Understanding the Actual Use of Anti-HIV Drugs in Japan from 2016 to 2019: Demonstrating Epidemiological Relevance of NDB Open Data Japan for Understanding Japanese Medical Care
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Backbone Drug and Anchor Drug
2.3. Data Processing and Analysis
2.4. Ethics
3. Results
3.1. Anti-HIV Drugs Included in This Study and Their Characteristics
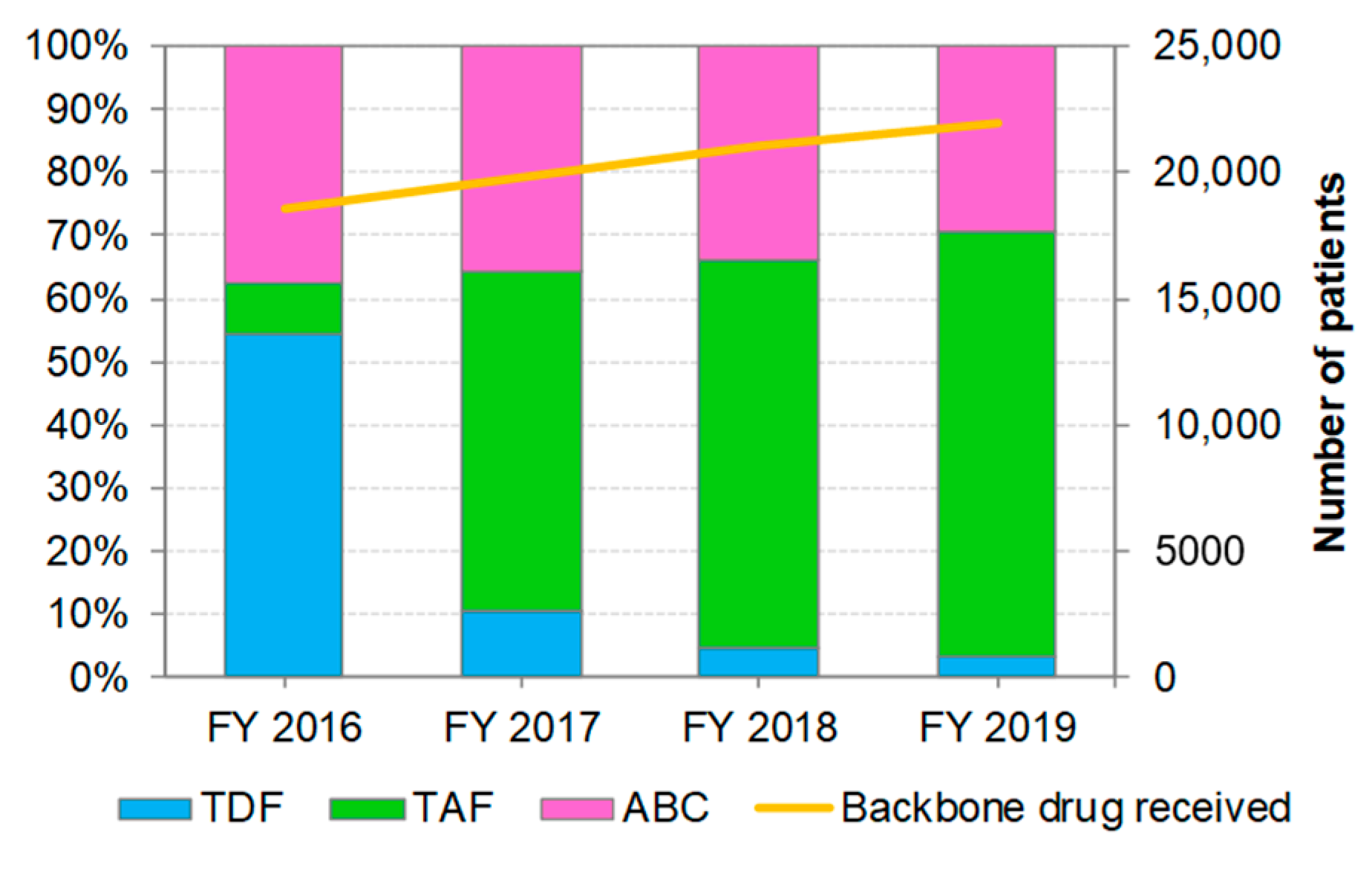
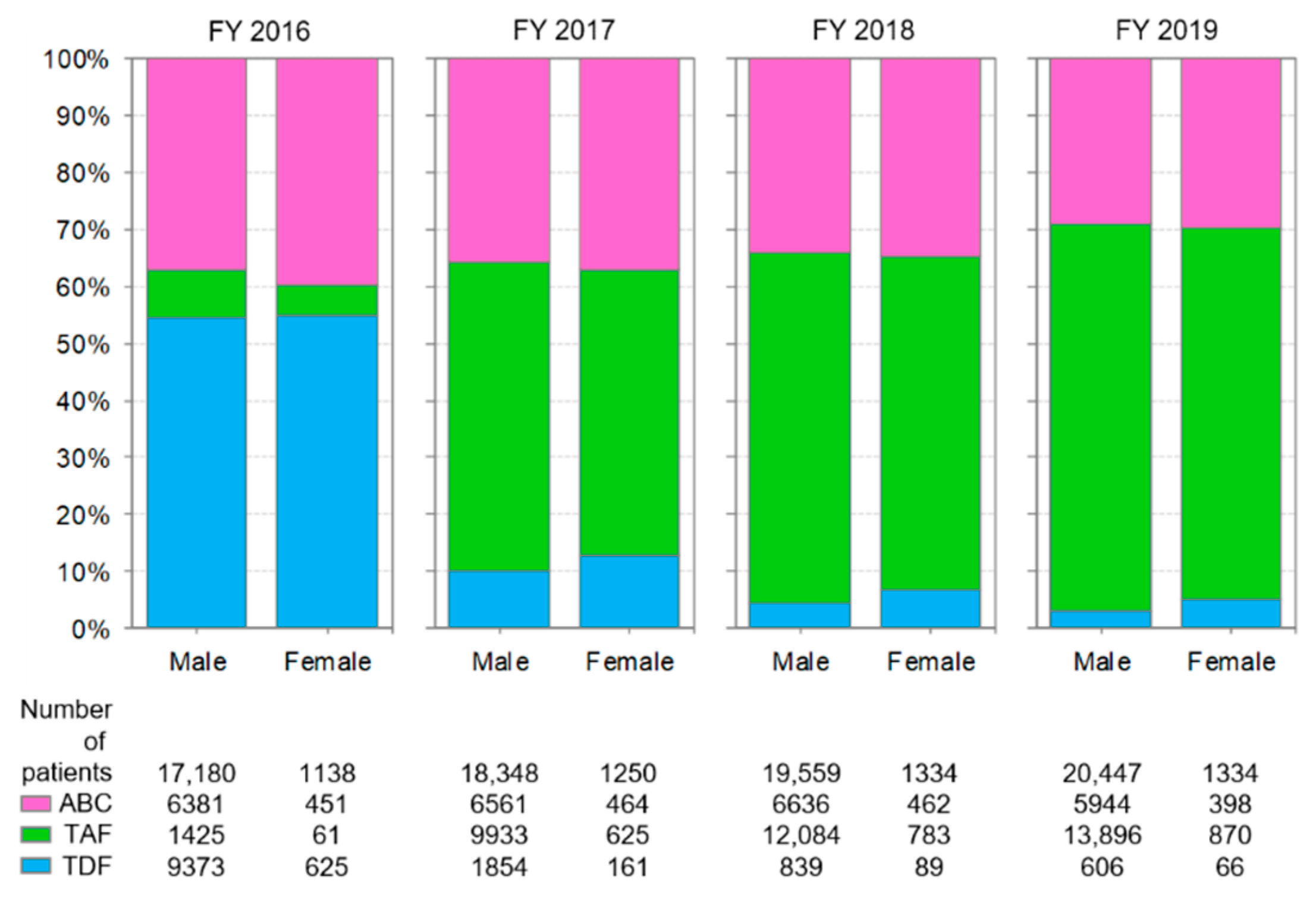
3.2. Distribution of Backbone Drug Prescriptions by Year
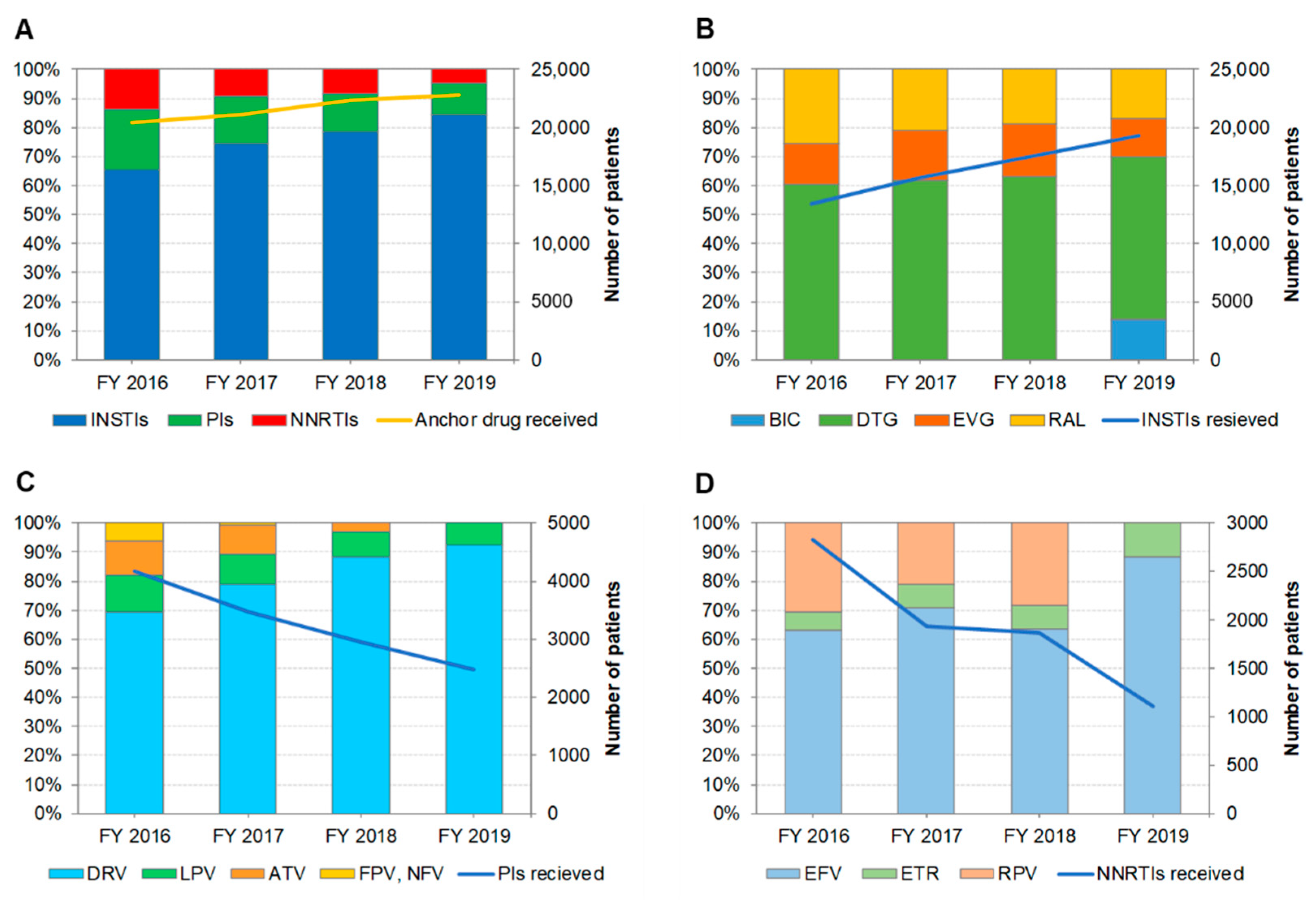
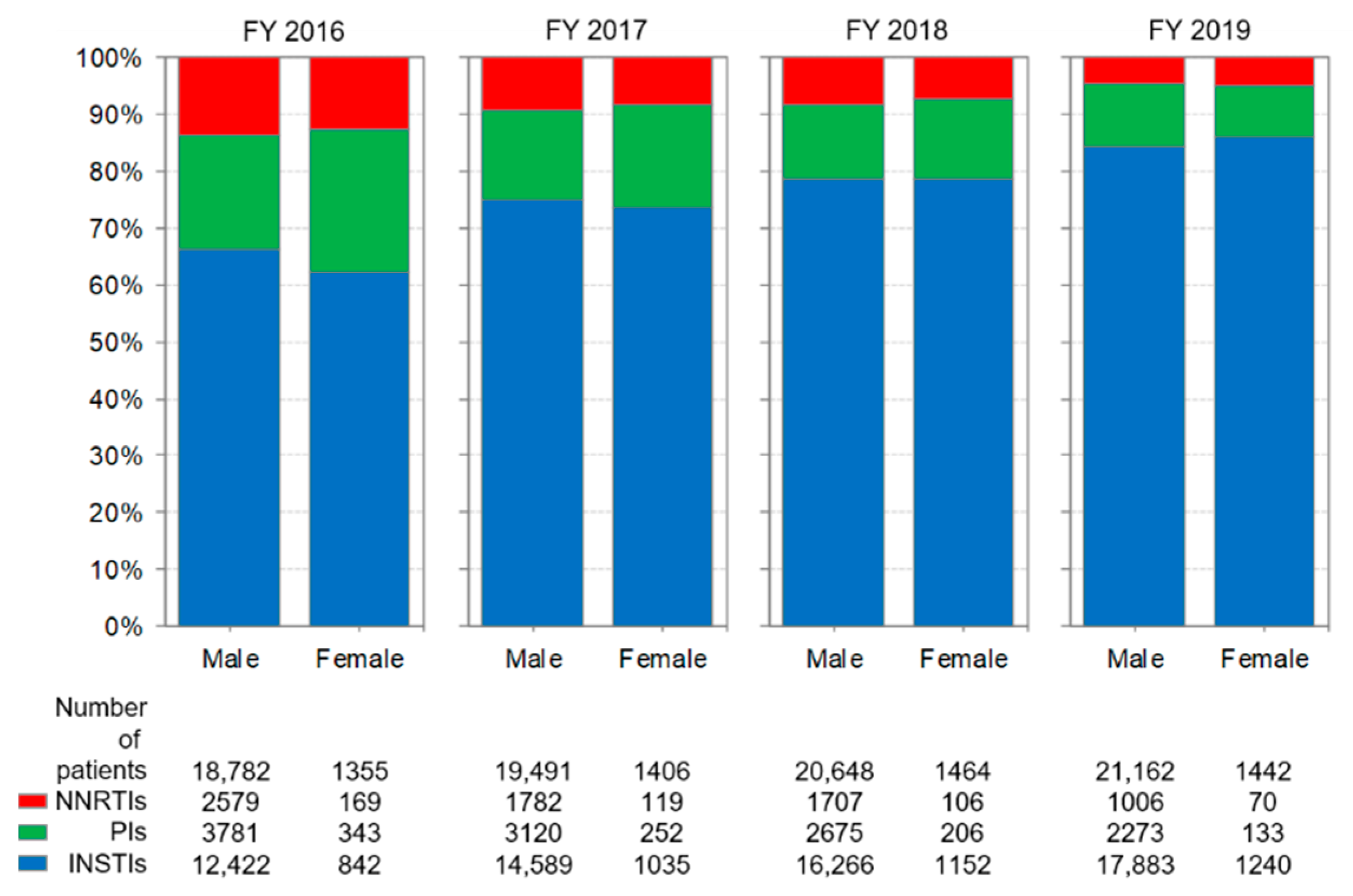
3.3. Distribution of Anchor Drug Prescriptions by Year (Figure 2)
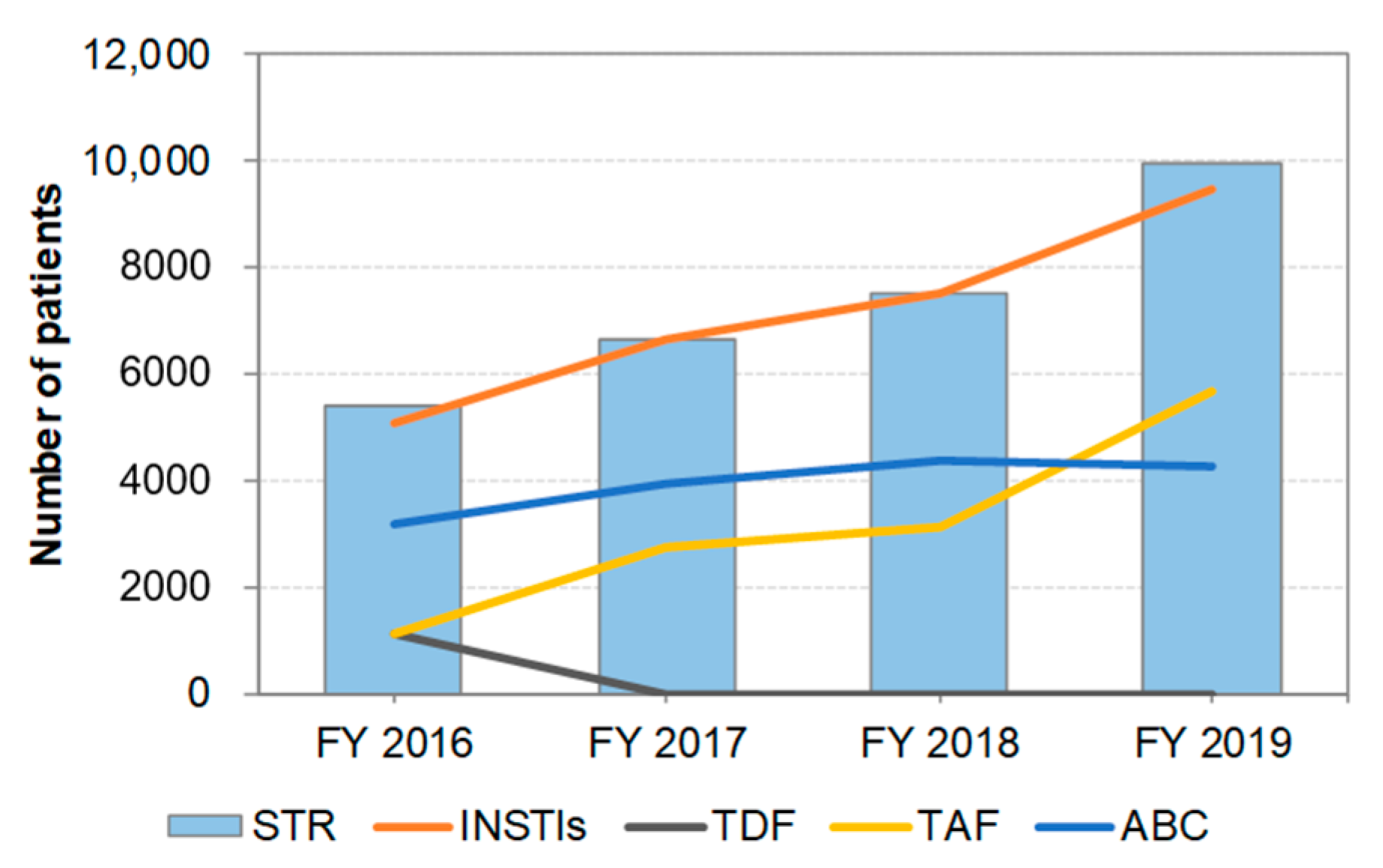
3.4. Distribution of STR Prescriptions by Year (Figure 3)
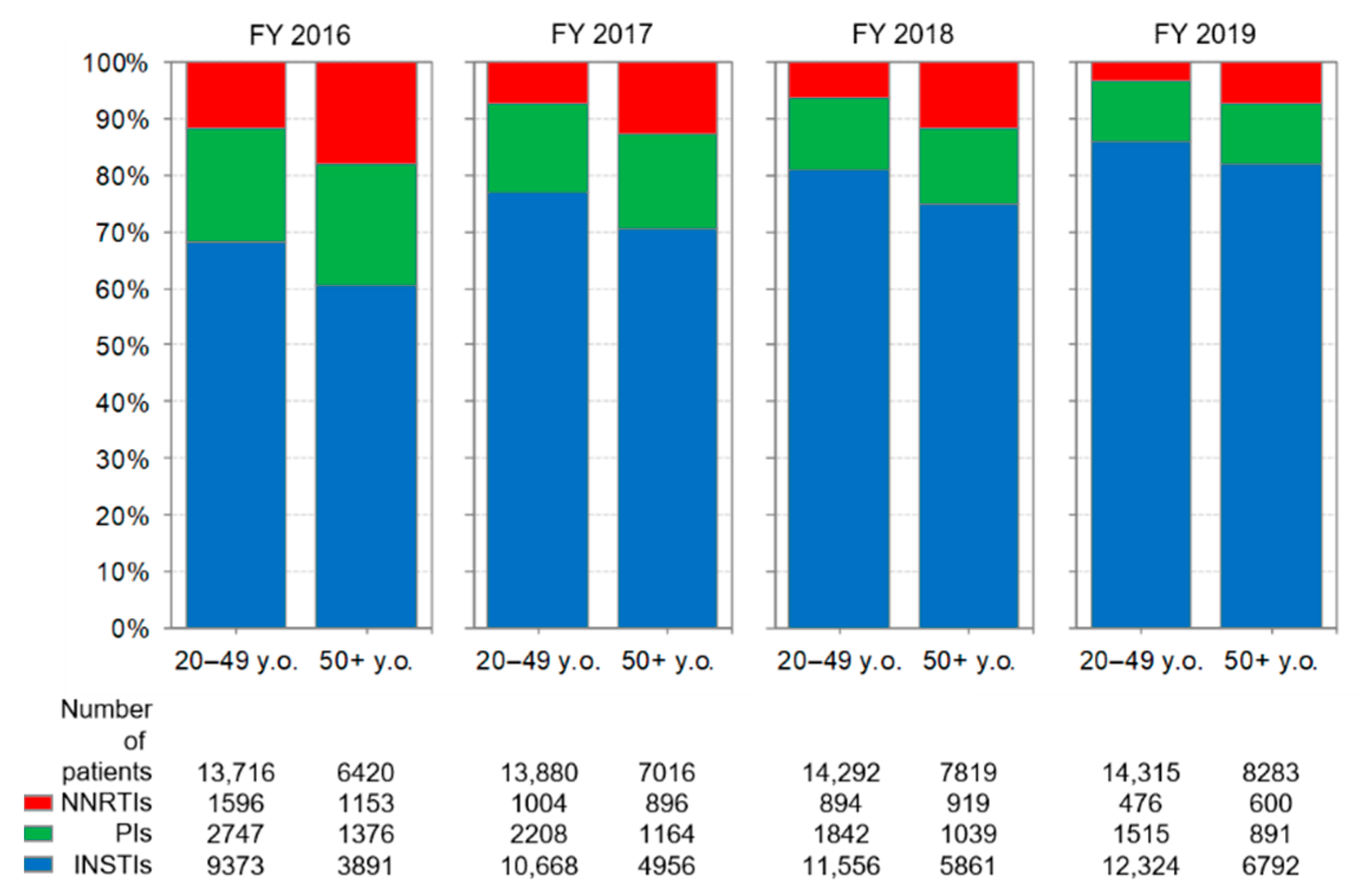
3.5. Comparison of Anti-HIV-Drug Prescriptions by Age Group
3.6. Comparison of Anti-HIV-Drug Prescriptions by Sex
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Department of Health and Human Services. Panel on Antiretroviral Guidelines for Adults and Adolescents, Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. Available online: https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/adult-adolescent-arv/guidelines-adult-adolescent-arv.pdf (accessed on 20 July 2022).
- Ministry of Health, Labour and Welfare. The Guidelines for the Treatment of HIV Infection, March 2022 Version. Available online: https://hiv-guidelines.jp/pdf/guideline2022.pdf (accessed on 20 July 2022). (In Japanese).
- Tanaka, H.; Wada, T.; Takayama, Y.; Matsumoto, K.; Atsuda, K.; Satoh, M. Evaluation of the efficacy and safety of changes in antiretroviral regimens for HIV-infected patients. J. Pharm. Sci. 2014, 17, 316–323. [Google Scholar] [CrossRef] [PubMed][Green Version]
- De Clercq, E.; Li, G. Approved antiviral drugs over the past 50 years. Clin. Microbiol. Rev. 2016, 29, 695–747. [Google Scholar] [CrossRef]
- Nakagawa, F.; May, M.; Phillips, A. Life expectancy living with HIV: Recent estimates and future implications. Curr. Opin. Infect. Dis. 2013, 26, 17–25. [Google Scholar] [CrossRef]
- Naito, T.; Suzuki, M.; Fukushima, S.; Yuda, M.; Fukui, N.; Tsukamoto, S.; Fujibayashi, K.; Goto-Hirano, K.; Kuwatsuru, R. Comorbidities and co-medications among 28,089 people living with HIV: A nationwide cohort study from 2009 to 2019 in Japan. HIV Med. 2022, 23, 485–493. [Google Scholar] [CrossRef]
- Pau, A.K.; George, J.M. Antiretroviral therapy: Current drugs. Infect. Dis. Clin. North Am. 2014, 28, 371–402. [Google Scholar] [CrossRef] [PubMed]
- Demessine, L.; Peyro-Saint-Paul, L.; Gardner, E.M.; Ghosn, J.; Parienti, J.J. Risk and cost associated with drug-drug interactions among aging HIV patients receiving combined antiretroviral therapy in France. Open Forum Infect. Dis. 2019, 6, ofz051. [Google Scholar] [CrossRef] [PubMed]
- Naito, T.; Mori, H.; Fujibayashi, K.; Fukushima, S.; Yuda, M.; Fukui, N.; Tsukamoto, S.; Suzuki, M.; Goto-Hirano, K.; Kuwatsuru, R. Analysis of antiretroviral therapy switch rate and switching pattern for people living with HIV from a national database in Japan. Sci. Rep. 2022, 12, 1732. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health, Labour and Welfare. The 6th NDB Open Data Commentary. Available online: https://www.mhlw.go.jp/content/12400000/000821378.pdf (accessed on 20 July 2022). (Abstract in English).
- Tanaka, H.; Ishii, T. Investigation of prescription pattern of antiretroviral using the national database of health insurance claims specific health checkups of Japan open data. J. AIDS Res. 2019, 21, 173–180. (In Japanese) [Google Scholar]
- Nojiri, S.; Itoh, H.; Kasai, T.; Fujibayashi, K.; Saito, T.; Hiratsuka, Y.; Okuzawa, A.; Naito, T.; Yokoyama, K.; Daida, H. Comorbidity status in hospitalized elderly in Japan: Analysis from National Database of Health Insurance Claims and Specific Health Checkups. Sci. Rep. 2019, 9, 20237. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, A.; Sakai, R.; Inoue, E.; Harigai, M. Prevalence of patients with rheumatoid arthritis and age-stratified trends in clinical characteristics and treatment, based on the National Database of Health Insurance Claims and Specific Health Checkups of Japan. Int. J. Rheum. Dis. 2020, 23, 1676–1684. [Google Scholar] [CrossRef] [PubMed]
- Tsukiji, M.; Yamazaki, S.; Taniguchi, T.; Nakamura, T.; Suzuki, T.; Igari, H.; Ishii, I. Adjusted for optimal Truvada® dosage by Tenofovir therapeutic drug monitoring—Two cases of HIV-infected patients who were undergoing hemodialysis. J. AIDS Res. 2018, 20, 132–137. (In Japanese) [Google Scholar]
- Uchitsubo, K.; Masuda, J.; Akazawa, T.; Inoue, R.; Tsukada, K.; Gatanaga, H.; Terakado, H.; Oka, S. Nucleos(t)ide reverse transcriptase inhibitor-sparing regimens in the era of standard 3-drug combination therapies for HIV-1 infection. Glob. Health Med. 2020, 2, 384–387. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, A.; Taira, R.; Yokomaku, Y.; Koibuchi, T.; Rahman, M.; Izumi, Y.; Tadokoro, K. The HIV care cascade: Japanese perspectives. PLoS ONE 2017, 12, e0174360. [Google Scholar] [CrossRef] [PubMed]
- Buti, M.; Riveiro-Barciela, M.; Esteban, R. Tenofovir Alafenamide Fumarate: A new Tenofovir prodrug for the treatment of chronic Hepatitis B infection. J. Infect. Dis. 2017, 216, S792–S796. [Google Scholar] [CrossRef]
- Domingo, P.; Mateo, M.G.; Gutierrez, M.D.M.; Vidal, F. Tolerability of current antiretroviral single-tablet regimens. AIDS Rev. 2018, 20, 141–149. [Google Scholar] [CrossRef]
- Cohen, J.; Beaubrun, A.; Bashyal, R.; Huang, A.; Li, J.; Baser, O. Real-world adherence and persistence for newly-prescribed HIV treatment: Single versus multiple tablet regimen comparison among US medicaid beneficiaries. AIDS Res. Ther. 2020, 17, 12. [Google Scholar] [CrossRef]
- Cotte, L.; Ferry, T.; Pugliese, P.; Valantin, M.A.; Allavena, C.; Cabié, A.; Poizot-Martin, I.; Rey, D.; Duvivier, C.; Cheret, A.; et al. Effectiveness and tolerance of single tablet versus once daily multiple tablet regimens as first-line antiretroviral therapy—Results from a large french multicenter cohort study. PLoS ONE 2017, 12, e0170661. [Google Scholar] [CrossRef]
- Pandit, N.S.; Chastain, D.B.; Pallotta, A.M.; Badowski, M.E.; Huesgen, E.C.; Michienzi, S.M. Simplifying ARV therapy in the setting of resistance. Curr. Infect. Dis. Rep. 2019, 21, 38. [Google Scholar] [CrossRef]
- Avihingsanon, A.; Kerr, S.J.; Punyawudho, B.; van der Lugt, J.; Gorowara, M.; Ananworanich, J.; Lange, J.M.; Cooper, D.A.; Phanuphak, P.; Burger, D.M.; et al. Short communication: Aging not gender is associated with high atazanavir plasma concentrations in Asian HIV-infected patients. AIDS Res. Hum. Retrovir. 2013, 29, 1541–1546. [Google Scholar] [CrossRef]
- Greig, J.M.; Anderson, J. Optimizing antiretroviral therapy for women living with HIV. Curr. Opin. Infect. Dis. 2014, 27, 46–52. [Google Scholar] [CrossRef]
- Floridia, M.; Giuliano, M.; Palmisano, L.; Vella, S. Gender differences in the treatment of HIV infection. Pharmacol. Res. 2008, 58, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Van De Ven, N.S.; Pozniak, A.L.; Levi, J.A.; Clayden, P.; Garratt, A.; Redd, C.; Mofenson, L.M.; Hill, A. Analysis of pharmacovigilance databases for dolutegravir safety in pregnancy. Clin. Infect. Dis. 2020, 70, 2599–2606. [Google Scholar] [CrossRef] [PubMed]

| Product Name | Ingredients | Mechanism of Action | STR | NDB Open Data Japan | |||||
|---|---|---|---|---|---|---|---|---|---|
| Anchor Drug | Backbone Drug | Others | 3rd | 4th | 5th | 6th | |||
| BIKTARVY® Combination Tablets | BIC, TAF, FTC | INSTI | NRTI | Yes | 〇 | ||||
| CELSENTRI® Tablets 150 mg | MVC | CCR5 antagonist | No | 〇 | 〇 | 〇 | 〇 | ||
| Combivir® Combination Tablets | AZT, 3TC | NRTI | No | 〇 | 〇 | ||||
| COMPLERA® Combination Tablets | RPV, TDF, FTC | NNRTI | NRTI | Yes | 〇 | ||||
| Descovy® Combination Tablets LT | TAF, FTC | NRTI | No | 〇 | 〇 | 〇 | |||
| Descovy® Combination Tablets HT | TAF, FTC | NRTI | No | 〇 | 〇 | 〇 | 〇 | ||
| EDURANT® Tablets 25 mg | RPV | NNRTI | No | 〇 | 〇 | 〇 | |||
| Epivir® Tablets 150 mg | 3TC | NRTI | No | 〇 | 〇 | 〇 | 〇 | ||
| Epzicom® Combination Tablets | ABC, 3TC | NRTI | No | 〇 | 〇 | 〇 | 〇 | ||
| Genvoya® Combination Tablets | EVG/Cobi, TAF, FTC | INSTI | NRTI | Cobi: Booster | Yes | 〇 | 〇 | 〇 | 〇 |
| ISENTRESS® Tablets 400 mg | RAL | INSTI | No | 〇 | 〇 | 〇 | 〇 | ||
| ISENTRESS® Tablets 600 mg | RAL | INSTI | No | 〇 | 〇 | ||||
| INTELENCE® Tablets 100 mg | ETR | NNRTI | No | 〇 | 〇 | 〇 | 〇 | ||
| Kaletra® Combination Tablets | LPV/RTV | PI | RTV: Booster | No | 〇 | 〇 | 〇 | 〇 | |
| Kaletra® Combination Oral Solution | LPV/RTV | PI | RTV: Booster | No | 〇 | 〇 | 〇 | 〇 | |
| Lexiva® Tablets 700 mg | FPV | PI | No | 〇 | |||||
| Norvir® Tablets | RTV | PI | Booster | No | 〇 | 〇 | 〇 | 〇 | |
| PREZCOBIX® Combination Tablets | DRV/Cobi | PI | Cobi: Booster | No | 〇 | 〇 | 〇 | ||
| PREZISTA® Tablets 600 mg | DRV | PI | No | 〇 | 〇 | 〇 | |||
| PREZISTANAIVE® Tablets 800 mg | DRV | PI | No | 〇 | 〇 | 〇 | 〇 | ||
| Retrovir® Capsules 100 mg | AZT | NRTI | No | 〇 | 〇 | 〇 | 〇 | ||
| REYATAZ® Capsules 150 mg | ATV | PI | No | 〇 | 〇 | 〇 | |||
| STOCRIN® Tablets 200 mg | EFV | NNRTI | No | 〇 | 〇 | 〇 | 〇 | ||
| STOCRIN® Tablets 600 mg | EFV | NNRTI | No | 〇 | 〇 | 〇 | 〇 | ||
| Stribild® Combination Tablets | EVG/Cobi, TDF, FTC | INSTI | NRTI | Cobi: Booster | Yes | 〇 | |||
| SYMTUZA® Combination Tablets | DRV/Cobi, TAF, FTC | PI | NRTI | Cobi: Booster | Yes | 〇 | |||
| Tivicay® Tablets | DTG | INSTI | No | 〇 | 〇 | 〇 | 〇 | ||
| Triumeq® Combination Tablets | DTG, ABC, 3TC | INSTI | NRTI | Yes | 〇 | 〇 | 〇 | 〇 | |
| Truvada® Combination Tablets | TDF, FTC | NRTI | No | 〇 | 〇 | 〇 | 〇 | ||
| VIRACEPT® Tablets 250 mg | NFV | PI | No | 〇 | 〇 | ||||
| Viread® Tablets 300 mg | TDF | NRTI | No | 〇 | |||||
| Ziagen® Tablets 300 mg | ABC | NRTI | No | 〇 | 〇 | 〇 | 〇 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanaka, H.; Onoda, T.; Ishii, T. Understanding the Actual Use of Anti-HIV Drugs in Japan from 2016 to 2019: Demonstrating Epidemiological Relevance of NDB Open Data Japan for Understanding Japanese Medical Care. Int. J. Environ. Res. Public Health 2022, 19, 12130. https://doi.org/10.3390/ijerph191912130
Tanaka H, Onoda T, Ishii T. Understanding the Actual Use of Anti-HIV Drugs in Japan from 2016 to 2019: Demonstrating Epidemiological Relevance of NDB Open Data Japan for Understanding Japanese Medical Care. International Journal of Environmental Research and Public Health. 2022; 19(19):12130. https://doi.org/10.3390/ijerph191912130
Chicago/Turabian StyleTanaka, Hiroyuki, Toshihisa Onoda, and Toshihiro Ishii. 2022. "Understanding the Actual Use of Anti-HIV Drugs in Japan from 2016 to 2019: Demonstrating Epidemiological Relevance of NDB Open Data Japan for Understanding Japanese Medical Care" International Journal of Environmental Research and Public Health 19, no. 19: 12130. https://doi.org/10.3390/ijerph191912130
APA StyleTanaka, H., Onoda, T., & Ishii, T. (2022). Understanding the Actual Use of Anti-HIV Drugs in Japan from 2016 to 2019: Demonstrating Epidemiological Relevance of NDB Open Data Japan for Understanding Japanese Medical Care. International Journal of Environmental Research and Public Health, 19(19), 12130. https://doi.org/10.3390/ijerph191912130






