Nine-Year Trends in Prevention of Thromboembolic Complications in Elderly Patients with Atrial Fibrillation Treated with NOACs
Abstract
:1. Introduction
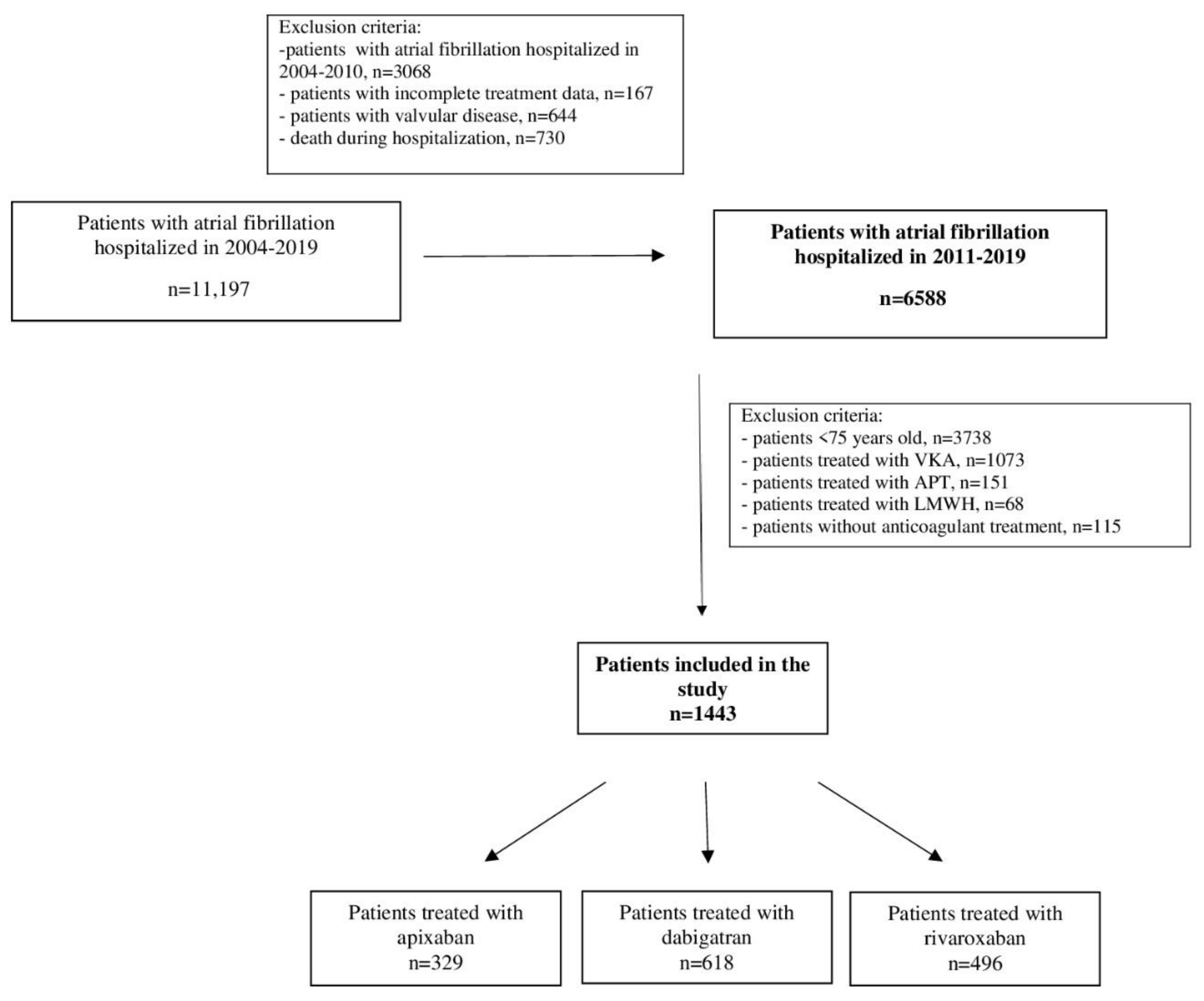
2. Materials and Methods
2.1. Study Group
2.2. Assessed Parameters
2.3. Assessment of the Thromboembolic Risk and Bleeding Risk
2.4. Prophylaxis of Thromboembolic Complications
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the Study Group
3.2. Predictors of the Use of Individual NOACs
4. Discussion
5. Study Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Go, A.S.; Mozaffarian, D.; Roger, V.L.; Benjamin, E.J.; Berry, J.D.; Borden, W.B.; Bravata, D.M.; Dai, S.; Ford, E.S.; Fox, C.S.; et al. Heart disease and stroke statistics—2013 update: A report from the American Heart Association. Circulation 2013, 127, e6–e245. [Google Scholar] [CrossRef] [PubMed]
- Wolf, P.A.; Abbott, R.D.; Kannel, W.B. Atrial fibrillation as an independent risk factor for stroke: The Framingham Study. Stroke 1991, 22, 938–983. [Google Scholar] [CrossRef] [PubMed]
- Bauersachs, R.M.; Herold, J. Oral Anticoagulation in the Elderly and Frail. Hamostaseologie 2020, 40, 74–83. [Google Scholar] [CrossRef]
- Tayaa, S.; Berrut, G.; de Decker, L.; Chevalet, P. Direct oral anticoagulants in non-valvular atrial fibrillation in elderly: For a treatment adapted to patient profile. Geriatr. Psychol. Neuropsychiatr. Vieil. 2018, 16, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Miyasaka, Y.; Barnes, M.E.; Gersh, B.J.; Cha, S.S.; Bailey, K.R.; Abhayaratna, W.P.; Seward, J.B.; Tsang, T.S.M. Secular trends in the prevalence of atrial fibrillation in Olmsted County, Minnesota 1980–2000 and implications for prognosis of future prevalence. Circulation 2006, 114, 119–125. [Google Scholar] [CrossRef]
- Lee, H.; Kim, T.H.; Baek, Y.S.; Uhm, J.S.; Pak, H.N.; Lee, M.H.; Joung, B. Inpatient visit trends for atrial fibrillation and their cost, treatment regimens and mortality in Korea: 10-year national cohort data. Korean Circ. J. 2017, 47, 56–64. [Google Scholar] [CrossRef]
- Siguret, V.; Gouin-Thibault, I.; Gaussem, P.; Pautas, E. Optimizing the use of anticoagulants (heparins and oral anticoagulants) in the elderly. Drugs Aging 2013, 30, 687–699. [Google Scholar] [CrossRef]
- Deedwania, P.C. New oral anticoagulants in elderly patients with atrial fibrillation. Am. J. Med. 2013, 126, 289–296. [Google Scholar] [CrossRef]
- Debray, M.; Pautas, E.; Couturier, P.; Franco, A.; Siguret, V. Oral anticoagulants in the elderly. Rev. Med. Interne 2003, 24, 107–117. [Google Scholar] [CrossRef]
- Camm, A.J.; Kirchhof, P.; Camm, A.J.; Schotten, U.; Savelieva, I.; Ernst, S.; Van Gelder, I.C.; Al-Attar, N.; Hindricks, G.; Prendergast, B.; et al. Guidelines for the management of atrial fibrillation: The task force for the management of atrial fibrillation of the European society of cardiology (ESC). Eur. Heart J. 2010, 31, 2369–2429. [Google Scholar] [CrossRef]
- Huiart, L.; Ferdynus, C.; Renoux, C.; Beaugrand, A.; Lafarge, S.; Bruneau, L.; Suissa, S.; Maillard, O.; Ranouil, X. Trends in initiation of direct oral anticoagulant therapies for atrial fibrillation in a national population- based cross-sectional study in the French health insurance databases. BMJ Open 2018, 8, e018180. [Google Scholar] [CrossRef] [PubMed]
- Adeboyeje, G.; Sylwestrzak, G.; Barron, J.J.; White, J.; Rosenberg, A.; Abarca, J.; Crawford, G.; Redberg, R. Major Bleeding Risk During Anticoagulation with Warfarin, Dabigatran, Apixaban, or Rivaroxaban in Patients with Nonvalvular Atrial Fibrillation. J. Manag. Care Spec. Pharm. 2017, 23, 968–978. [Google Scholar] [CrossRef] [PubMed]
- Yong, C.M.; Liu, Y.; Apruzzese, P.; Doros, G.; Cannon, C.P.; Maddox, T.M.; Gehi, A.; Hsu, J.C.; Lubitz, S.A.; Virani, S.; et al. Association of insurance type with receipt of oral anticoagulation in insured patients with atrial fibrillation: A report from the American College of Cardiology NCDR PINNACLE registry. Am. Heart J. 2018, 195, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, O.C.W.; Jonasson, C.; Ghanima, W.; Söderdahl, F.; Halvorsen, S. Comparison of dabigatran, rivaroxaban, and apixaban for effectiveness and safety in atrial fibrillation: A nationwide cohort study. Eur. Heart J. Cardiovasc. Pharmacother. 2020, 6, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, O.C.W.; Jonasson, C.; Ghanima, W.; Söderdahl, F.; Halvorsen, S. Effectiveness and safety of oral anticoagulants in elderly patients with atrial fibrillation. Heart 2022, 108, 345–352. [Google Scholar] [CrossRef]
- Zeitouni, M.; Giczewska, A.; Lopes, R.D.; Wojdyla, D.M.; Christersson, C.; Siegbahn, A.; De Caterina, R.; Steg, P.G.; Granger, C.B.; Wallentin, L.; et al. Clinical and Pharmacological Effects of Apixaban Dose Adjustment in the ARISTOTLE Trial. J. Am. Coll. Cardiol. 2020, 75, 1145–1155. [Google Scholar] [CrossRef]
- Steinberg, B.A.; Shrader, P.; Thomas, L.; Ansell, J.; Fonarow, G.C.; Gersh, B.J.; Hylek, E.; Kowey, P.R.; Mahaffey, K.W.; O’Brien, E.C.; et al. Factors associated with non-vitamin K antagonist oral anticoagulants for stroke prevention in patients with new-onset atrial fibrillation: Results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation II (ORBIT-AF II). Am. Heart J. 2017, 189, 40–47. [Google Scholar] [CrossRef]
- Yao, X.; Abraham, N.S.; Sangaralingham, L.R.; Bellolio, M.F.; McBane, R.D.; Shah, N.D.; Noseworthy, P.A. Effectiveness and Safety of Dabigatran, Rivaroxaban, and Apixaban Versus Warfarin in Nonvalvular Atrial Fibrillation. J. Am. Heart Assoc. 2016, 5, e003725. [Google Scholar] [CrossRef]
- Black-Maier, E.; Kim, S.; Steinberg, B.A.; Fonarow, G.C.; Freeman, J.V.; Kowey, P.R.; Ansell, J.; Gersh, B.J.; Mahaffey, K.W.; Naccarelli, G.; et al. Oral anticoagulation management in patients with atrial fibrillation undergoing cardiac implantable electronic device implantation. Clin. Cardiol. 2017, 40, 746–751. [Google Scholar] [CrossRef]
- Deharo, J.C.; Sciaraffia, E.; Leclercq, C.; Amara, W.; Doering, M.; Bongiorni, M.G.; Chen, J.; Dagres, M.; Estner, H.; Larsen, T.B.; et al. Perioperative management of antithrombotic treatment during implantation or revision of cardiac implantable electronic devices: The European Snapshot Survey on Procedural Routines for Electronic Device Implantation (ESS-PREDI). Europace 2016, 18, 778–784. [Google Scholar] [CrossRef]
- Tsai, C.T.; Liao, J.N.; Chao, T.F.; Lin, Y.J.; Chang, S.L.; Lo, L.W.; Hu, Y.F.; Chung, F.P.; Tuan, T.C.; Chen, S.A. Uninterrupted non-vitamin K antagonist oral anticoagulants during implantation of cardiac implantable electronic devices in patients with atrial fibrillation. J. Chin. Med. Assoc. 2019, 82, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Kosiuk, J.; Koutalas, E.; Doering, M.; Sommer, P.; Rolf, S.; Breithardt, O.; Nedios, S.; Dinov, B.; Hindricks, G.; Richter, S.; et al. Treatment with novel oral anticoagulants in a real-world cohort of patients undergoing cardiac rhythm device implantations. Europace 2014, 16, 1028–1032. [Google Scholar] [CrossRef] [PubMed]
- Shurrab, M.; Dennis, T.K.; McElhaney, J.; Henderson, M.; Danon, A.; Quinn, K.L.; O’Donnell, D.; Crystal, E.; Newman, D. Identifying Factors That Predict the Prescription of Non-vitamin K Antagonist Oral Anticoagulants in Older Individuals with Atrial Fibrillation. J. Am. Med. Dir. Assoc. 2019, 20, 984–987. [Google Scholar] [CrossRef] [PubMed]
- Pisters, R.; Lane, D.A.; Nieuwlaat, R.; de Vos, C.B.; Crijns, H.J.G.M.; Lip, G.Y.H. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: The Euro Heart Survey. Chest 2010, 138, 1093–1100. [Google Scholar] [CrossRef]
- Lip, G.Y.H.; Nieuwlaat, R.; Pisters, R.; Lane, D.A.; Crijns, H.J.G.M. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: The euro heart survey on atrial fibrillation. Chest 2010, 137, 263–272. [Google Scholar] [CrossRef]
- Ishii, M.; Ogawa, H.; Unoki, T.; An, Y.; Iguchi, M.; Masunaga, N.; Esato, M.; Chun, Y.H.; Tsuji, H.; Wada, H.; et al. Relationship of Hypertension and Systolic Blood Pressure with the Risk of Stroke or Bleeding in Patients with Atrial Fibrillation: The Fushimi AF Registry. Am. J. Hypertens. 2017, 30, 1073–1082. [Google Scholar] [CrossRef]
- Matsumoto, M.; Hori, M.; Tanahashi, N.; Momomura, S.I.; Uchiyama, S.; Goto, S.; Izumi, T.; Koretsune, Y.; Kajikawa, M.; Kato, M.; et al. Rivaroxaban versus warfarin in Japanese patients with non-valvular atrial fibrillation in relation to hypertension: A subgroup analysis of the J-ROCKET AF trial. Hypertens. Res. 2014, 37, 457–462. [Google Scholar] [CrossRef]
- Diener, H.C.; Aisenberg, J.; Ansell, J.; Atar, D.; Breithardt, G.; Eikelboom, J.; Ezekowitz, M.D.; Granger, C.B.; Halperin, J.L.; Hohnloser, S.H.; et al. Choosing a particular oral anticoagulant and dose for stroke prevention in individual patients with non-valvular atrial fibrillation: Part 2. Eur. Heart J. 2017, 38, 860–868. [Google Scholar] [CrossRef]
- Granger, C.B.; Alexander, J.H.; McMurray, J.J.V.; Lopes, R.D.; Hylek, E.M.; Hanna, M.; Al-Khalidi, H.R.; Ansell, J.; Atar, D.; Avezum, A.; et al. Apixaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2011, 365, 981–992. [Google Scholar] [CrossRef]
- Malik, A.H.; Yandrapalli, S.; Aronow, W.S.; Panza, J.A.; Cooper, H.A. Meta-Analysis of Direct-Acting Oral Anticoagulants Compared with Warfarin in Patients >75 Years of Age. Am. J. Cardiol. 2019, 123, 2051–2057. [Google Scholar] [CrossRef]
- Sharma, M.; Cornelius, V.R.; Patel, J.P.; Davies, J.G.; Molokhia, M. Efficacy and Harms of Direct Oral Anticoagulants in the Elderly for Stroke Prevention in Atrial Fibrillation and Secondary Prevention of Venous Thromboembolism: Systematic Review and Meta-Analysis. Circulation 2015, 132, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Hijazi, Z.; Hohnloser, S.H.; Oldgren, J.; Andersson, U.; Connolly, S.J.; Eikelboom, J.W.; Ezekowitz, M.D.; Reilly, P.A.; Siegbahn, A.; Yusuf, S.; et al. Efficacy and safety of dabigatran compared with warfarin in relation to baseline renal function in patients with atrial fibrillation: A RE-LY (Randomized Evaluation of Long-term Anticoagulation Therapy) trial analysis. Circulation 2014, 129, 961–970. [Google Scholar] [CrossRef]
- Noseworthy, P.A.; Yao, X.; Abraham, N.S.; Sangaralingham, L.R.; McBane, R.D.; Shah, N.D. Direct Comparison of Dabigatran, Rivaroxaban, and Apixaban for Effectiveness and Safety in Nonvalvular Atrial Fibrillation. Chest 2016, 150, 1302–1312. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.J.; Reichman, M.E.; Wernecke, M.; Hsueh, Y.H.; Izem, R.; Southworth, M.R.; Wei, Y.; Liao, J.; Goulding, M.G.; Mottet, K.; et al. Stroke, bleeding, and mortality risks in elderly Medicare beneficiaries treated with dabigatran or rivaroxaban for nonvalvular atrial fibrillation. JAMA Intern. Med. 2016, 176, 1662–1671. [Google Scholar] [CrossRef] [PubMed]



| Clinical Characteristic | All NOAC n = 1443 | Apixaban n = 329 | Dabigatran n = 618 | Rivaroxaban n = 496 | p |
|---|---|---|---|---|---|
| Age | |||||
| Mean (SD), years | 82.3 (5) | 83.8 (5.3) | 81.7 (4.8) | 81.9 (4.8) | <0.001 |
| Median (IQR) | 82 (8) | 84 (8) a | 81 (7) b | 82 (7) b | |
| Gender | |||||
| Female, n (%) | 836 (57.9) | 193 (58.7) | 338 (54.7) | 305 (61.5) | 0.070 |
| Type of atrial fibrillation n(%) | |||||
| Paroxysmal | 657 (45.5) | 148 (45) | 295 (47.7) | 214 (43.1) | 0.303 |
| Persistent | 154 (10.7) | 35 (10.6) | 57 (9.2) | 62 (12.5) | 0.212 |
| Permanent | 632 (43.8) | 146 (44.4) | 266 (43) | 220 (44.4) | 0.882 |
| Non-permanent | 811 (56.2) | 183 (55.6) | 352 (57) | 276 (55.6) | 0.882 |
| Medical history n(%) | |||||
| Hypertension | 1174 (81.3) | 259 (78.7) ab | 524 (84.8) b | 391 (78.8) a | 0.015 |
| Heart failure | 992 (68.7) | 237 (72) | 419 (67.8) | 336 (67.7) | 0.342 |
| Vascular disease | 693 (48) | 164 (49.8) | 301 (48.7) | 228 (46) | 0.498 |
| Previous myocardial infarction | 351 (24.3) | 93 (28.3) | 149 (24.1) | 109 (22) | 0.118 |
| PAD | 159 (11) | 45 (13.7) | 71 (11.5) | 43 (8.7) | 0.071 |
| Previous stroke/TIA/peripheral embolism | 244 (16.9) | 55 (16.7) | 111 (18) | 78 (15.7) | 0.610 |
| Diabetes mellitus | 439 (30.4) | 106 (32.2) | 183 (29.6) | 150 (30.2) | 0.704 |
| Any previous bleeding | 59 (4.1) | 27 (8.2) a | 20 (3.2) b | 12 (2.4) b | <0.001 |
| Ulcer | 36 (2.5) | 4 (1.2) | 15 (2.4) | 17 (3.4) | 0.136 |
| Malignancy | 81 (5.6) | 22 (6.7) | 31 (5) | 28 (5.6) | 0.568 |
| Thromboembolic risk | |||||
| CHA2DS2-VASC score | |||||
| Mean (SD) | 5.2 (1.4) | 5.3 (1.5) | 5.2 (1.4) | 5.2 (1.4) | 0.644 |
| Bleeding risk | |||||
| HAS-BLED score | |||||
| Mean (SD) | 2.2 (0.8) | 2.2 (0.7) | 2.3 (0.7) | 2.2 (0.8) | 0.026 |
| Median (IQR) | 2 (1) | 2 (1) a | 2 (1) b | 2 (1) ab | |
| ≥3, n (%) | 466 (32.3) | 88 (26.7) a | 214 (34.6) b | 164 (33.1) ab | 0.043 |
| Reason for hospitalisation, n(%) | |||||
| Electrical cardioversion | 96 (6.6) | 18 (5.5) | 42 (6.8) | 36 (7.3) | 0.591 |
| Planned coronarography/PCI/ACS | 115 (8) | 31 (9.4) | 44 (7.1) | 40 (8.1) | 0.458 |
| Heart failure | 414 (28.7) | 120 (36.5) a | 171 (27.7) b | 123 (24.8) b | 0.001 |
| Ablation | 17 (1.2) | 2 (0.6) | 5 (0.8) | 10 (2) | 0.122 |
| CIED | 301 (20.8) | 51 (15.5) a | 133 (21.5) ab | 116 (23.4) b | 0.017 |
| AF attack | 157 (10.9) | 32 (9.7) | 78 (12.6) | 47 (9.5) | 0.183 |
| Other | 343 (23.8) | 75 (22.8) | 145 (23.5) | 123 (24.8) | 0.781 |
| Laboratory tests | |||||
| Haemoglobin | |||||
| Mean (SD), g/dl | 12.9 (3.3) n = 1418 | 12.5 (1.7) n = 319 | 13.1 (4.7) n = 609 | 12.8 (1.6) n = 490 | <0.001 |
| Median (IQR) | 12.8 (2.1) | 12.4 (2.2) a | 12.9 (2.1) b | 12.9 (2) b | |
| Platelet | |||||
| Mean (SD), K/uL | 209.9 (75.3) n = 1410 | 207.6 (79.5) n = 318 | 207.2 (72.6) n = 604 | 214.6 (75.7) n = 488 | 0.113 |
| Median (IQR) | 198 (78) | 197.5 (93.8) | 194 (72) | 202.5 (76) | |
| eGFR | |||||
| Mean (SD), mL/min/1.73 m2 | 49.9 (14.8) n = 1438 | 45.8 (17.3) n = 327 | 52.3 (12.6) n = 616 | 49.7 (14.9) n = 495 | <0.001 |
| Median (IQR) | 49.3 (19.3) | 43 (24.8) a | 51.2 (17.2) b | 49.2 (19.6) c | |
| <60 mL/min/1.73 m2, n (%) | 1107 (77) n = 1438 | 264 (80.7) N = 327 | 458 (74.4) n = 616 | 385 (77.8) n = 495 | 0.088 |
| Echocardiographic findings | |||||
| Ejection fraction, mm | |||||
| Mean (SD) | 49.2 (20.3) n = 1185 | 47 (12.5) n = 269 | 50.3 (27.4) n = 516 | 49.4 (12) n = 400 | 0.021 |
| Median (IQR) | 50 (18) | 50 (17) a | 52 (17) b | 50 (15) ab | |
| Left ventricular systolic diameter, mm | |||||
| Mean (SD) | 36.3 (9.5) n = 1160 | 36.1 (9.2) n = 263 | 37 (10) n = 509 | 35.7 (8.8) n = 388 | 0.352 |
| Median (IQR) | 35 (11) | 35 (12) | 35 (12) | 34 (10) | |
| Left ventricular diastolic diameter, mm | |||||
| Mean (SD) | 50.2 (8) n = 1168 | 49.2 (8.5) n = 268 | 50.9 (8) n = 511 | 49.9 (7.6) n = 389 | 0.016 |
| Median (IQR) | 49 (10) | 48.5 (12) a | 50 (11) b | 49 (10) ab | |
| Reduced dose, n (%) | 920 (63.7) | 137 (41.6) a | 487 (78.8) b | 296 (59.7) c | <0.001 |
| Antiplatelet with NOAC, n (%) | 104 (7.2) | 31 (9.4) | 39 (6.3) | 34 (6.9) | 0.197 |
| Apixaban | Dabigatran | Rivaroxaban | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Factors | OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p |
| Age | 1.08 | 1.06–1.11 | <0.001 | 0.96 | 0.94–0.98 | <0.001 | 0.98 | 0.96–1.00 | 0.078 |
| Gender | 1.04 | 0.81–1.33 | 0.761 | 0.79 | 0.64–0.98 | 0.031 | 1.25 | 1.00–1.56 | 0.048 |
| Type of atrial fibrillation | |||||||||
| Paroxysmal | 0.97 | 0.76–1.24 | 0.821 | 1.17 | 0.95–1.44 | 0.146 | 0.86 | 0.69–1.08 | 0.188 |
| Persistent | 1.00 | 0.67–1.48 | 0.982 | 0.76 | 0.54–1.08 | 0.124 | 1.33 | 0.94–1.87 | 0.104 |
| Permanent | 1.03 | 0.81–1.32 | 0.810 | 0.95 | 0.77–1.17 | 0.617 | 1.04 | 0.83–1.29 | 0.758 |
| Non-permanent | 0.97 | 0.76–1.24 | 0.810 | 1.06 | 0.86–1.30 | 0.617 | 0.97 | 0.78–1.20 | 0.758 |
| Medical history | |||||||||
| Hypertension | 0.81 | 0.59–1.09 | 0.805 | 1.50 | 1.14–1.98 | 0.004 | 0.78 | 0.59–1.03 | 0.075 |
| Heart failure | 1.23 | 0.93–1.61 | 0.143 | 0.93 | 0.74–1.16 | 0.502 | 0.93 | 0.74–1.18 | 0.552 |
| Vascular disease | 1.10 | 0.86–1.41 | 0.451 | 1.05 | 0.85–1.29 | 0.654 | 0.88 | 0.71–1.10 | 0.258 |
| Previous myocardial infarction | 1.31 | 0.99–1.73 | 0.058 | 0.98 | 0.77–1.25 | 0.870 | 0.82 | 0.63–1.06 | 0.133 |
| PAD | 1.39 | 0.96–2.01 | 0.081 | 1.09 | 0.78–1.52 | 0.622 | 0.68 | 0.47–0.98 | 0.040 |
| Previous stroke/TIA/peripheral embolism | 0.98 | 0.71–1.37 | 0.916 | 1.14 | 0.86–1.50 | 0.356 | 0.58 | 0.26–1.29 | 0.183 |
| Diabetes mellitus | 1.16 | 0.86–1.45 | 0.420 | 0.94 | 0.75–1.17 | 0.562 | 0.99 | 0.78–1.25 | 0.914 |
| Any previous bleeding | 3.03 | 1.78–5.13 | <0.001 | 0.67 | 0.39–1.17 | 0.159 | 0.48 | 0.25–0.90 | 0.023 |
| Ulcer | 0.42 | 0.15–1.19 | 0.101 | 0.95 | 0.49–1.86 | 0.887 | 1.73 | 0.89–3.37 | 0.104 |
| Malignancy | 1.28 | 0.77–2.13 | 0.337 | 0.82 | 0.52–1.30 | 0.394 | 1.01 | 0.63–1.62 | 0.970 |
| Thromboembolic risk | |||||||||
| CHA2DS2-VASC score | 1.03 | 0.94–1.12 | 0.539 | 1.01 | 0.94–1.09 | 0.736 | 0.97 | 0.89–1.04 | 0.372 |
| Bleeding risk | |||||||||
| HAS-BLED score | 0.91 | 0.78–1.08 | 0.283 | 1.21 | 1.05–1.39 | 0.008 | 0.87 | 0.76–1.01 | 0.068 |
| ≥3, n (%) | 0.71 | 0.54–0.94 | 0.015 | 1.20 | 0.96–1.50 | 0.101 | 1.06 | 0.84–1.33 | 0.650 |
| Reason for hospitalisation | |||||||||
| Electrical cardioversion | 0.77 | 0.45–1.30 | 0.329 | 1.04 | 0.69–1.58 | 0.850 | 1.16 | 0.75–1.78 | 0.505 |
| Planned coronarography/PCI/ACS | 1.28 | 0.83–1.96 | 0.269 | 0.81 | 0.55–1.20 | 0.303 | 1.02 | 0.68–1.52 | 0.923 |
| Heart failure | 1.60 | 1.23–2.08 | <0.001 | 0.92 | 0.73–1.16 | 0.458 | 0.74 | 0.58–0.95 | 0.018 |
| Ablation | 0.45 | 0.10–1.97 | 0.288 | 0.55 | 0.19–1.58 | 0.268 | 2.76 | 1.05–7.30 | 0.040 |
| CIED | 0.63 | 0.46–0.88 | 0.007 | 1.07 | 0.83–1.39 | 0.592 | 1.28 | 0.99–1.66 | 0.065 |
| AF attack | 0.85 | 0.57–1.28 | 0.445 | 1.36 | 0.98–1.90 | 0.067 | 0.80 | 0.56–1.14 | 0.216 |
| Laboratory test | |||||||||
| Haemoglobin | 0.86 | 0.79–0.93 | <0.001 | 1.10 | 1.03–1.17 | 0.006 | 0.99 | 0.96–1.03 | 0.749 |
| Platelet | 1.00 | 1.00–1.00 | 0.999 | 1.00 | 1.00–1.00 | 0.249 | 1.00 | 1.00–1.00 | 0.085 |
| eGFR | 0.97 | 0.97–0.98 | <0.001 | 1.10 | 1.01–1.03 | <0.001 | 1.00 | 0.99–1.01 | 0.724 |
| eGFR < 60 mL/min/1.73 m2 | 1.31 | 0.96–1.77 | 0.085 | 0.78 | 0.61–0.99 | 0.043 | 1.08 | 0.83–1.40 | 0.556 |
| Echocardiographic findings | |||||||||
| Ejection fraction | 0.99 | 0.98–1.00 | 0.007 | 1.01 | 1.00–1.01 | 0.166 | 1.00 | 1.00–1.01 | 0.857 |
| Left ventricular systolic diameter | 1.00 | 0.98–1.01 | 0.633 | 1.01 | 1.00–1.03 | 0.048 | 0.99 | 0.98–1.00 | 0.097 |
| Left ventricular diastolic diameter | 0.98 | 0.96–1.00 | 0.015 | 1.02 | 1.01–1.04 | 0.005 | 0.99 | 0.98–1.01 | 0.440 |
| Reduced dose | 0.30 | 0.23–0.39 | <0.001 | 3.37 | 2.66–4.26 | <0.001 | 0.77 | 0.61–0.96 | 0.020 |
| Antiplatelet with NOAC | 1.48 | 0.96–2.30 | 0.079 | 0.79 | 0.52–1.19 | 0.255 | 0.92 | 0.60–1.41 | 0.708 |
| Apixaban 1 | Dabigatran 2 | Rivaroxaban 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Factors | OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p |
| Age | 1.08 | 1.05–1.11 | <0.001 | 0.96 | 0.94–0.98 | <0.001 | 0.98 | 0.96–1.00 | 0.094 |
| Gender | 0.99 | 0.76–1.29 | 0.916 | 0.82 | 0.65–1.02 | 0.071 | 1.25 | 0.99–1.58 | 0.058 |
| Hypertension | 0.80 | 0.58–1.10 | 0.168 | 1.56 | 1.18–2.06 | 0.022 | 0.74 | 0.56–0.98 | 0.034 |
| PAD | 1.20 | 0.82–1.76 | 0.351 | 1.17 | 0.83–1.64 | 0.373 | 0.72 | 0.49–1.04 | 0.081 |
| Any previous bleeding | 2.94 | 1.71–5.06 | <0.001 | 0.68 | 0.39–1.18 | 0.167 | 0.50 | 0.26–0.95 | 0.034 |
| Heart failure | 1.28 | 0.96–1.71 | 0.093 | 0.98 | 0.76–1.26 | 0.873 | 0.83 | 0.63–1.08 | 0.157 |
| Ablation | 0.59 | 0.13–2.64 | 0.491 | 0.52 | 0.18–1.51 | 0.228 | 2.39 | 0.89–6.41 | 0.084 |
| CIED | 0.63 | 0.44–0.90 | 0.011 | 1.11 | 0.84–1.46 | 0.470 | 1.25 | 0.94–1.66 | 0.126 |
| eGFR < 60 mL/min/1.73 m2 | 1.20 | 0.87–1.66 | 0.266 | 0.83 | 0.65–1.08 | 0.164 | 1.07 | 0.82–1.41 | 0.604 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bielecka, B.; Gorczyca-Głowacka, I.; Wożakowska-Kapłon, B. Nine-Year Trends in Prevention of Thromboembolic Complications in Elderly Patients with Atrial Fibrillation Treated with NOACs. Int. J. Environ. Res. Public Health 2022, 19, 11938. https://doi.org/10.3390/ijerph191911938
Bielecka B, Gorczyca-Głowacka I, Wożakowska-Kapłon B. Nine-Year Trends in Prevention of Thromboembolic Complications in Elderly Patients with Atrial Fibrillation Treated with NOACs. International Journal of Environmental Research and Public Health. 2022; 19(19):11938. https://doi.org/10.3390/ijerph191911938
Chicago/Turabian StyleBielecka, Bernadetta, Iwona Gorczyca-Głowacka, and Beata Wożakowska-Kapłon. 2022. "Nine-Year Trends in Prevention of Thromboembolic Complications in Elderly Patients with Atrial Fibrillation Treated with NOACs" International Journal of Environmental Research and Public Health 19, no. 19: 11938. https://doi.org/10.3390/ijerph191911938
APA StyleBielecka, B., Gorczyca-Głowacka, I., & Wożakowska-Kapłon, B. (2022). Nine-Year Trends in Prevention of Thromboembolic Complications in Elderly Patients with Atrial Fibrillation Treated with NOACs. International Journal of Environmental Research and Public Health, 19(19), 11938. https://doi.org/10.3390/ijerph191911938








