Evaluation of Trophic Structure and Energy Flow in a Pelteobagrus fulvidraco Integrated Multi-Trophic Aquaculture System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Construction of FMRP
2.2. Culture Management
2.3. Construction of Ecopath Model
2.4. Functional Group Settings
2.5. Model Parameters and Data Collection
2.6. Model Balancing
2.7. Carrying Capacity Assessment
2.8. Ecosystem Analysis Derived from Ecopath Model Application
3. Results
3.1. Parameter Estimation of the Ecopath Model
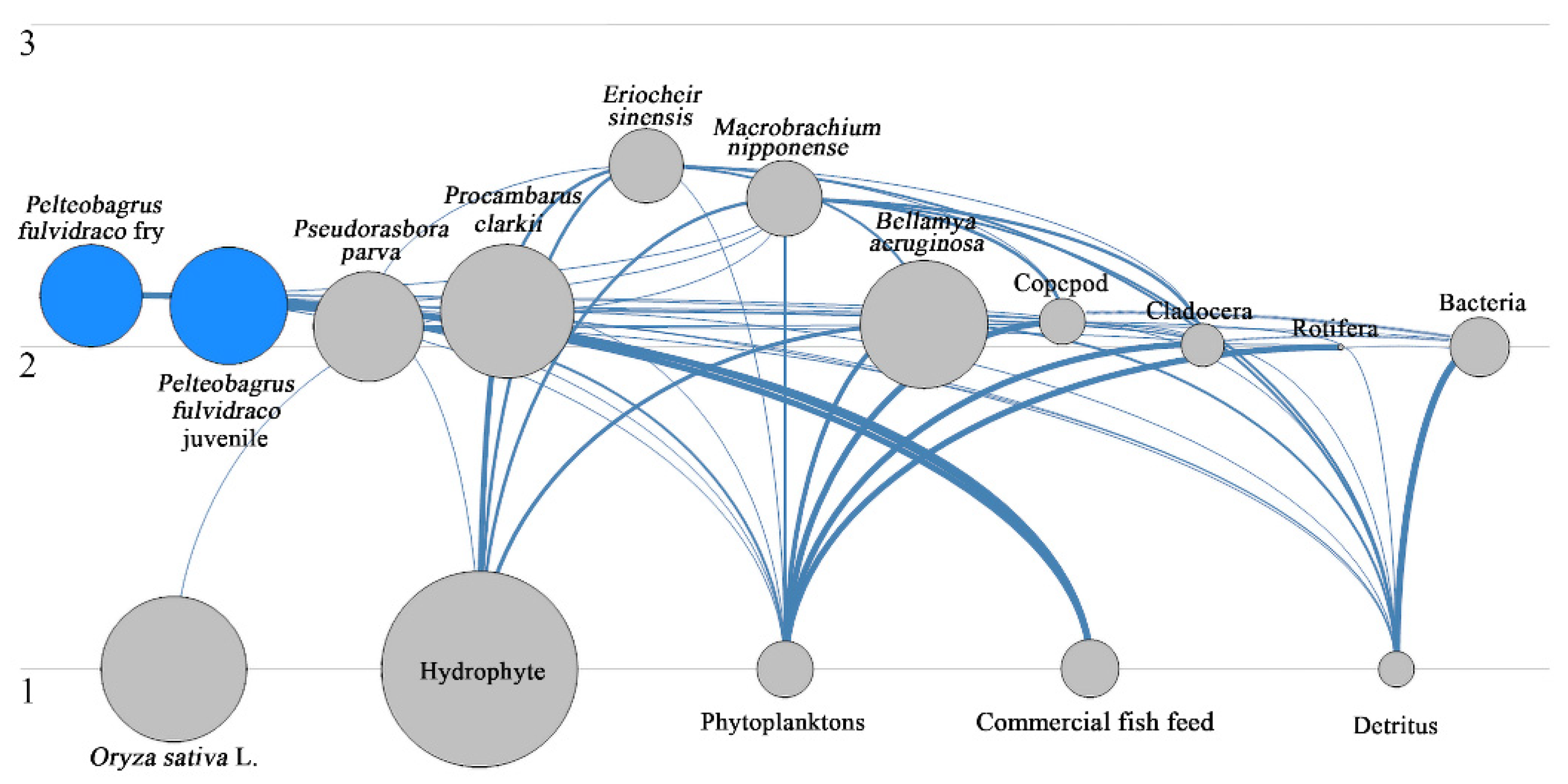
3.2. Features of Food Web
3.3. System Characteristics
3.4. Energy Consumption by Consumers
3.5. Characteristics of Energy Conversion
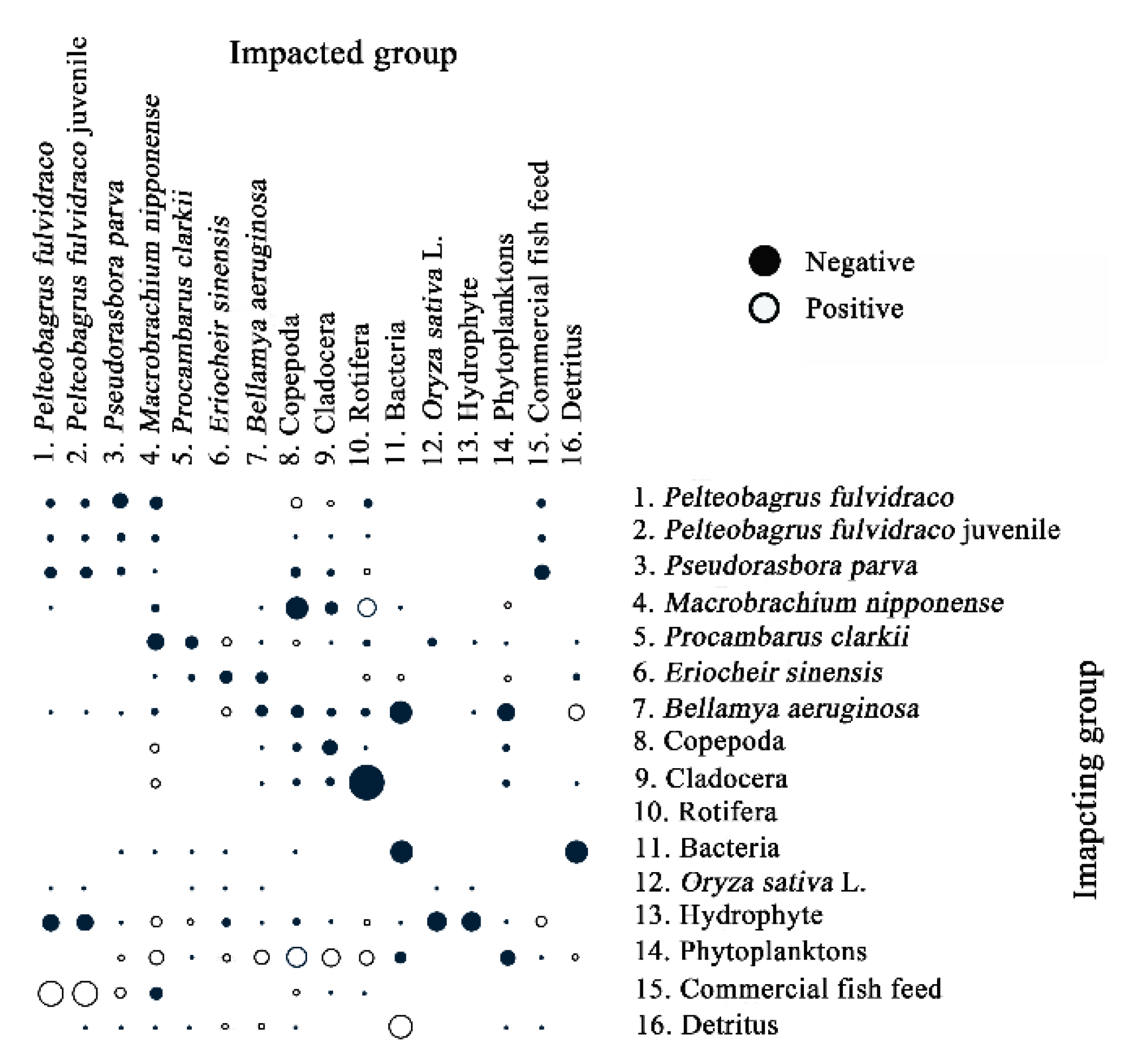
3.6. Analysis of Mixed Trophic Impact
3.7. Keystone Functional Group Analysis
3.8. Carrying Capacity Estimation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAMA (Fisheries Administration of the Ministry of Agriculture and Rural Affairs of the People’s Republic of China); NTEC (National Fisheries Technology Extension Center); CSF (China Society of Fisheries). China Fisheries Statistics Yearbook; China Agricultural Press: Beijing, China, 2021. (In Chinese) [Google Scholar]
- Li, L.L.; Yao, R.; Olsen, R.H.; Zhang, Y.; Meng, H.C. Antibiotic resistance and polymyxin B resistance mechanism of Aeromonas spp. isolated from yellow catfish, hybrid snakeheads and associated water from intensive fish farms in Southern China. LWT-Food Sci. Technol. 2022, 166, 113802. [Google Scholar] [CrossRef]
- Zhang, M.Z.; Li, M.; Li, X.; Qian, Y.X.; Wang, R.X.; Hong, M.L. The protective effects of selenium on chronic ammonia toxicity in yellow catfish (Pelteobagrus fulvidraco). Fish Shellfish Immunol. 2020, 107, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Zhong, A.H.; Gao, T.X. Characterization of immunomodulatory lncRNAs in the head kidney of yellow catfish (Pelteobagrus fulvidraco) infected by Aeromonas hydrophila. Aquac. Rep. 2022, 23, 101091. [Google Scholar] [CrossRef]
- Wang, S.D.; Li, X.; Zhang, M.Z.; Jiang, H.B.; Wang, R.X.; Qian, Y.X.; Li, M. Ammonia stress disrupts intestinal microbial community and amino acid metabolism of juvenile yellow catfish (Pelteobagrus fulvidraco). Ecotoxicol. Environ. Saf. 2021, 227, 112932. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Tian, X.L.; Dong, S.L.; He, R.P.; Zhang, K.; Zhang, D.X.; Li, L.; Zhang, Q.Q.; Zhang, T. Comparative analysis of the energy fluxes and trophic structure of polyculture ecosystems of Portunus trituberculatus based on Ecopath model. Aquaculture 2018, 496, 185–196. [Google Scholar] [CrossRef]
- Liu, X.G. Pond Aquaculture Ecological Engineering; Agriculture Press: Beijing, China, 2018. [Google Scholar]
- Zhu, H.; Cheng, S.P.; Wang, J.; Liu, X.G.; Lai, Q.F. Construction and operation evaluation of ecologically engineered cultivation system of saline-alkaline pond. Fish Mod 2018, 45, 27–33. [Google Scholar]
- Liu, X.G.; Che, X.; Wang, X.D.; Zhu, H.; Cheng, G.F.; Gu, Z.J.; Liu, C. The technology and method for green and clean pond aquaculture. Chin. Fish. Qual. Stand. 2018, 8, 1–8. [Google Scholar]
- Tong, L. Ecopath—A mass-balance modeling for ecosystem estimation. Prog. Fish Sci. 1999, 20, 102–107. [Google Scholar]
- Colléter, M.; Valls, A.; Guitton, J.; Gascuel, D.; Pauly, D.; Christensen, V. Global overview of the applications of the Ecopath with Ecosim modeling approach using the EcoBase models repository. Ecol. Model. 2015, 302, 42–53. [Google Scholar] [CrossRef]
- Byron, C.; Link, J.; Costa-Pierce, B.; Bengtson, D. Calculating ecological carrying capacity of shellfish aquaculture using mass-balance modeling: Narragansett Bay, Rhode Island. Ecol. Model. 2011, 222, 1743–1755. [Google Scholar] [CrossRef]
- Kluger, L.C.; Taylor, M.H.; Mendo, J.; Tam, J.; Wolff, M. Carrying capacity simulations as a tool for ecosystem-based management of a scallop aquaculture system. Ecol. Model. 2011, 331, 44–55. [Google Scholar] [CrossRef]
- Kluger, L.C.; Taylor, M.H.; Rivera, E.B.; Silva, E.T.; Wolff, M. Assessing the ecosystem impact of scallop bottom culture through a community analysis and trophic modelling approach. Mar. Ecol. Prog. Ser. 2016, 547, 121–135. [Google Scholar] [CrossRef]
- Coll, M.; Libralato, S. Contributions of food web modelling to the ecosystem approach to marine resource management in the Mediterranean Sea. Fish Fish. 2012, 13, 60–88. [Google Scholar] [CrossRef]
- Stäbler, M.; Kempf, A.; Mackinson, S.; Poos, J.J.; Garcia, C.; Temming, A. Combining efforts to make maximum sustainable yields and good environmental status match in a food-web model of the southern North Sea. Ecol. Model. 2016, 331, 17–30. [Google Scholar] [CrossRef]
- Tucker, L.; Boyd, C.E.; Mccoy, E.W. Effects of feeding rate on water quality, production of channel catfish, and economic returns. Trans. Am. Fish. Soc. 1979, 108, 389–396. [Google Scholar] [CrossRef]
- Jia, P.Q. Ecopath Model Construction and Ecological Effect of Pen Culture of Silver Carp and Bighead Carp in Gehu Lake; Shanghai Ocean University: Shanghai, China, 2012. [Google Scholar]
- Zhao, X.H.; Xu, D.P.; Ren, L.; Xu, P. Assessment of the ecological carrying capacity of silver and bighead carp in the Taihu Lake based on Ecopath model. J. Fish. Sci. China 2021, 28, 785–795. [Google Scholar]
- Li, Y.K.; Liu, E.S.; Wang, H.; Gong, Y. Analysis on the ecosystem structure and function of Lake Taihu based on Ecopath model. J. Appl. Ecol. 2014, 25, 2033–2040. [Google Scholar]
- Wang, Q.; Hu, G.D.; Song, C.; Li, D.D.; Song, Y.; Qiu, L.P.; Fan, L.M.; Meng, S.L.; Chen, J.C.; Bing, X.W. Evaluation of nutritional structure and energy flow of crab-rice complex ecosystem based on an ecopath model. Acta Ecol. Sin. 2020, 40, 4852–4862. [Google Scholar]
- Zhou, B. Research on Grass Carp (Ctenopharyngodon Idella) Integrated Aquaculture Pond Ecosystem: Based on EwE Model; Ocean University of China: Qingdao, China, 2015. [Google Scholar]
- Yan, J. Recovery, conversion, reuse and recycling of organic wastewater from a pharmaceutical factory. Ecol. Eng. 1999, 2, 297–307. [Google Scholar]
- Vögler, R.; Miless, A.C.; Quiñones, R.A. Influence of environmental variables on the distribution of Squatina guggenheim (Chondrichthyes, Squatinidae) in the Argentine-Uruguayan Common Fishing Zone. Fish. Res. 2008, 91, 212–221. [Google Scholar] [CrossRef]
- Milessi, A.C.; Danilo, C.; Laura, R.G.; Daniel, D.; Javier, S.; Rodríguez-Gallego, L. Trophic mass-balance model of a subtropical coastal lagoon, including a comparison with a stable isotope analysis of the food-web. Ecol. Model. 2010, 221, 2859–2869. [Google Scholar] [CrossRef]
- Heymans, J.J.; Coll, M.; Link, J.S.; Mackinson, S.; Steenbeek, J.; Walters, C.; Christensen, V. Best practice in Ecopath with Ecosim food-web models for ecosystem-based management. Ecol. Model. 2016, 331, 173–184. [Google Scholar] [CrossRef]
- Zhang, T.L.; Cui, Y.B. Methods and advances on fish production. J. Fish. China 1999, 13, 409–414. [Google Scholar]
- Zhang, T.L.; Cui, Y.B.; Fang, R.L.; Xie, S.G.; Li, Z.J. Population biology of topmouth gudgeon in the Baoan Lake V. production. Acta Hydrobiol. Sin. 2001, 25, 331–337. [Google Scholar]
- Diana, J.S.; Lin, C.K.; Schneeberger, P.J. Relationships among nutrient inputs, water nutrient concentrations, primary production, and yield of Oreochromis niloticus in ponds. Aquaculture 1991, 92, 323–341. [Google Scholar] [CrossRef]
- Dong, S.P.; Gao, Y.F.; Gao, Y.P.; He, M.D.; Liu, F.; Yan, F.J.; Wang, F. Evaluation of the trophic structure and energy flow of a rice-crayfish integrated farming ecosystem based on the Ecopath model. Aquaculture 2021, 539, 736626. [Google Scholar] [CrossRef]
- Feng, D.X.; Chen, L.; Li, Y.K.; Yu, N.; Chen, L.Q. Structure and energy flow of Dianshan Lake ecosystem based on the Ecopath model. J. Fish. Sci. China 2011, 18, 867–876. [Google Scholar] [CrossRef]
- Liu, Z.J. Studies on Differences in Age, Growth, Reproduction and Feeding Habits of Pelteobagrus Nitidus between the Upstream and Downstream of Caojie Hydropower in Jialing River; Southwest University: Chongqing, China, 2020. [Google Scholar]
- Huang, X.F.; Bing, X.W.; Chen, J.C. Energy flow and trophic structure of the Wuli Lake ecosystem based on an ecopath model. J. Fish. Sci. China 2012, 19, 471–481. [Google Scholar] [CrossRef]
- Wen, Z.R.; Xie, P. Studies on feeding habits and source of dietary carbon of Macrobrachium nipponense and Exopalaemon modestus in Taihu Lake. J. Yangtze Univ. (Nat. Sci. Ed.) 2013, 10, 36–42+7–8. [Google Scholar]
- Song, B. Ecosystem Modeling Study on the Fishery and Environment of Taihu Lake; East China Normal University: Shanghai, China, 2004. [Google Scholar]
- Li, L.P. Study on Feeding Habit, Growth and Burrowing Behavior of Procambarus Clarkii; Huazhong Agricultural University: Wuhan, China, 2006. [Google Scholar]
- Xu, J.J. Study on the Feeding Habit and Intestinal Microbes of Eriocheir Sinensis in the Yangtze River Estuary; Shanghai Ocean University: Shanghai, China, 2019. [Google Scholar]
- Duan, X.J. Feeding Habits and Selectivity of Bellamya Purificata in Ecological Ditch; Huazhong Agricultural University: Wuhan, China, 2013. [Google Scholar]
- Zeng, X.L.; Wei, B.C.; Liu, X.G.; Gu, Z.J.; Lu, S.M.; Che, X. Analysis of compound culturing pond build based on Ecopath model. J. Fish. China 2018, 42, 711–719. [Google Scholar]
- Chamberlain, P.; Patterson, G.; Reid, J.; Kavanagh, K.; Forgatch, M. Observation of client resistance. Behav. Ther. 1984, 15, 144–155. [Google Scholar] [CrossRef]
- Christensen, V.; Walters, C.J. Ecopath with Ecosim: Methods, capabilities and limitations. Ecol. Model. 2004, 172, 109–139. [Google Scholar] [CrossRef]
- Jiang, W.; Gibbs, M.T. Predicting the carrying capacity of bivalve shellfish culture using a steady, linear food web model. Aquaculture 2005, 244, 171–185. [Google Scholar] [CrossRef]
- Lalli, C.M.; Parsons, T.R. Biological Oceanography: An Introduction; Pergamon Press: New York, NY, USA, 1993. [Google Scholar]
- Christensen, V.; Walters, C.J.; Pauly, D. Ecopath with Ecosim: A User’s Guide; University of British Columbia: Vancouver, BC, Canada, 2005. [Google Scholar]
- Ulanowicz, R.E. Growth and Development: Ecosystem Phenomenology; Springer Verlag: New York, NY, USA, 1986. [Google Scholar]
- Ulanowicz, R.E.; Norden, J.S. Symmetrical overhead in flow networks. Int. J. Syst. Sci. 1990, 21, 429–437. [Google Scholar] [CrossRef]
- Finn, J.T. Measures of ecosystem structure and function derived from analysis of flows. J. Theor. Biol. 1976, 56, 363–380. [Google Scholar] [CrossRef]
- Ulanowicz, R.E.; Puccia, C.J. Mixed trophic impacts in ecosystems. Coenoses 1990, 5, 7–16. [Google Scholar]
- Libralato, S.; Christensen, V.; Pauly, D. A method for identifying keystone species in food web models. Ecol. Model. 2006, 195, 153–171. [Google Scholar] [CrossRef]
- Morissett, L. Complexity, Cost and Quality of Ecosystem Models and Their Impact on Resilience: A Comparative Analysis, with Emphasis on Marine Mannals and the Gulf of St; University of British Columbia: Vancouver, BC, Canada, 2007. [Google Scholar]
- Mi, W.J.; Hu, J.X.; Zhao, X.F. Application of Ecopath model in aquatic ecosystems assessment and management. J. Hydroecol. 2012, 33, 127–130. [Google Scholar]
- Hannon, B. The structure of ecosystems. J. Theor. Biol. 1973, 41, 535–546. [Google Scholar] [CrossRef]
- Wang, Y.F.; Peng, X.Z.; Zhou, S.Q.; Zhang, J.D.; Chen, S.J. Increasing ecological energy conversion efficiency in freshwater culture pond with photosynthetic bacteria. J. Fish. China 1993, 3, 253–256. [Google Scholar]
- Cruz-Escalona, V.H.; Arreguin-Sanchez, F.; Zetina-Rejon, M. Analysis of the ecosystem structure of Laguna Alvarado, western Gulf of Mexico, by means of a mass balance model. Estuar. Coast. Shelf Sci. 2007, 72, 155–167. [Google Scholar] [CrossRef]
- Rehren, J.; Wolff, M.; Jiddawi, M. Holistic assessment of Chwaka Bay’s multi-gear fishery—Using a trophic modeling approach. J. Mar. Syst. 2018, 180, 265–278. [Google Scholar] [CrossRef]
- Vasconcellos, M.; Mackinsons, S.; Sloman, K.; Pauly, D. The stability of trophic mass-balance models of marine ecosystems: A comparative analysis. Ecol. Model. 1997, 100, 125–134. [Google Scholar] [CrossRef]
- Odum, E.P. The strategy of ecosystem development. Science 1969, 164, 262–270. [Google Scholar] [CrossRef] [Green Version]
- Phong, L.T.; van Dam, A.A.; Udo, H.M.J.; van Mensvoort, M.E.F.; Tri, L.Q.; Steenstra, F.A.; van der Zijpp, A.J. An agro-ecological evaluation of aquaculture integration into farming systems of the Mekong Delta. Agric. Ecosyst. Environ. 2010, 138, 232–241. [Google Scholar] [CrossRef]
- Mcqueen, D.J.; Post, J.R.; Mills, E.L. Trophic relationships in freshwater pelagic ecosystems. Can. J. Fish. Aquat. Sci. 1986, 43, 1571–1581. [Google Scholar] [CrossRef]
- Hu, G.Y. Research on Integrated Aquaculture Mode of Shrimp and Shellfish Pond Based on Ecopath Model; Shanghai Ocean University: Shanghai, China, 2020. [Google Scholar]
- Nhan, D.K.; Milstein, A.; Verdegem, M.C.J.; Verreth, J.A.J. Food inputs, water quality and nutrient accumulation in integrated pond systems: A multivariate approach. Aquaculture 2006, 261, 160–173. [Google Scholar] [CrossRef]




| Prey | Predator | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
| 1 Pelteobagrus fulvidraco fry | |||||||||||
| 2 Pelteobagrus fulvidraco juvenile | |||||||||||
| 3 Pseudorasbora parva | 0.0900 | 0.0700 | 0.0010 | 0.0080 | |||||||
| 4 Macrobrachium nipponense | 0.0400 | 0.0300 | 0.0110 | ||||||||
| 5 Procambarus clarkii | 0.0770 | 0.2500 | |||||||||
| 6 Eriocheir sinensis | 0.0200 | ||||||||||
| 7 Bellamya aeruginosa | 0.1800 | ||||||||||
| 8 Copepoda | 0.0033 | 0.0040 | 0.0320 | 0.2350 | 0.0060 | 0.0300 | |||||
| 9 Cladocera | 0.0042 | 0.0032 | 0.0280 | 0.2040 | 0.0030 | 0.0200 | 0.0750 | ||||
| 10 Rotifera | 0.0018 | 0.0086 | |||||||||
| 11 Bacteria | 0.0700 | 0.0900 | 0.0290 | 0.0370 | |||||||
| 12 Oryza sativa L. | 0.1170 | ||||||||||
| 13 Hydrophyte | 0.0320 | 0.2400 | 0.6120 | 0.2600 | 0.2800 | ||||||
| 14 Phytoplankton | 0.0008 | 0.0008 | 0.152 | 0.1700 | 0.06700 | 0.0900 | 0.5000 | 0.8020 | 0.8510 | 0.9130 | |
| 15 Commercial fish feed | 0.8000 | 0.8200 | 0.641 | ||||||||
| 16 Detritus | 0.0621 | 0.0720 | 0.1140 | 0.1510 | 0.1070 | 0.1420 | 0.1500 | 0.0310 | 0.1110 | 0.0500 | 1.0000 |
| Sum | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 |
| Functional Groups | Biomass (g/m2) | P/B (150 Days) | Q/B (150 Days) | Feed Import (g/m2·150 Days) | Ecotrophic Efficiency | Effective Trophic Level |
|---|---|---|---|---|---|---|
| 1 Pelteobagrus fulvidraco fry | 12.17 | 1.96 | 8.31 | 0.988 | 2.164 | |
| 2 Pelteobagrus fulvidraco juvenile | 24.73 | 1.55 | 2.37 | 0.980 | 2.127 | |
| 3 Pseudorasbora parva | 16.38 | 2.25 | 11.00 | 0.489 | 2.068 | |
| 4 Macrobrachium nipponense | 3.23 | 4.50 | 24.40 | 0.705 | 2.487 | |
| 5 Procambarus clarkii | 50.46 | 3.24 | 8.00 | 0.309 | 2.112 | |
| 6 Eriocheir sinensis | 3.13 | 2.46 | 24.74 | 0.201 | 2.566 | |
| 7 Bellamya aeruginosa | 39.84 | 1.33 | 10.61 | 0.263 | 2.070 | |
| 8 Copepoda | 0.77 | 48.00 | 120.00 | 0.856 | 2.170 | |
| 9 Cladocera | 0.62 | 57.00 | 143.00 | 0.876 | 2.038 | |
| 10 Rotifera | 0.01 | 117.00 | 293.00 | 0.785 | 2.037 | |
| 11 Bacteria | 1.50 | 217.00 | 543.00 | 0.123 | 2.000 | |
| 12 Oryza sativa L. | 92.26 | 2.42 | 0.991 | 1.000 | ||
| 13 Hydrophyte | 952.70 | 2.25 | 0.769 | 1.000 | ||
| 14 Phytoplankton | 1.27 | 367.19 | 0.931 | 1.000 | ||
| 15 Commercial fish feed | 1.33 | 245.30 | 0.997 | 1.000 | ||
| 16 Detritus | 0.42 | 0.592 | 1.000 |
| Parameter | Unit | |
|---|---|---|
| Total system throughput (TST) | 6626.25 | g/m2·150 days |
| Sum of all consumption (TC) | 2315.04 | g/m2·150 days |
| Sum of all respiratory flows (TR) | 919.22 | g/m2·150 days |
| Sum of all flows into detritus (TD) | 1914.74 | g/m2·150 days |
| Sum of all production (TP) | 3567.19 | g/m2·150 days |
| Total net primary production (TPP) | 2832.87 | g/m2·150 days |
| Net system production | 1913.65 | g/m2·150 days |
| Total biomass (excluding detritus) (TB) | 1198.99 | g/m2 |
| Total primary production/Total respiration (TPP/TR) | 3.08 | |
| Total primary production/Total biomass (TPP/TB) | 2.36 | |
| Total biomass/Total throughput (TB/TP) | 0.18 | |
| Proportion of total flow originating from detritus | 0.42 | % of total throughput |
| Proportion of total flow originating from primary producers | 0.58 | % of total throughput |
| Ascendency (A) | 8786.00 | flowbits/m2·150 days |
| Overhead (O) | 17,910.00 | flowbits/m2·150 days |
| Total development capacity (TDC) | 30,606.00 | flowbits/m2·150 days |
| Ascendency/Total development capacity (A/TDC) | 0.29 | |
| Overhead/Total development capacity (O/TDC) | 0.59 | |
| Connectance index (CI) | 0.28 | |
| System omnivory index (SOI) | 0.11 | |
| Ecopath pedigree index (EPI) | 0.78 | |
| Finn’s cycling index (FCI, %) | 20.41 | % of total throughput |
| Finn’s cycling mean path length (FCL) | 2.77 |
| Trophic Level | Throughput (g/m2·150 Days) | Biomass (g/m2·150 Days) |
|---|---|---|
| VII | 0.00014 | 0.0000060 |
| VI | 0.021 | 0.0021 |
| V | 0.77 | 0.11 |
| IV | 12.07 | 1.28 |
| III | 146.00 | 10.91 |
| II | 2123.00 | 140.50 |
| I | 4311.00 | 1046.00 |
| Sum | 6593.00 | 1187.89 |
| Functional Groups | Keystone Index |
|---|---|
| 1 Pelteobagrus fulvidraco fry | −0.21 |
| 2 Pelteobagrus fulvidraco juvenile | −0.58 |
| 3 Pseudorasbora parva | −0.42 |
| 4 Macrobrachium nipponense | −0.19 |
| 5 Procambarus clarkii | −0.48 |
| 6 Eriocheir sinensis | −0.32 |
| 7 Bellamya aeruginosa | −0.27 |
| 8 Copepoda | −0.47 |
| 9 Cladocera | −0.09 |
| 10 Rotifera | −2.11 |
| 11 Bacteria | −1.03 |
| 12 Oryza sativa L. | −1.00 |
| 13 Hydrophyte | −0.79 |
| 14 Phytoplankton | −0.12 |
| Increased Species | Increment (g/m2) |
|---|---|
| Pelteobagrus fulvidraco fry | 0.12 |
| Pelteobagrus fulvidraco juvenile | 0.42 |
| Pelteobagrus fulvidraco fry and juvenile | 0.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Liu, X.; Lu, M.; Zhou, R.; Sun, Z.; Xiao, S. Evaluation of Trophic Structure and Energy Flow in a Pelteobagrus fulvidraco Integrated Multi-Trophic Aquaculture System. Int. J. Environ. Res. Public Health 2022, 19, 12027. https://doi.org/10.3390/ijerph191912027
Zhao Y, Liu X, Lu M, Zhou R, Sun Z, Xiao S. Evaluation of Trophic Structure and Energy Flow in a Pelteobagrus fulvidraco Integrated Multi-Trophic Aquaculture System. International Journal of Environmental Research and Public Health. 2022; 19(19):12027. https://doi.org/10.3390/ijerph191912027
Chicago/Turabian StyleZhao, Yuxi, Xingguo Liu, Ming Lu, Runfeng Zhou, Zhaoyun Sun, and Shuwen Xiao. 2022. "Evaluation of Trophic Structure and Energy Flow in a Pelteobagrus fulvidraco Integrated Multi-Trophic Aquaculture System" International Journal of Environmental Research and Public Health 19, no. 19: 12027. https://doi.org/10.3390/ijerph191912027
APA StyleZhao, Y., Liu, X., Lu, M., Zhou, R., Sun, Z., & Xiao, S. (2022). Evaluation of Trophic Structure and Energy Flow in a Pelteobagrus fulvidraco Integrated Multi-Trophic Aquaculture System. International Journal of Environmental Research and Public Health, 19(19), 12027. https://doi.org/10.3390/ijerph191912027








