The Bright Side of Skin Autofluorescence Determination in Children and Adolescents with Newly Diagnosed Type 1 Diabetes Mellitus: A Potential Predictor of Remission?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Size Calculation
2.2. Participants
2.3. Demographic and Clinical Data
2.4. Biochemical Analyses
2.5. Skin Autofluorescence
2.6. Hospitalization and Initiation of the Insulin Treatment
2.7. Statistical Analysis
3. Results
3.1. Group Characteristics
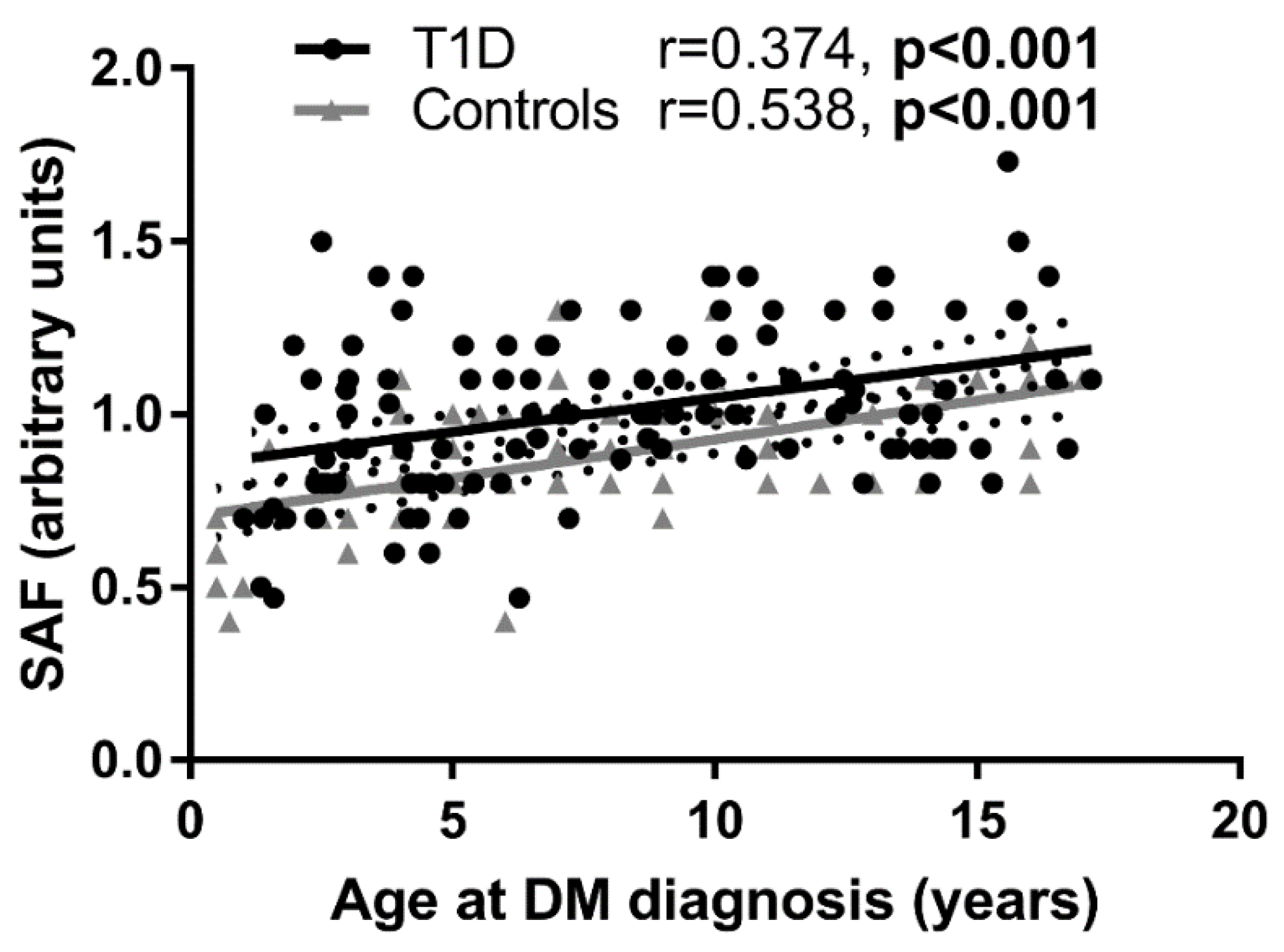
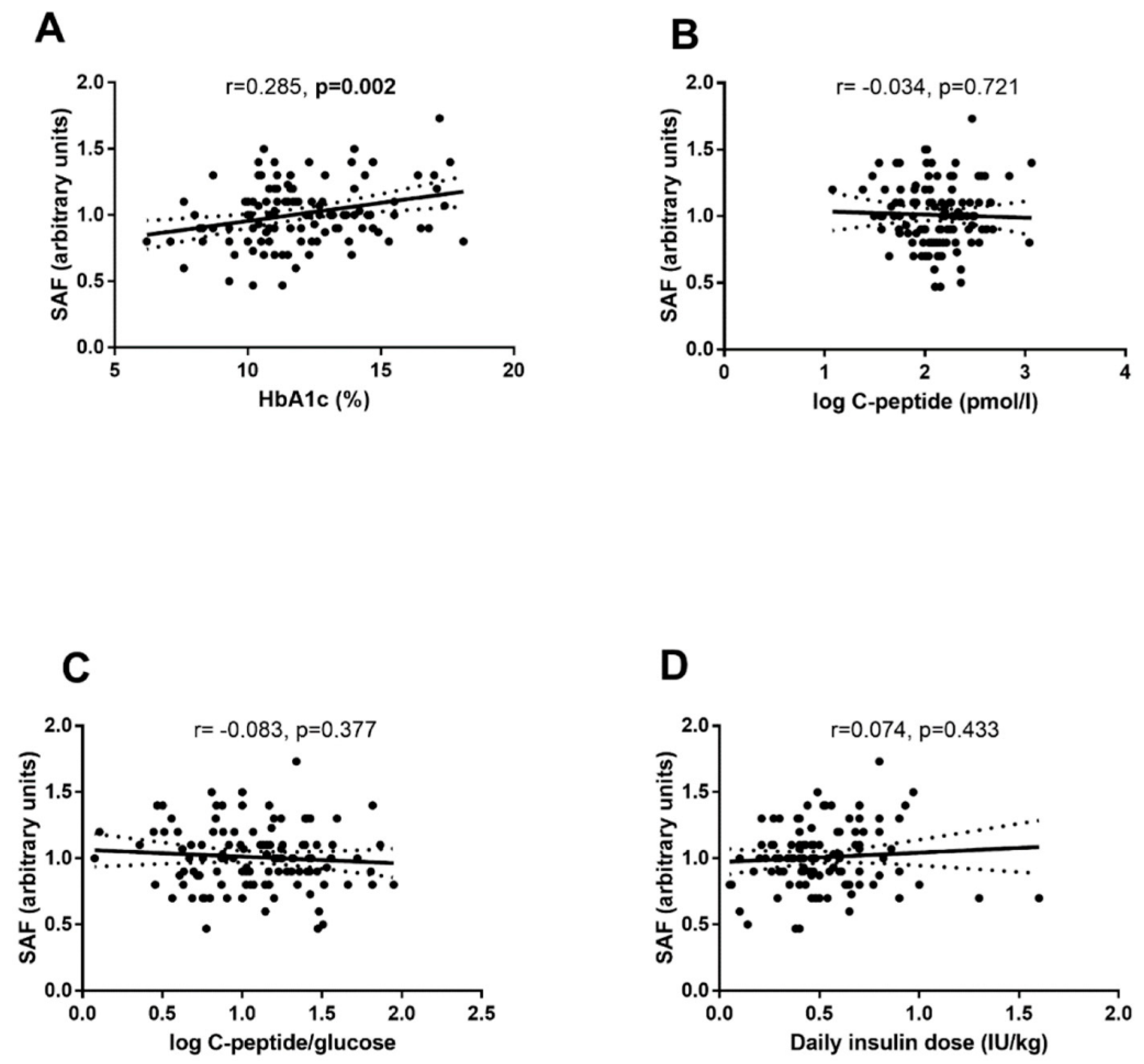
3.2. Variables at the Time of DM Diagnosis
3.3. Follow-Up Study
3.4. Effects of Diabetic Ketoacidosis
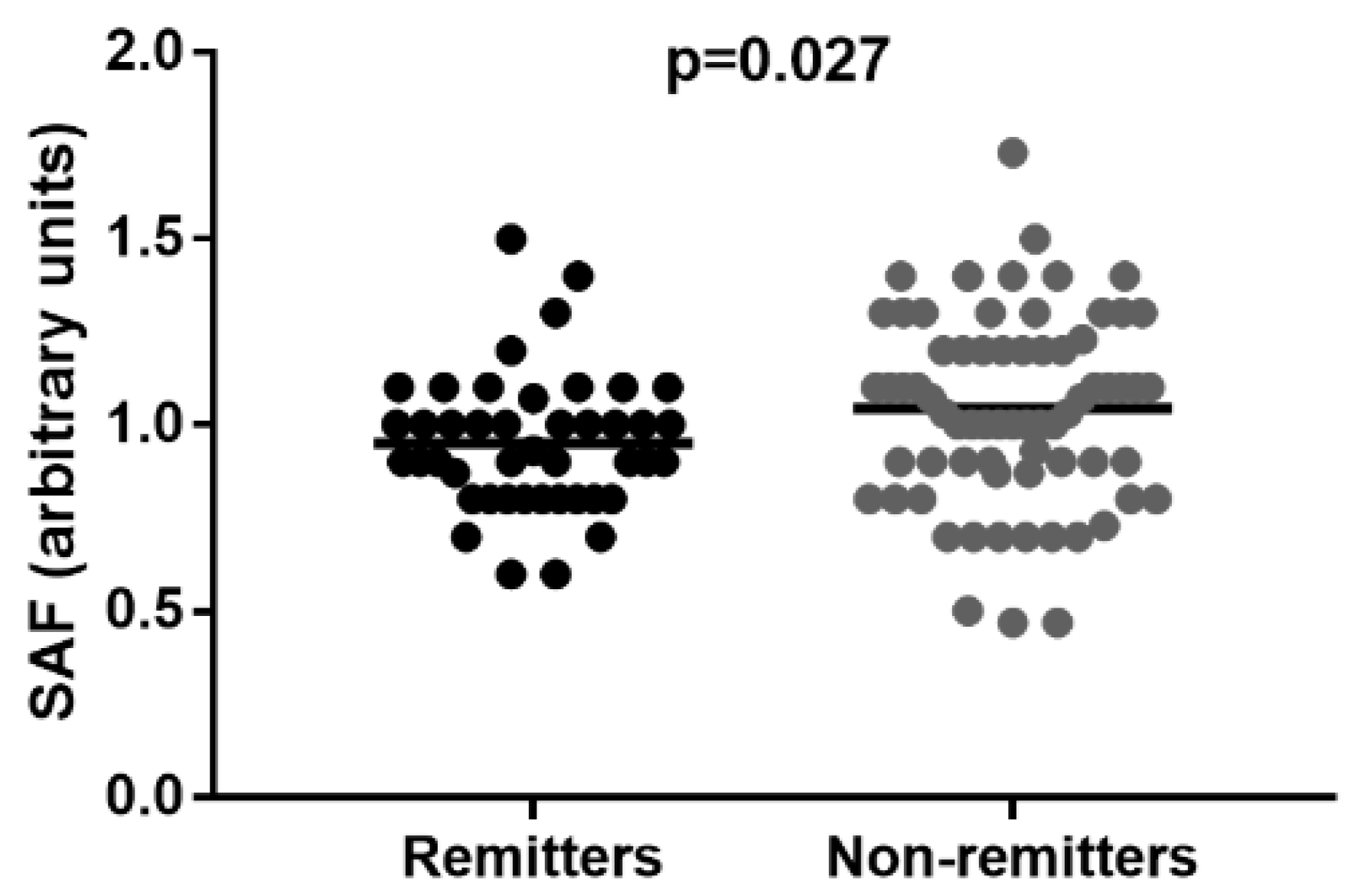
3.5. Remitters vs. Non-Remitters Group
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mayer-Davis, E.J.; Kahkoska, A.R.; Jefferies, C.; Dabelea, D.; Balde, N.; Gong, C.X.; Aschner, P.; Craig, M.E. ISPAD Clinical Practice Consensus Guidelines 2018: Definition, epidemiology, and classification of diabetes in children and adolescents. Pediatr. Diabetes 2018, 19 (Suppl. S27), 7–19. [Google Scholar] [CrossRef] [PubMed]
- Donaghue, K.C.; Marcovecchio, M.L.; Wadwa, R.P.; Chew, E.Y.; Wong, T.Y.; Calliari, L.E.; Zabeen, B.; Salem, M.A.; Craig, M.E. ISPAD Clinical Practice Consensus Guidelines 2018: Microvascular and macrovascular complications in children and adolescents. Pediatr. Diabetes 2018, 19 (Suppl. S27), 262–274. [Google Scholar] [CrossRef] [PubMed]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Nowotny, K.; Jung, T.; Hohn, A.; Weber, D.; Grune, T. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules 2015, 5, 194–222. [Google Scholar] [CrossRef]
- Cerami, C.; Founds, H.; Nicholl, I.; Mitsuhashi, T.; Giordano, D.; Vanpatten, S.; Lee, A.; Al-Abed, Y.; Vlassara, H.; Bucala, R.; et al. Tobacco smoke is a source of toxic reactive glycation products. Proc. Natl. Acad. Sci. USA 1997, 94, 13915–13920. [Google Scholar] [CrossRef]
- Cagalova, A.; Ticha, L.; Gaal Kovalcikova, A.; Sebekova, K.; Podracka, L. Bone mineral density and oxidative stress in adolescent girls with anorexia nervosa. Eur. J. Pediatr. 2022, 181, 311–321. [Google Scholar] [CrossRef]
- Sebekova, K.; Kupcova, V.; Schinzel, R.; Heidland, A. Markedly elevated levels of plasma advanced glycation end products in patients with liver cirrhosis—amelioration by liver transplantation. J. Hepatol. 2002, 36, 66–71. [Google Scholar] [CrossRef]
- Sebekova, K.; Klenovicsova, K.; Ferenczova, J.; Hedvig, J.; Podracka, L.; Heidland, A. Advanced oxidation protein products and advanced glycation end products in children and adolescents with chronic renal insufficiency. J. Ren. Nutr. 2012, 22, 143–148. [Google Scholar] [CrossRef]
- Domingueti, C.P.; Dusse, L.M.; Carvalho, M.; de Sousa, L.P.; Gomes, K.B.; Fernandes, A.P. Diabetes mellitus: The linkage between oxidative stress, inflammation, hypercoagulability and vascular complications. J. Diabetes Complicat. 2016, 30, 738–745. [Google Scholar] [CrossRef]
- Tahara, Y.; Shima, K. Kinetics of HbA1c, glycated albumin, and fructosamine and analysis of their weight functions against preceding plasma glucose level. Diabetes Care 1995, 18, 440–447. [Google Scholar] [CrossRef]
- DiMeglio, L.A.; Acerini, C.L.; Codner, E.; Craig, M.E.; Hofer, S.E.; Pillay, K.; Maahs, D.M. ISPAD Clinical Practice Consensus Guidelines 2018: Glycemic control targets and glucose monitoring for children, adolescents, and young adults with diabetes. Pediatr. Diabetes 2018, 19 (Suppl. S27), 105–114. [Google Scholar] [CrossRef] [PubMed]
- Banser, A.; Naafs, J.C.; Hoorweg-Nijman, J.J.; van de Garde, E.M.; van der Vorst, M.M. Advanced glycation end products, measured in skin, vs. HbA1c in children with type 1 diabetes mellitus. Pediatr. Diabetes 2016, 17, 426–432. [Google Scholar] [CrossRef]
- Verzijl, N.; DeGroot, J.; Thorpe, S.R.; Bank, R.A.; Shaw, J.N.; Lyons, T.J.; Bijlsma, J.W.; Lafeber, F.P.; Baynes, J.W.; TeKoppele, J.M. Effect of collagen turnover on the accumulation of advanced glycation end products. J. Biol. Chem. 2000, 275, 39027–39031. [Google Scholar] [CrossRef] [PubMed]
- van der Heyden, J.C.; Birnie, E.; Mul, D.; Bovenberg, S.; Veeze, H.J.; Aanstoot, H.J. Increased skin autofluorescence of children and adolescents with type 1 diabetes despite a well-controlled HbA1c: Results from a cohort study. BMC Endocrinol. Disord. 2016, 16, 49. [Google Scholar] [CrossRef] [PubMed]
- Vlassara, H.; Striker, G.E. Advanced glycation endproducts in diabetes and diabetic complications. Endocrinol. Metab. Clin. N. Am. 2013, 42, 697–719. [Google Scholar] [CrossRef]
- Bos, D.C.; de Ranitz-Greven, W.L.; de Valk, H.W. Advanced glycation end products, measured as skin autofluorescence and diabetes complications: A systematic review. Diabetes Technol. Ther. 2011, 13, 773–779. [Google Scholar] [CrossRef]
- Ahmed, N.; Thornalley, P.J. Advanced glycation endproducts: What is their relevance to diabetic complications? Diabetes Obes. Metab. 2007, 9, 233–245. [Google Scholar] [CrossRef]
- Goh, S.Y.; Cooper, M.E. Clinical review: The role of advanced glycation end products in progression and complications of diabetes. J. Clin. Endocrinol. Metab. 2008, 93, 1143–1152. [Google Scholar] [CrossRef]
- Genuth, S.; Sun, W.; Cleary, P.; Sell, D.R.; Dahms, W.; Malone, J.; Sivitz, W.; Monnier, V.M.; Group, D.S.C.A.S. Glycation and carboxymethyllysine levels in skin collagen predict the risk of future 10-year progression of diabetic retinopathy and nephropathy in the diabetes control and complications trial and epidemiology of diabetes interventions and complications participants with type 1 diabetes. Diabetes 2005, 54, 3103–3111. [Google Scholar] [CrossRef]
- Barat, P.; Cammas, B.; Lacoste, A.; Harambat, J.; Vautier, V.; Nacka, F.; Corcuff, J.B. Advanced glycation end products in children with type 1 diabetes: Family matters? Diabetes Care 2012, 35, e1. [Google Scholar] [CrossRef] [Green Version]
- Cho, Y.H.; Craig, M.E.; Januszewski, A.S.; Benitez-Aguirre, P.; Hing, S.; Jenkins, A.J.; Donaghue, K.C. Higher skin autofluorescence in young people with Type 1 diabetes and microvascular complications. Diabet. Med. 2017, 34, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Klenovics, K.S.; Kollarova, R.; Hodosy, J.; Celec, P.; Sebekova, K. Reference values of skin autofluorescence as an estimation of tissue accumulation of advanced glycation end products in a general Slovak population. Diabet. Med. 2014, 31, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Kobzova, J.; Vignerova, J.; Blaha, P.; Krejcovsky, L.; Riedlova, J. The 6th nationwide anthropological survey of children and adolescents in the Czech Republic in 2001. Cent. Eur. J. Public Health 2004, 12, 126–130. [Google Scholar] [PubMed]
- Koetsier, M.; Lutgers, H.L.; de Jonge, C.; Links, T.P.; Smit, A.J.; Graaff, R. Reference Values of Skin Autofluorescence. Diabetes Technol. Ther. 2010, 12, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Wolfsdorf, J.I.; Glaser, N.; Agus, M.; Fritsch, M.; Hanas, R.; Rewers, A.; Sperling, M.A.; Codner, E. ISPAD Clinical Practice Consensus Guidelines 2018: Diabetic ketoacidosis and the hyperglycemic hyperosmolar state. Pediatr. Diabetes 2018, 19 (Suppl. S27), 155–177. [Google Scholar] [CrossRef]
- Couper, J.J.; Haller, M.J.; Greenbaum, C.J.; Ziegler, A.G.; Wherrett, D.K.; Knip, M.; Craig, M.E. ISPAD Clinical Practice Consensus Guidelines 2018: Stages of type 1 diabetes in children and adolescents. Pediatr. Diabetes 2018, 19 (Suppl. S27), 20–27. [Google Scholar] [CrossRef]
- Hoffman, W.H.; Kappler, F.; Passmore, G.G.; Mehta, R. Diabetic ketoacidosis and its treatment increase plasma 3-deoxyglucosone. Clin. Biochem. 2003, 36, 269–273. [Google Scholar] [CrossRef]
- Turk, Z.; Nemet, I.; Varga-Defteardarović, L.; Car, N. Elevated level of methylglyoxal during diabetic ketoacidosis and its recovery phase. Diabetes Metab. 2006, 32, 176–180. [Google Scholar] [CrossRef]
- Neelofar, K.; Ahmad, J. Amadori albumin in diabetic nephropathy. Indian J. Endocrinol. Metab. 2015, 19, 39–46. [Google Scholar] [CrossRef]
- Shah, S.; Baez, E.A.; Felipe, D.L.; Maynard, J.D.; Hempe, J.M.; Chalew, S.A. Advanced glycation endproducts in children with diabetes. J. Pediatr. 2013, 163, 1427–1431. [Google Scholar] [CrossRef]
- Mortensen, H.B.; Hougaard, P.; Swift, P.; Hansen, L.; Holl, R.W.; Hoey, H.; Bjoerndalen, H.; de Beaufort, C.; Chiarelli, F.; Danne, T.; et al. New definition for the partial remission period in children and adolescents with type 1 diabetes. Diabetes Care 2009, 32, 1384–1390. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| All | No Remission | Remission | p | |
|---|---|---|---|---|
| n (%, CI) | 114 | 70 (61.4, CI: 52.2–69.8) | 44 (38.6, CI: 30.2–47.8) | NA |
| Sex, boys (%) | 64 (56) | 39 (56) | 25 (57) | 1.000 |
| AT DIABETES DIAGNOSIS | ||||
| BMI SDS | −0.5 ± 1.1 | −0.7 ± 0.9 | −0.1 ± 1.0 | <0.001 |
| Age, years | 8.0 ± 4.5 | 7.3 ± 4.3 | 9.1 ± 4.7 | 0.041 |
| HbA1c, % (mmol/mol) | 11.9 ± 2.4 (107 ± 27) | 12.0 ± 2.6 (108 ± 28) | 11.9 ± 2.2 (106 ± 24) | 0.786 |
| C-peptide, pmol/L | 133.5; 80.5–219.5 | 121.0; 65.2–193.7 | 158.5; 99.7–255.7 | 0.019 |
| C-peptide/glucose ratio | 13.7; 6.5–24.8 | 10.3; 5.5–22.1 | 14.8; 9.8–27.0 | 0.032 |
| Diabetic ketoacidosis, yes (%) | 53 (46) | 35 (50) | 18 (41) | 0.441 |
| Antibodies, positive (%) | 107 (94) | 66 (94) | 41 (93) | 1.000 |
| Skin autofluorescence, arbitrary units | 1.01 ± 0.23 | 1.04 ± 0.26 | 0.95 ± 0.18 | 0.027 |
| Insulin daily dose at discharge (IU/kg/24 h) | 0.5 ± 0.2 | 0.6 ± 0.3 | 0.5 ± 0.2 | 0.034 |
| Duration of remission (days) | NA | NA | 201 ± 199 | 0.001 |
| FOLLOW-UP AFTER 1 YEAR OF DIABETES DURATION | ||||
| HbA1c, % (mmol/mol) | 7.6 ± 1.2 (60 ± 13) | 7.9 ± 1.2 (63 ± 13) | 7.1 ± 1.0 (54 ± 11) | 0.236 |
| Delta HbA1c, % (mmol/mol) | −4.3 ± 2.6 (−47 ± 29) | −4.1 ± 2.8 (−45 ± 31) | −4.7 ± 2.2 (−50 ± 25) | <0.001 |
| Mean HbA1c, % (mmol/mol) | 8.4 ± 0.9 (68 ± 11) | 8.7 ± 0.9 (72 ± 10) | 7.8 ± 0.8 (62 ± 9) | <0.001 |
| C-peptide, pmol/L | 121.0; 12.7–297.0 | 78.0; 9.2–202.5 | 263.5; 99.2–425.7 | <0.001 |
| C-peptide/glucose ratio | 13.7; 1.4–30.4 | 6.5; 1.0–17.6 | 20.4; 9.7–53.8 | <0.001 |
| Insulin daily dose, IU/kg/24 h | 0.6 ± 0.2 | 0.7 ± 0.2 | 0.5 ± 0.2 | <0.001 |
| All (n = 74) | Siblings (n = 43) | Non-Siblings (n = 31) | p | |
|---|---|---|---|---|
| Age, years | 7.9 ± 4.5 | 7.3 ± 3.9 | 8.6 ± 5.1 | 0.228 |
| Sex, boys (%) | 38 (51) | 21 (49) | 17 (55) | 0.644 |
| SAF (arbitrary units) | 0.88 ± 0.19 | 0.88 ± 0.2 | 0.88 ± 0.18 | 0.883 |
| T1D (n = 114) | Controls (n = 74) | p | |
|---|---|---|---|
| Age, years | 8.0 ± 4.5 | 7.9 ± 4.5 | 0.805 |
| Sex, boys (%) | 64 (56) | 38 (51) | 0.551 |
| SAF (arbitrary units) | 1.01 ± 0.23 | 0.88 ± 0.19 | p < 0.001 |
| Step | Parameter | ΔR2 | Standardized β | p Value |
|---|---|---|---|---|
| Independent variables: age, HbA1c, and SAF at DM diagnosis | ||||
| Dependent variable: HbA1c change in 1 year of DM duration (R2 = 0.786; p < 0.001; n = 113) | ||||
| 1 | HbA1c | 0.786 | 0.232 | <0.001 |
| Dependent variable: C-peptide after 1 year of DM duration (R2 = 0.434; p < 0.001; n = 114) | ||||
| 1 | age | 0.413 | 0.601 | <0.001 |
| HbA1c | 0.021 | 0.167 | 0.025 | |
| Dependent variable: C-peptide/glucose ratio after 1 year of DM duration (R2 = 0.421; p < 0.001; n = 114) | ||||
| 1 | age | 0.401 | 0.593 | <0.001 |
| 2 | HbA1c | 0.020 | 0.165 | 0.029 |
| Step | Parameter | ΔR2 | β (CI) | p Value |
|---|---|---|---|---|
| Dependent variable: remission (R2 = 0.140; p = 0.002; n = 114) | ||||
| Independent variables: age, HbA1c, and SAF at DM diagnosis | ||||
| 1 | SAF | 0.051 | 0.051 (0.007–0.390) | 0.004 |
| 2 | age | 0.089 | 1.151 (1.040–1.273) | 0.007 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Podolakova, K.; Barak, L.; Jancova, E.; Stanik, J.; Sebekova, K.; Podracka, L. The Bright Side of Skin Autofluorescence Determination in Children and Adolescents with Newly Diagnosed Type 1 Diabetes Mellitus: A Potential Predictor of Remission? Int. J. Environ. Res. Public Health 2022, 19, 11950. https://doi.org/10.3390/ijerph191911950
Podolakova K, Barak L, Jancova E, Stanik J, Sebekova K, Podracka L. The Bright Side of Skin Autofluorescence Determination in Children and Adolescents with Newly Diagnosed Type 1 Diabetes Mellitus: A Potential Predictor of Remission? International Journal of Environmental Research and Public Health. 2022; 19(19):11950. https://doi.org/10.3390/ijerph191911950
Chicago/Turabian StylePodolakova, Kristina, Lubomir Barak, Emilia Jancova, Juraj Stanik, Katarina Sebekova, and Ludmila Podracka. 2022. "The Bright Side of Skin Autofluorescence Determination in Children and Adolescents with Newly Diagnosed Type 1 Diabetes Mellitus: A Potential Predictor of Remission?" International Journal of Environmental Research and Public Health 19, no. 19: 11950. https://doi.org/10.3390/ijerph191911950
APA StylePodolakova, K., Barak, L., Jancova, E., Stanik, J., Sebekova, K., & Podracka, L. (2022). The Bright Side of Skin Autofluorescence Determination in Children and Adolescents with Newly Diagnosed Type 1 Diabetes Mellitus: A Potential Predictor of Remission? International Journal of Environmental Research and Public Health, 19(19), 11950. https://doi.org/10.3390/ijerph191911950





