim, M.R. Leprosy Reactions and Neuropathic Pain in Pure Neural Leprosy in a Reference Center in Rio de Janeiro—Brazil. Front. Med. 2022, 9, 865485. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.A.G.; Silveira, V.M.; França, E.R. Características epidemiológicas e clínicas das reações hansênicas em indivíduos paucibacilares e multibacilares, atendidos em dois centros de referência para hanseníase, na Cidade de Recife, Estado de Pernambuco. Rev. Soc. Bras. Med. Trop. 2010, 43, 287–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nery, J.; Vieira, L.; Matos, H.; Gallo, M.; Sarno, E. Reactional states in multibacillary hansen disease patients during multidrug therapy. Rev. Inst. Med. Trop. São Paulo 1998, 40, 363–370. [Google Scholar] [CrossRef]
- Wagenaar, I.; Post, E.; Brandsma, W.; Bowers, B.; Alam, K.; Shetty, V.; Pai, V.; Husain, S.; Prakoeswa, C.R.S.; Astari, L.; et al. Effectiveness of 32 versus 20 weeks of prednisolone in leprosy patients with recent nerve function impairment: A randomized controlled trial. PLoS Negl. Trop. Dis. 2017, 11, e0005952. [Google Scholar] [CrossRef]
- Silva, S.F.; Griep, R.H. Reação hansênica em pacientes portadores de hanseníase em centros de saúde da área de planejamento 3.2 do município do Rio de Janeiro. Hansen Int. 2007, 32, 155–162. [Google Scholar]
- Rodrigues, A.L.P.; Almeida, A.P.; Rodrigues, B.F.; Pinheiro, C.A.; Borges, D.S.; Mendonça, M.L.H.; Silva, V.E.F.; Goulartet, I.M.R. Ocorrência de reações em pacientes pós-alta por cura de hanseníase: Subsídios para implementação de um programa de atenção específica. Hansen Int. 2000, 25, 7–16. [Google Scholar]
- Croft, R.P.; Nicholls, P.G.; Steyerberg, E.W.; Richardus, J.H.; Withington, S.G.; Smith, W.C.S. A clinical prediction rule for nerve function impairment in leprosy patients—revisited after 5 years of follow-up. Lepr. Rev. 2003, 74, 35–41. [Google Scholar] [CrossRef]
- Ramos, J.M.H.; Souto, F.J.D. Incapacidade pós-tratamento em pacientes hansenianos em Várzea Grande, Estado de Mato Grosso. Rev. Soc. Bras. Med. Trop. 2010, 43, 293–297. [Google Scholar] [CrossRef]
- Pradeep, N.; Mathew, R. Grade 2 disability in leprosy: Scenario in the post-elimination phase of leprosy from a tertiary care center. Indian J. Lepr. 2017, 89, 127–137. [Google Scholar]
- Chukwu, J.N.; Ekeke, N.; Nwafor, C.C.; Meka, A.O.; Alphonsus, C.; Mbah, O.K.; Eze, C.C.; Ukwaja, K.N. Worsening of the disability grade during leprosy treatment: Prevalence and its determinants in Southern Nigeria. Trans. R Soc. Trop. Med. Hyg. 2018, 112, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Haefner, K.; Walther, F.; Chichava, O.A.; Ariza, L.; Alencar, C.H.; Alencar, M.D.J.F.; Ramos Jr, A.N.; Richter, J.; Heukelbach, J. High occurrence of disabilities caused by leprosy: Census from a hyperendemic area in Brazil’s savannah region. Lepr. Rev. 2017, 88, 520–532. [Google Scholar] [CrossRef]
- Rodrigues, N.C.; Castro, L.E.; Silva, J.G.; Fontana, A.P.; Neto, B.C.; William, S.V.; Gomes, M.K. Physical disability and its social and functional repercussions in patients with leprosy after discharge from multidrug therapy. Lepr. Rev. 2017, 88, 85–94. [Google Scholar] [CrossRef]
- Raposo, M.T.; Reis, M.C.; Caminha, A.V.Q.; Heukelbach, J.; Parker, L.A.; Pastor-Valero, M.; Nemes, M.I.B. Grade 2 disabilities in leprosy patients from Brazil: Need for follow-up after completion of multidrug therapy. PLoS Negl. Trop. Dis. 2018, 12, e0006645. [Google Scholar] [CrossRef] [PubMed]
- Indrayan, A.; Tripathi, C.B. Survival Analysis: Where, Why, What and How? Indian Pediatr. 2021, 59, 74–79. [Google Scholar] [CrossRef]
- Espinosa, O.A.; Ferreira, S.M.B.; Palacio, F.G.L.; Cortela, D.D.C.B.; Ignotti, E. Accuracy of Enzyme-Linked Immunosorbent Assays (elisas) in Detecting Antibodies against Mycobacterium leprae in Leprosy Patients: A Systematic Review and Meta-Analysis. Can. J. Infect. Dis. Med. Microbiol. 2018, 2018, 9828023. [Google Scholar] [CrossRef] [Green Version]
- Romero, C.P.; Castro, R.; Brasil, P.E.A.; Pereira, D.R.; Pinheiro, R.O.; Toscano, C.M.; Oliveira, M.R.F. Accuracy of rapid point-of-care serological tests for leprosy diagnosis: A systematic review and meta-analysis. Mem. Inst. Oswaldo Cruz. 2022, 8, e220317. [Google Scholar] [CrossRef]
| Study/Year of Publication | Study Design | Country | Follow Up Duration (Years) | Study Population (Total) | Main Outcome (Event) | Event/Total | Mean Follow-Up (Years) | Incidence Density/100 Person-Years | Survival Curve | Survival Curve Comparison Method | Regression Model | Measures of Association/Effect |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pattyn et al. (1994) a [16] | Clinical trial | The Republic of Zaire (now the Republic of Congo) | 4 | Leprosy patients/paucibacillary | Cure/Relapse/Grade 2 disability | Cure: C2: 214/317; C4: 206/305, Relapse: C2: 9/317; C4: 6/305, Grade 2 disability: C2: 175/317; C4: 157/305 | NA | Relapse: C2: 3.3; C4: 1.9 | Kaplan–Meier method | log-rank test | NA | Relative Risk |
| Croft, Nicholls, Richardus et al. (2000) [17] | Cohort | Bangladesh | 2 | Leprosy patients | Nerve Function Impairment | 166/2510 | NA | 3.7 | NA | log-rank test | Cox’s proportional hazards regression | Hazard Ratio |
| Croft, Nicholls, Steyerberg et al. (2000) [18] | Cohort/prospective | Bangladesh | 2 | Leprosy patients | Nerve Function Impairment | 166/2510 | NA | NA | Kaplan–Meier method | NA | Cox’s proportional hazards regression | Hazard Ratio |
| Girdhar et al. (2000) b [19] | NA | India | FDT: 3, TNS: 4 | Leprosy patients/multibacillary | Relapse | FDT: 20/260, TSN: 12/301 | NA | FDT: 2.04, TSN: 1.11 | Kaplan–Meier method | NA | NA | NA |
| Cellona et al. (2003) [20] | Cohort/prospective | The Philippines | 16 | Leprosy patients/multibacillary/cured/after the use of multidrug therapy | Relapse | 15/500 | 10.8 | 0.28 | Kaplan–Meier method | NA | NA | NA |
| Richardus et al. (2004) [21] | Cohort/prospective | Bangladesh | 5 | Leprosy patients | Nerve Function Impairment | Paucibacillary: 54/2153, Multibacillary: 121/357 | NA | Paucibacillary: 0.85, Multibacillary: 16.1 | NA | log-rank test | NA | NA |
| Smith et al. (2004) c [22] | Clinical trial | Bangladesh and Nepal | 3 | Leprosy patients/multibacillary | Nerve Function Impairment | 153/636 | NA | NA | Kaplan–Meier method | NA | NA | Relative Risk |
| Bakker et al. (2005) d [23] | Clinical trial | Indonesia | 4 | Leprosy contacts/household/neighbor | Leprosy | Control group: 11/1252, Contact group: 15/1633, Blanket group: 3/1080 | NA | NA | Kaplan–Meier method | log-rank test | Cox’s proportional hazards regression | Hazard Ratio |
| Bakker et al. (2006) e [24] | Cohort | Indonesia | 4 | Leprosy contacts/household/neighbor | Leprosy | 44/4903 | NA | 0.298 | NA | NA | Cox’s proportional hazards regression | Hazard Ratio |
| Kumar et al. (2007) f [25] | NA | India | 4 | Leprosy contacts/household/neighbor | Leprosy | 77/42,113, non-familial contacts: 56/41.119, familial contacts: 21/994 | NA | 0.062, non-familial contacts: 0.046, familial contacts: 0.676 | Kaplan–Meier method | log-rank test | NA | NA |
| Gomes et al. (2008) g [26] | Cohort/prospective | Brazil | 3 | Leprosy patients/paucibacillary | Leprosy reactions/Neuritis/Onset and increase of new wounds/Change of operational classification. | 46/259 | NA | 0.069/100 person-months | Kaplan–Meier method | log-rank test | NA | NA |
| Gonçalves et al. (2008) [27] | Cohort/Retrospective | Brazil | 11 | Leprosy patients | Neuritis | 281/529 | NA | NA | Kaplan–Meier method | log-rank test | Cox’s proportional hazards regression | Hazard Ratio |
| Schuring et al. (2008) h [28] | Cohort/prospective | Bangladesh | 4 | Leprosy patients | Nerve Function Impairment | 115/864 | NA | NA | Kaplan–Meier method | NA | Cox’s proportional hazards regression | Hazard Ratio |
| Smith et al. (2009) [29] | Cohort | India | 2 | Leprosy patients/multibacillary | Nerve Function Impairment/Leprosy reactions | 74/188 | NA | NA | Kaplan–Meier method | NA | Cox’s proportional hazards regression | Hazard Ratio |
| Girdhar et al. (2011) i [30] | Clinical trial | India | 5 | Leprosy patients/paucibacillary | Relapse | 9/300, ROM: 05/151, C-ROM: 04/149 | ROM: 1.6 C-ROM: 1.7 | ROM: 1.05, C-ROM: 0.90 | Kaplan–Meier method | log-rank test | NA | NA |
| Guerrero-Guerrero et al. (2012) [31] | Cohort/Retrospective | Colombia | 11 | Leprosy patients/multibacillary | Relapse | 33/243 | NA | 6.74 | Kaplan–Meier method | log-rank test | Cox’s proportional hazards regression | Hazard Ratio |
| Kumar et al. (2012) [32] | Cohort/prospective | India | 6 | Leprosy patients/multibacillary | Physical disability pre-multidrug therapy e post-multidrug therapy | 24/205 | 4.28 | 2.74 | NA | log-rank test | NA | Odds Ratio |
| Penna et al. (2012) j [33] | Clinical trial | Brazil | 6 | Leprosy patients/multibacillary | Nerve Function Impairment/Leprosy reactions | U-MTD: 120/306, R-MDT: 90/272 | First leprosy reactions: 5.2 | NA | Kaplan–Meier method | log-rank test | Multivariable Poisson regression | Relative Risk |
| Sales et al. (2013) [34] | Cohort | Brazil | 14 | Leprosy patients/multibacillary | Physical disability post-multidrug therapy | 103/368 | 4.3 | 6.5 | Kaplan–Meier method | NA | Cox’s proportional hazards regression | Hazard Ratio |
| Penna et al. (2017) j [35] | Clinical trial | Brazil | 8 | Leprosy patients/multibacillary | Leprosy reactions/Physical disability/Bacilloscopic index (≥4 and <4)/Relapse | U-MTD: NA/323, R-MDT: NA/290 | U-MTD: 4.86, R-MDT: 4.77 | NA | Kaplan–Meier method | log-rank test | Negative binomial regression | NA |
| Gomes et al. (2019) [36] | Cohort/Retrospective | Brazil | 16 | Leprosy contacts/household | Leprosy | 92/5061 | without BCG vaccine scar: 1.91, one BCG vaccine scar: 1.97, two BCG vaccine scars: 3.00 | NA | Kaplan–Meier method | log-rank, Breslow, and Tarone–Ware tests | NA | Relative Risk and Hazard Ratio |
| Manta et al. (2019) [37] | Cohort | Brazil | 7 | Leprosy contacts/household | Leprosy | 69/2437 | NA | NA | Kaplan–Meier method | log-rank test | NA | Hazard Ratio |
| Santos et al. (2020) k [7] | Cohort/Retrospective | Brazil | 17 | Leprosy patients/cured | Physical disability post-multidrug therapy | 188/385 | Paucibacillary: 13.5. multibacillary 12.6, Leprosy reactions: 10.8, reports of complaints during treatment 11.6 | NA | Kaplan–Meier method | log-rank test | Cox’s proportional hazards regression | Hazard Ratio |
| Coriolano et al. (2021) [38] | Cohort | Brazil | 9 | Leprosy patients/leprosy reactions during and after the use of multidrug therapy | First leprosy reactions during multidrug therapy and post-multidrug therapy | NA/1621 | NA | NA | Kaplan–Meier method | log-rank test | Cox’s proportional hazards regression | Hazard Ratio |
| Hacker et al. (2021) l [8] | Cohort | Brazil | 33 | Leprosy contacts | Leprosy | 192/9024 | NA | 0.141 | NA | NA | Cox’s proportional hazards regression | Hazard Ratio |
| Pepito et al. (2021) m [11] | Cohort/Retrospective | The Philippines | 7 | Leprosy patients/multibacillary | Treatment completion/Treatment default | Treatment completion: 590/1034, Treatment default: 383/1034 | Treatment completion: 1.1, Treatment default: 0.3 | NA | Kaplan–Meier method | log-rank test | Cox’s proportional hazards regression | NA |
| Rajkumar et al. (2021) n [39] | Cohort | India | 10 | Leprosy patients/cured | Relapse | 69/2177 | NA | 0.542 | Kaplan–Meier method | Mid-p exact test | Andersen–Gill method | Hazard Ratio |
| Nascimento et al. (2022) o [40] | Retrospective | Brazil | 6 | Leprosy patients/cured | Relapse | 126/1059 | 11.6 | NA | Kaplan–Meier method | log-rank, Breslow and Tarone–Ware tests. | NA | NA |
| Study/Year of Publication | Outcome: Leprosy Household Contact | |||||||
|---|---|---|---|---|---|---|---|---|
| Paucibacillary | Multibacillary | |||||||
| Mean Follow-Up (Years) | Incidence Density/100 Person-Years (95% CI) | Curve Kaplan Meier (Years) | Hazard Ratio (95% CI) | Mean Follow-Up (Years) | Incidence Density/100 Person-Years (95% CI) | Curve Kaplan–Meier (Years) | Hazard Ratio (95% CI) | |
| Bakker et al. (2005) [23] | NA | NA | NA | NA | NA | NA | NA | NA |
| Bakker et al. (2006) a [24] | 233 | 0.215 (0.030–1.52) | NA | No contact-1.0; 0.97 (0.13–7.32) | 217 | 1.15 (0.480–2.77) | NA | No contact-1.0; 4.60 (1.65–12.9) |
| Kumar et al. (2007) b [25] | NA | 0.410 (NA) | NA | NA | NA | 1.313 (NA) | NA | NA |
| Gomes et al. (2019) [36] | NA | NA | NA | NA | NA | NA | NA | NA |
| Manta et al. (2019) [37] | NA | NA | NA | NA | NA | NA | NA | NA |
| Hacker et al. (2021) [8] | NA | NA | NA | NA | NA | NA | NA | NA |
| Study/Year of Publication | Outcome: Relapse | |||||||
|---|---|---|---|---|---|---|---|---|
| Paucibacillary | Multibacillary | |||||||
| Mean Follow-Up (Years) | Incidence Density/100 Person-Years (95% CI) | Curve Kaplan–Meier (Years) | Hazard Ratio (95% CI) | Mean Follow-Up (Years) | Incidence Density/100 Person-Years (95% CI) | Curve Kaplan–Meier (Years) | Hazard Ratio (95% CI) | |
| Pattyn et al. (1994) a [16] | NA | C2: 3.3 (1.1–5.4); C4: 1.9 (0.7–4.0) | NA | NA | NA | NA | NA | NA |
| Girdhar et al. (2000) b [19] | NA | NA | NA | NA | NA | FDT: 2.04 (NA); TSN: 1.11 (NA) | FDT < 90.0% (8); TSN < 100.0% (8) relapse-free | NA |
| Cellona et al. (2003) [20] | NA | NA | NA | NA | NA | 0.28 | 4.0% (12) | NA |
| Girdhar et al. (2011) c [30] | ROM: 1.6 C-ROM: 1.7 | ROM: 1.05 (NA), C-ROM: 0.90 (NA) | NA | NA | NA | NA | NA | NA |
| Guerrero-Guerrero et al. (2012) [31] | NA | NA | NA | NA | NA | 6.70 | <75.0% (10) | NA |
| Penna et al. (2017) d [35] | NA | NA | NA | NA | NA | U-MDT: 0.446 (NA); R-MDT: 0.044 (NA) | NA | NA |
| Rajkumar et al. (2021) e [39] | NA | 0.506 (NA) | NA | NA | 0.595 (NA) | NA | NA | |
| Nascimento et al. (2022) f [40] | 10 | NA | MDT-PB 6 dose: 64.28% (10); 85.71% (15) | NA | 14 | NA | MDT-MB 12 dose: 70.58% (10); 90.19% (15) MDT-MB 24 dose: 38.6% (10); 63.6% (15) | NA |
| Study/Year of Publication | Outcome: Clinical Manifestations before, during and after Treatment—Leprosy Reactions | |||||||
|---|---|---|---|---|---|---|---|---|
| Paucibacillary | Multibacillary | |||||||
| Mean Follow-Up (Years) | Incidence Density/100 Person-Years (95% CI) | Curve Kaplan–Meier (Years) | Hazard Ratio (95% CI) | Mean Follow-Up (Years) | Incidence Density/100 Person-Years (95% CI) | Curve Kaplan–Meier (Years) | Hazard Ratio (95% CI) | |
| Gomes et al. (2008) [26] | NA | NA | NA | NA | NA | NA | NA | NA |
| Smith et al. (2009) [29] | NA | NA | NA | NA | NA | NA | NA | NA |
| Penna et al. (2012) a [33] | NA | NA | NA | NA | 5.2 | NA | NA | NA |
| Penna et al. (2017) a [35] | NA | NA | NA | NA | NA | NA | U-MDT: 64.14% (6 months) R-MDT: 62.23% (6 months) reaction-free | NA |
| Coriolano et al. (2021) [38] | 6 months | NA | NA | 1.244 (1.108–1.397) | 8 months | NA | NA | 1.0 |
| Study/Year of Publication | Outcome: Clinical Manifestations before, during and after Treatment—Nerve Function Impairment | |||||||
|---|---|---|---|---|---|---|---|---|
| Paucibacillary | Multibacillary | |||||||
| Mean Follow-Up (Years) | Incidence Density/100 Person-Years (95% CI) | Curve Kaplan–Meier (Years) | Hazard Ratio (95% CI) | Mean Follow-Up (Years) | Incidence Density/100 Person-Years (95% CI) | Curve Kaplan–Meier (Years) | Hazard Ratio (95% CI) | |
| Croft, Nicholls, Richardus et al. (2000) [17] | NA | 1.3 | NA | 1.0 | NA | 24.4 | NA | 8.8 (6.2–12.5) |
| Croft, Nicholls, Steyerberg et al. (2000) [18] | NA | NA | 2.6% (2) | 1.0 | NA | NA | 37.0% (2) | 7.5 (5.3–11.0) |
| Richardus et al. (2004) [21] | NA | 0.85 | NA | NA | NA | 16.1 | NA | NA |
| Smith et al. (2004) [22] | NA | NA | NA | NA | NA | NA | NA | Relative Risk: 2.0 (0.8–4.5) |
| Schuring et al. (2008) [28] | NA | NA | NA | 1.0 | NA | NA | NA | 8.0 (5.0–13.0) |
| Smith et al. (2009) [29] | NA | NA | NA | NA | NA | NA | NA | |
| Penna et al. (2012) [33] | NA | NA | NA | NA | NA | NA | NA | NA |
| Study/Year of Publication | Outcome: Clinical Manifestations before, during and after Treatment—Physical Disabilities | |||||||
|---|---|---|---|---|---|---|---|---|
| Paucibacillary | Multibacillary | |||||||
| Mean Follow-Up (Years) | Incidence Density/100 Person-Years (95% CI) | Curve Kaplan–Meier (Years) | Hazard Ratio (95% CI) | Mean Follow-Up (Years) | Incidence Density/100 Person-Years (95% CI) | Curve Kaplan–Meier (Years) | Hazard Ratio (95% CI) | |
| Pattyn et al. (1994) a [16] | NA | NA | NA | Relative Risk: C2: 1.0; C4: 1.6 (0.9–3.0) physical disability-free | NA | NA | NA | NA |
| Kumar et al. (2012) [32] | NA | NA | NA | NA | NA | 2.74 | NA | NA |
| Sales et al. (2013) [34] | NA | NA | NA | NA | 4.3 | 6.5 | <60.0% (10) | Grade 0: 1.0; Grade 1: 2.03 (1.32–3.14); Grade 2: 2.80 (1.65–4.74) |
| Penna et al. (2017) b [35] | NA | NA | NA | NA | NA | NA | U-MDT: 33.8% (5) R-MDT: 30.06% (5) | NA |
| Santos et al. (2020) c [7] | NA | NA | < 80.0% (17) | 1.0 | 12.6 | NA | <80.0% (17) | 0.82 (0.60–1.11) |
| Studies | Criteria | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Question/Objective Sufficiently Described? | Study Design Evident and Appropriate? | Method of Subject/Comparison Group Selection or Source of Information/Input Variables Described and Appropriate? | Subject (and Comparison Group, if Applicable) Characteristics Sufficiently Described? | If Interventional and Random Allocation Was Possible, Was It Described? | If Interventional and Blinding of Investigators Was Possible, Was It Reported? | If Interventional and Blinding of Subjects Was Possible, Was It Reported? | Outcome and (If Applicable) Exposure Measure(s) Well Defined and Robust to Measurement/Misclassification Bias? Means of Assessment Reported? | Sample Size Appropriate? | Analytic Methods Described/Justified and Appropriate? | Some Estimate of Variance Is Reported for the Main Results? | Controlled for Confounding? | Results Reported in Sufficient Detail? | Conclusions Supported by the Results? | Maximum Points | Total Points | Summary Score (%) | |
| Pattyn et al. (1994) [16] | 2 | 2 | 2 | 2 | 1 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 2 | 1 | 28 | 15 | 53.6 |
| Croft, Nicholls, Steyerberg et al. (2000) [17] | 2 | 2 | 2 | 2 | N/A | N/A | N/A | 2 | 0 | 2 | 1 | 0 | 2 | 1 | 22 | 16 | 72.7 |
| Croft, Nicholls, Richardus et al. (2000) [18] | 1 | 2 | 2 | 2 | N/A | N/A | N/A | 0 | 0 | 2 | 1 | 0 | 2 | 1 | 22 | 13 | 59.1 |
| Girdhar et al. (2000) [19] | 2 | 1 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 2 | 2 | 28 | 13 | 46.4 |
| Cellona et al. (2003) [20] | 2 | 2 | 2 | 2 | N/A | N/A | N/A | 0 | 0 | 2 | 1 | 0 | 2 | 2 | 22 | 15 | 68.2 |
| Richardus et al. (2004) [21] | 2 | 2 | 2 | 2 | N/A | N/A | N/A | 0 | 0 | 2 | 0 | 0 | 2 | 2 | 22 | 14 | 63.6 |
| Smith et al. (2004) [22] | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 1 | 0 | 2 | 2 | 28 | 24 | 85.7 |
| Bakker et al. (2005) [23] | 2 | 2 | 1 | 1 | 1 | 1 | 1 | 2 | 0 | 2 | 1 | 0 | 2 | 2 | 28 | 18 | 64.3 |
| Bakker et al. (2006) [24] | 2 | 2 | 1 | 1 | 1 | 1 | 1 | 2 | 0 | 2 | 1 | 0 | 2 | 2 | 28 | 18 | 64.3 |
| Kumar et al. (2007) [25] | 2 | 2 | 2 | 1 | N/A | N/A | N/A | 0 | 2 | 2 | 1 | 0 | 2 | 2 | 22 | 16 | 72.7 |
| Gomes et al. (2008) [26] | 1 | 0 | 1 | 1 | N/A | N/A | N/A | 0 | 1 | 1 | 0 | 0 | 2 | 2 | 22 | 9 | 40.9 |
| Gonçalves et al. (2008) [27] | 2 | 2 | 2 | 1 | N/A | N/A | N/A | 0 | 0 | 2 | 1 | 0 | 2 | 2 | 22 | 14 | 63.6 |
| Schuring et al. (2008) [28] | 2 | 1 | 2 | 1 | N/A | N/A | N/A | 0 | 0 | 2 | 1 | 0 | 2 | 2 | 22 | 13 | 59.1 |
| Smith et al. (2009) [29] | 2 | 1 | 2 | 1 | N/A | N/A | N/A | 0 | 2 | 2 | 1 | 0 | 2 | 2 | 22 | 15 | 68.2 |
| Girdhar et al. (2011) [30] | 2 | 2 | 2 | 2 | 1 | 0 | 0 | 0 | 2 | 2 | 0 | 0 | 2 | 2 | 28 | 17 | 60.7 |
| Guerrero-Guerrero et al. (2012) [31] | 1 | 2 | 2 | 1 | N/A | N/A | N/A | 0 | 0 | 2 | 2 | 0 | 2 | 1 | 22 | 13 | 59.1 |
| Kumar et al. (2012) [32] | 2 | 1 | 2 | 2 | N/A | N/A | N/A | 1 | 0 | 2 | 1 | 0 | 2 | 2 | 22 | 15 | 68.2 |
| Penna et al. (2012) [33] | 1 | 2 | 2 | 1 | 1 | 1 | 2 | 0 | 0 | 2 | 1 | 0 | 2 | 1 | 28 | 16 | 57.1 |
| Sales et al. (2013) [34] | 2 | 2 | 2 | 1 | N/A | N/A | N/A | 0 | 0 | 2 | 1 | 0 | 2 | 2 | 22 | 14 | 63.6 |
| Penna et al. (2017) [35] | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 1 | 0 | 2 | 1 | 28 | 21 | 75.0 |
| Gomes et al. (2019) [36] | 2 | 2 | 2 | 1 | N/A | N/A | N/A | 1 | 2 | 2 | 1 | 0 | 2 | 2 | 22 | 17 | 77.3 |
| Manta et al. (2019) [37] | 2 | 2 | 2 | 2 | N/A | N/A | N/A | 0 | 0 | 1 | 0 | 0 | 2 | 1 | 22 | 12 | 54.5 |
| Santos et al. (2020) [7] | 2 | 2 | 2 | 1 | N/A | N/A | N/A | 0 | 0 | 2 | 1 | 0 | 2 | 2 | 22 | 14 | 63.6 |
| Coriolano et al. (2021) [38] | 2 | 2 | 2 | 2 | N/A | N/A | N/A | 1 | 0 | 2 | 1 | 0 | 2 | 1 | 22 | 15 | 68.2 |
| Hacker et al. (2021) [8] | 2 | 1 | 2 | 1 | N/A | N/A | N/A | 0 | 0 | 1 | 1 | 0 | 2 | 2 | 22 | 12 | 54.5 |
| Pepito et al. (2021) [11] | 2 | 2 | 2 | 1 | N/A | N/A | N/A | 0 | 0 | 2 | 1 | 0 | 2 | 2 | 22 | 14 | 63.6 |
| Rajkumar et al. (2021) [39] | 2 | 2 | 2 | 1 | N/A | N/A | N/A | 0 | 0 | 2 | 1 | 0 | 2 | 2 | 22 | 14 | 63.6 |
| Nascimento et al. (2022) [40] | 2 | 1 | 2 | 2 | N/A | N/A | N/A | 0 | 0 | 2 | 1 | 0 | 2 | 1 | 22 | 13 | 59.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbosa, C.C.; Bezerra, G.S.N.; Xavier, A.T.; Albuquerque, M.d.F.P.M.d.; Bonfim, C.V.d.; Medeiros, Z.M.d.; Souza, W.V.d. Systematic Review of Survival Analysis in Leprosy Studies—Including the Following Outcomes: Relapse, Impairment of Nerve Function, Reactions and Physical Disability. Int. J. Environ. Res. Public Health 2022, 19, 12155. https://doi.org/10.3390/ijerph191912155
Barbosa CC, Bezerra GSN, Xavier AT, Albuquerque MdFPMd, Bonfim CVd, Medeiros ZMd, Souza WVd. Systematic Review of Survival Analysis in Leprosy Studies—Including the Following Outcomes: Relapse, Impairment of Nerve Function, Reactions and Physical Disability. International Journal of Environmental Research and Public Health. 2022; 19(19):12155. https://doi.org/10.3390/ijerph191912155
Chicago/Turabian StyleBarbosa, Celivane Cavalcanti, Gilberto Silva Nunes Bezerra, Amanda Tavares Xavier, Maria de Fátima Pessoa Militão de Albuquerque, Cristine Vieira do Bonfim, Zulma Maria de Medeiros, and Wayner Vieira de Souza. 2022. "Systematic Review of Survival Analysis in Leprosy Studies—Including the Following Outcomes: Relapse, Impairment of Nerve Function, Reactions and Physical Disability" International Journal of Environmental Research and Public Health 19, no. 19: 12155. https://doi.org/10.3390/ijerph191912155
APA StyleBarbosa, C. C., Bezerra, G. S. N., Xavier, A. T., Albuquerque, M. d. F. P. M. d., Bonfim, C. V. d., Medeiros, Z. M. d., & Souza, W. V. d. (2022). Systematic Review of Survival Analysis in Leprosy Studies—Including the Following Outcomes: Relapse, Impairment of Nerve Function, Reactions and Physical Disability. International Journal of Environmental Research and Public Health, 19(19), 12155. https://doi.org/10.3390/ijerph191912155






