Using a Short Food Frequency Questionnaire to Evaluate Macronutrients, Fiber, Phosphorus, Potassium, and Calcium in Adults with Stages 3–5 Chronic Kidney Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Collection of General Patient Characteristic Data
2.3. FFQ Assessment of Food Intakes
2.4. Three-Day Dietary Record Assessment of Dietary Intakes
2.5. Measurement of Clinical Parameters
2.6. Statistical Analysis
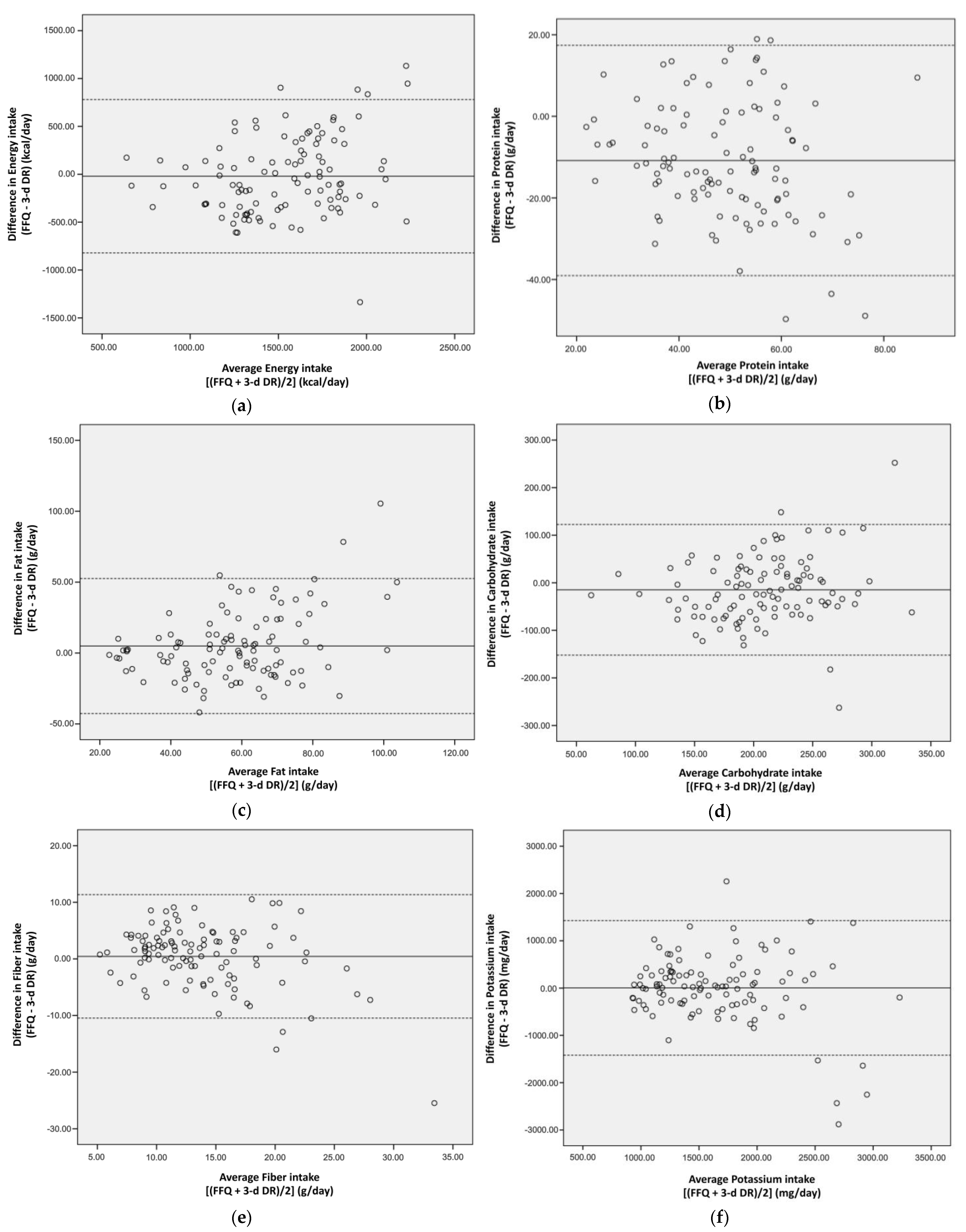
3. Results
3.1. Patient Characteristics
3.2. FFQ Validity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bikbov, B.; Purcell, C.A.; Levey, A.S.; Smith, M.; Abdoli, A.; Abebe, M.; Adebayo, O.M.; Afarideh, M.; Agarwal, S.K.; Agudelo-Botero, M.; et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef]
- Hill, N.R.; Fatoba, S.T.; Oke, J.L.; Hirst, J.A.; O’Callaghan, C.A.; Lasserson, D.S.; Hobbs, F.D. Global Prevalence of Chronic Kidney Disease—A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0158765. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.P.; Cheng, T.Y.; Tsai, M.K.; Chang, Y.C.; Chan, H.T.; Tsai, S.P.; Chiang, P.H.; Hsu, C.C.; Sung, P.K.; Hsu, Y.H.; et al. All-cause mortality attributable to chronic kidney disease: A prospective cohort study based on 462 293 adults in Taiwan. Lancet 2008, 371, 2173–2182. [Google Scholar] [CrossRef]
- Thurlow, J.S.; Joshi, M.; Yan, G.; Norris, K.C.; Agodoa, L.Y.; Yuan, C.M.; Nee, R. Global Epidemiology of End-Stage Kidney Disease and Disparities in Kidney Replacement Therapy. Am. J. Nephrol. 2021, 52, 98–107. [Google Scholar] [CrossRef]
- National Kidney, F. KDOQI Clinical Practice Guideline for Diabetes and CKD: 2012 Update. Am. J. Kidney Dis. 2012, 60, 850–886. [Google Scholar] [CrossRef]
- Webster, A.C.; Nagler, E.V.; Morton, R.L.; Masson, P. Chronic Kidney Disease. Lancet 2017, 389, 1238–1252. [Google Scholar] [CrossRef]
- Ash, S.; Campbell, K.L.; Bogard, J.; Millichamp, A. Nutrition prescription to achieve positive outcomes in chronic kidney disease: A systematic review. Nutrients 2014, 6, 416–451. [Google Scholar] [CrossRef]
- KDIGO Guidelines. Chapter 3: Management of progression and complications of CKD. Kidney Int. Suppl. 2013, 3, 73–90. [Google Scholar] [CrossRef]
- Fouque, D.; Pelletier, S.; Mafra, D.; Chauveau, P. Nutrition and chronic kidney disease. Kidney Int. 2011, 80, 348–357. [Google Scholar] [CrossRef]
- Bross, R.; Noori, N.; Kovesdy, C.P.; Murali, S.B.; Benner, D.; Block, G.; Kopple, J.D.; Kalantar-Zadeh, K. Dietary assessment of individuals with chronic kidney disease. Semin. Dial. 2010, 23, 359–364. [Google Scholar] [CrossRef] [Green Version]
- Gopinath, B.; Harris, D.C.; Flood, V.M.; Burlutsky, G.; Mitchell, P. Associations between dairy food consumption and chronic kidney disease in older adults. Sci. Rep. 2016, 6, 39532. [Google Scholar] [CrossRef] [PubMed]
- Joo, Y.S.; Kim, H.W.; Lee, S.; Nam, K.H.; Yun, H.R.; Jhee, J.H.; Han, S.H.; Yoo, T.H.; Kang, S.W.; Park, J.T. Dietary zinc intake and incident chronic kidney disease. Clin. Nutr. 2021, 40, 1039–1045. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Lopez, A.; Bullo, M.; Basora, J.; Martinez-Gonzalez, M.A.; Guasch-Ferre, M.; Estruch, R.; Warnberg, J.; Serra-Majem, L.; Aros, F.; Lapetra, J.; et al. Cross-sectional associations between macronutrient intake and chronic kidney disease in a population at high cardiovascular risk. Clin. Nutr. 2013, 32, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Pan, W.H.; Liu, K.L.; Yu, M.S. Reproducibility and validity of a Chinese food frequency questionnaire used in Taiwan. Asia Pac. J. Clin. Nutr. 2006, 15, 161–169. [Google Scholar]
- Huang, Y.C.; Lee, M.S.; Pan, W.H.; Wahlqvist, M.L. Validation of a simplified food frequency questionnaire as used in the Nutrition and Health Survey in Taiwan (NAHSIT) for the elderly. Asia Pac. J. Clin. Nutr. 2011, 20, 134–140. [Google Scholar]
- Chien, K.L.; Lee, M.S.; Tsai, Y.T.; Chen, P.R.; Lin, H.J.; Hsu, H.C.; Lee, Y.T.; Chen, M.F. A Taiwanese food frequency questionnaire correlates with plasma docosahexaenoic acid but not with plasma eicosapentaenoic acid levels: Questionnaires and plasma biomarkers. BMC Med. Res. Methodol. 2013, 13, 23. [Google Scholar] [CrossRef]
- Chiu, T.H.; Huang, H.Y.; Chen, K.J.; Wu, Y.R.; Chiu, J.P.; Li, Y.H.; Chiu, B.C.; Lin, C.L.; Lin, M.N. Relative validity and reproducibility of a quantitative FFQ for assessing nutrient intakes of vegetarians in Taiwan. Public Health Nutr. 2014, 17, 1459–1466. [Google Scholar] [CrossRef]
- Willett, W. Nutritional Epidemiology, 2nd ed.; Oxford University Press: New York, NY, USA, 1998. [Google Scholar]
- Duffrin, C.; Carraway-Stage, V.G.; Briley, A.; Christiano, C. Validation of a dietary intake tool for african-american dialysis patients with low literacy. J. Ren. Care 2015, 41, 126–133. [Google Scholar] [CrossRef]
- Affret, A.; Wagner, S.; El Fatouhi, D.; Dow, C.; Correia, E.; Niravong, M.; Clavel-Chapelon, F.; De Chefdebien, J.; Fouque, D.; Stengel, B.; et al. Validity and reproducibility of a short food frequency questionnaire among patients with chronic kidney disease. BMC Nephrol. 2017, 18, 297. [Google Scholar] [CrossRef]
- Bin Zarah, A.; Feraudo, M.C.; Andrade, J.M. Development and Relative Validity of the Chronic Kidney Disease Short Food Frequency Questionnaire (CKD SFFQ) to Determine Diet Quality and Dietary Habits among Adults with Chronic Kidney Disease. Nutrients 2021, 13, 3610. [Google Scholar] [CrossRef]
- Huang, M.C.; Lin, K.D.; Chen, H.J.; Wu, Y.J.; Chang, C.I.; Shin, S.J.; Hung, H.C.; Lee, C.H.; Huang, Y.F.; Hsu, C.C. Validity of a Short Food Frequency Questionnaire Assessing Macronutrient and Fiber Intakes in Patients of Han Chinese Descent with Type 2 Diabetes. Int. J. Environ. Res. Public Health 2018, 15, 1142. [Google Scholar] [CrossRef] [PubMed]
- Health Promotion Administration, Ministry of Health and Welfare. Obesity Prevention and Management. Available online: https://www.hpa.gov.tw/Pages/Detail.aspx?nodeid=542&pid=9737 (accessed on 8 September 2022).
- Alberti, K.G.; Zimmet, P.; Shaw, J.; IDF Epidemiology Task Force Consensus Group. The metabolic syndrome—A new worldwide definition. Lancet 2005, 366, 1059–1062. [Google Scholar] [CrossRef]
- Cade, J.; Thompson, R.; Burley, V.; Warm, D. Development, validation and utilisation of food-frequency questionnaires—A review. Public Health Nutr. 2002, 5, 567–587. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.; Stampfer, M.J. Total energy intake: Implications for epidemiologic analyses. Am. J. Epidemiol. 1986, 124, 17–27. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Measuring agreement in method comparison studies. Stat. Methods Med. Res. 1999, 8, 135–160. [Google Scholar] [CrossRef]
- Bonifacj, C.; Gerber, M.; Scali, J.; Daures, J.P. Comparison of dietary assessment methods in a southern French population: Use of weighed records, estimated-diet records and a food-frequency questionnaire. Eur. J. Clin. Nutr. 1997, 51, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Subar, A.F.; Thompson, F.E.; Kipnis, V.; Midthune, D.; Hurwitz, P.; McNutt, S.; McIntosh, A.; Rosenfeld, S. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires: The Eating at America’s Table Study. Am. J. Epidemiol. 2001, 154, 1089–1099. [Google Scholar] [CrossRef]
- Willett, W.C.; Sampson, L.; Browne, M.L.; Stampfer, M.J.; Rosner, B.; Hennekens, C.H.; Speizer, F.E. The use of a self-administered questionnaire to assess diet four years in the past. Am. J. Epidemiol. 1988, 127, 188–199. [Google Scholar] [CrossRef]
- Kesse-Guyot, E.; Castetbon, K.; Touvier, M.; Hercberg, S.; Galan, P. Relative validity and reproducibility of a food frequency questionnaire designed for French adults. Ann. Nutr. Metab. 2010, 57, 153–162. [Google Scholar] [CrossRef]
- Ahn, Y.; Kwon, E.; Shim, J.E.; Park, M.K.; Joo, Y.; Kimm, K.; Park, C.; Kim, D.H. Validation and reproducibility of food frequency questionnaire for Korean genome epidemiologic study. Eur. J. Clin. Nutr. 2007, 61, 1435–1441. [Google Scholar] [CrossRef]
- Barrat, E.; Aubineau, N.; Maillot, M.; Derbord, E.; Barthes, P.; Lescuyer, J.F.; Boisseau, N.; Peltier, S.L. Repeatability and relative validity of a quantitative food-frequency questionnaire among French adults. Food Nutr. Res. 2012, 56, 18472. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Xia, Y.; Wu, Q.; Chang, Q.; Niu, K.; Zhao, Y. A meta-analysis of the reproducibility of food frequency questionnaires in nutritional epidemiological studies. Int. J. Behav. Nutr. Phys. Act. 2021, 18, 12. [Google Scholar] [CrossRef] [PubMed]
- Lissner, L.; Troiano, R.P.; Midthune, D.; Heitmann, B.L.; Kipnis, V.; Subar, A.F.; Potischman, N. OPEN about obesity: Recovery biomarkers, dietary reporting errors and BMI. Int. J. Obes. 2007, 31, 956–961. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Demographic and Clinical Characteristics | Overall |
|---|---|
| Gender | |
| Men | 64 (59.8) |
| Women | 43 (40.2) |
| Years old | |
| <65 | 44 (41.1) |
| ≥65 | 63 (58.9) |
| CKD stage | |
| 3 | 33 (30.8) |
| 4 | 42 (39.3) |
| 5 | 32 (29.9) |
| Education years | |
| <high school | 21 (19.6) |
| >high school | 86 (80.4) |
| Smoking status | |
| Non-smokers | 66 (61.7) |
| Ex-smokers | 29 (27.1) |
| Current smokers | 12 (11.2) |
| Alcohol drinking | 3 (2.8) |
| BMIs (kg/m2) | 25.9 ± 4.6 |
| <24.0 | 35 (32.7) |
| ≥24.0 | 72 (67.3) |
| Waist circumferences (cm) | 89.2 ± 12.1 |
| Central obesity | |
| No | 40 (37.4) |
| Yes | 67 (62.6) |
| Systolic BP (mmHg) | 135.8 ± 22.7 |
| Diastolic BP (mmHg) | 72.8 ± 9.0 |
| Triglycerides (mg/dL) | 145.1 ± 96.1 |
| Total cholesterol (mg/dL) | 157.0 ± 36.2 |
| HbA1c (%) | 6.2 ± 1.2 |
| Blood urea nitrogen (mg/dL) | 45.6 ± 23.2 |
| Serum creatinine (mg/dL) | 3.3 ± 2.0 |
| eGFR (mL/min/1.73 m2) | 24.4 ± 12.9 |
| Nutrient | FFQ 1 | 3-Day DR 1 | % Difference 2 | p3,4 | Pearson Correlation Coefficient(r) 5 | |
|---|---|---|---|---|---|---|
| Crude 6 | Adjusted 7 | |||||
| Energy (kcal/day) | 1522.9 ± 435.7 | 1543.1 ± 350.3 | 0.7 | 0.273 3 | 0.562 ** | - |
| Protein (g/day) | 43.5 ± 13.2 | 54.3 ± 15.9 | −16.7 | <0.001 3 | 0.573 ** | 0.722 ** |
| Fat (g/day) | 60.6 ± 24.9 | 55.7 ± 17.5 | 14.5 | 0.157 3 | 0.494 ** | 0.619 ** |
| Carbohydrate (g/day) | 200.7 ± 64.0 | 215.5 ± 53.9 | −3.5 | 0.006 3 | 0.426 ** | 0.593 ** |
| Fiber (g/day) | 13.1 (10.6–16.8) | 12.5 (8.8–16.3) | 15.3 | 0.077 4 | 0.571 ** | 0.572 ** |
| Potassium (mg/day) | 1560.8 (1240.6–1910.5) | 1525.0 (1119.9–1925.9) | 11.8 | 0.574 4 | 0.374 ** | 0.450 ** |
| Phosphorus (mg/day) | 774.3 (594.5–985.0) | 632.4 (514.1–829.3) | 27.1 | <0.001 4 | 0.366 ** | 0.611 ** |
| Calcium (mg/day) | 378.9 (252.3–595.5) | 378.1 (244.7–561.3) | 26.6 | 0.879 4 | 0.385 ** | 0.410 ** |
| Nutrient | Cross-Classification (%) | ||||
|---|---|---|---|---|---|
| Same Quartile | Adjacent Quartile | Same or an Adjacent Quartile | One Quartile Apart | Extreme Quartile | |
| Protein (g/day) | 25.2 | 38.3 | 63.5 | 25.2 | 11.2 |
| Fat (g/day) | 31.8 | 35.5 | 67.3 | 21.5 | 11.2 |
| Carbohydrate (g/day) | 33.6 | 35.5 | 69.1 | 21.5 | 9.3 |
| Fiber (g/day) | 35.5 | 47.7 | 83.2 | 15.9 | 0.9 |
| Potassium (mg/day) | 36.4 | 38.3 | 74.7 | 21.5 | 3.7 |
| Phosphorus (mg/day) | 35.5 | 34.6 | 70.1 | 23.4 | 6.5 |
| Calcium (mg/day) | 32.7 | 44.9 | 77.6 | 19.6 | 2.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, M.-C.; Hung, S.-C.; Tai, T.-H.; Lin, T.-Y.; Chang, C.-I.; Hsu, C.-C. Using a Short Food Frequency Questionnaire to Evaluate Macronutrients, Fiber, Phosphorus, Potassium, and Calcium in Adults with Stages 3–5 Chronic Kidney Disease. Int. J. Environ. Res. Public Health 2022, 19, 11998. https://doi.org/10.3390/ijerph191911998
Huang M-C, Hung S-C, Tai T-H, Lin T-Y, Chang C-I, Hsu C-C. Using a Short Food Frequency Questionnaire to Evaluate Macronutrients, Fiber, Phosphorus, Potassium, and Calcium in Adults with Stages 3–5 Chronic Kidney Disease. International Journal of Environmental Research and Public Health. 2022; 19(19):11998. https://doi.org/10.3390/ijerph191911998
Chicago/Turabian StyleHuang, Meng-Chuan, Szu-Chun Hung, Tsen-Hua Tai, Ting-Yun Lin, Chiao-I Chang, and Chih-Cheng Hsu. 2022. "Using a Short Food Frequency Questionnaire to Evaluate Macronutrients, Fiber, Phosphorus, Potassium, and Calcium in Adults with Stages 3–5 Chronic Kidney Disease" International Journal of Environmental Research and Public Health 19, no. 19: 11998. https://doi.org/10.3390/ijerph191911998
APA StyleHuang, M.-C., Hung, S.-C., Tai, T.-H., Lin, T.-Y., Chang, C.-I., & Hsu, C.-C. (2022). Using a Short Food Frequency Questionnaire to Evaluate Macronutrients, Fiber, Phosphorus, Potassium, and Calcium in Adults with Stages 3–5 Chronic Kidney Disease. International Journal of Environmental Research and Public Health, 19(19), 11998. https://doi.org/10.3390/ijerph191911998






