Experimental Investigation on Red Mud from the Bayer Process for Cemented Paste Backfill
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
- (1)
- Tailings
- (2)
- Binder
- (3)
- Activator
2.2. Methods
2.2.1. Experimental Programs
- (1)
- Self-consolidation characteristics of red mud
- (2)
- Cementitious characteristics of the binders
- (3)
- Cementitious characteristics of binders with activators
2.2.2. Specimen Preparation
2.2.3. Experimental Methods
- (1)
- UCS tests
- (2)
- Water immersion tests
- (3)
- Leaching experiments
- (4)
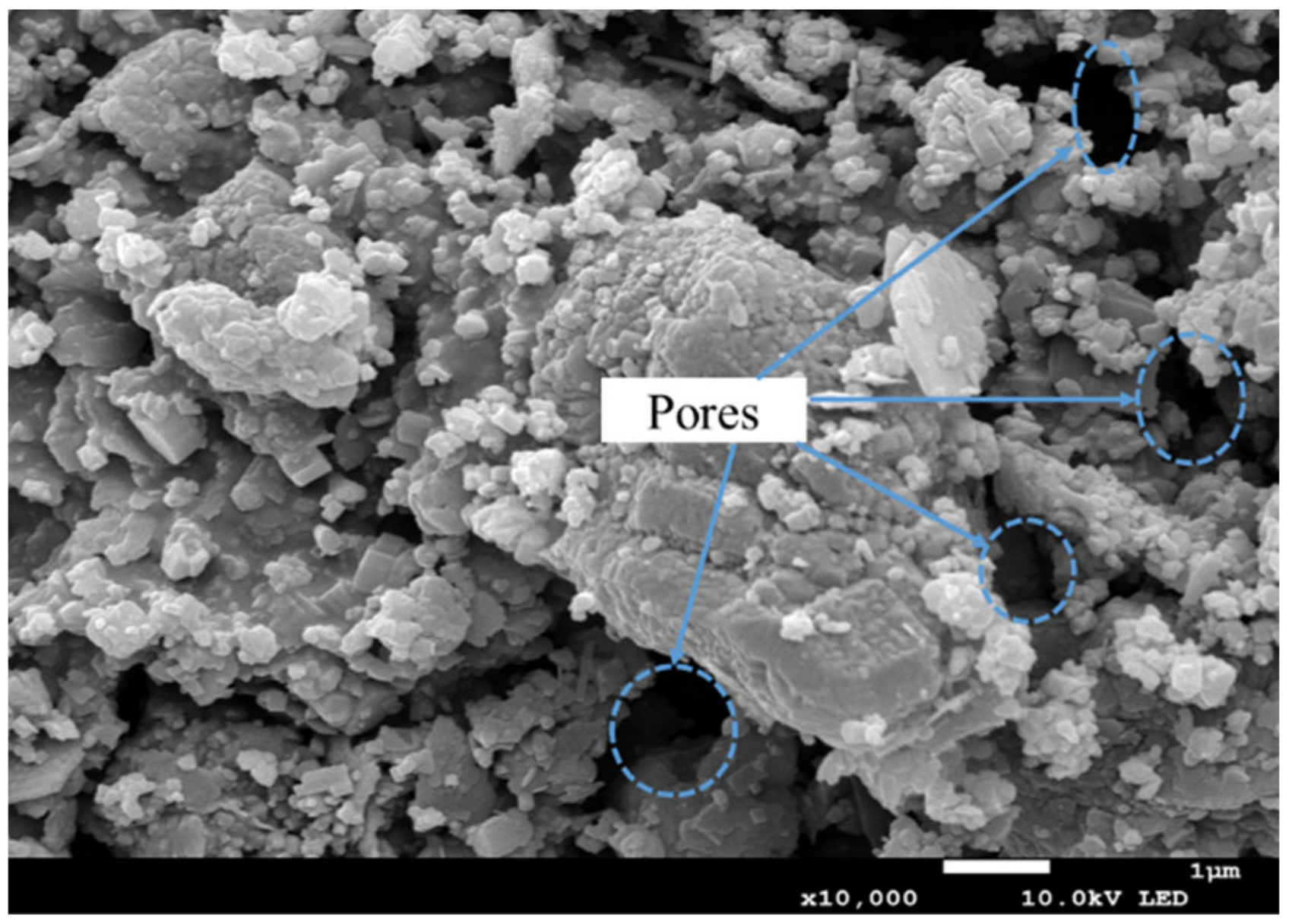
- Microstructural analysis
3. Results and Discussion
3.1. Strength Development of Red Mud-Based CPB under Standard Conditions
3.1.1. Strength Development of Specimens with Red Mud
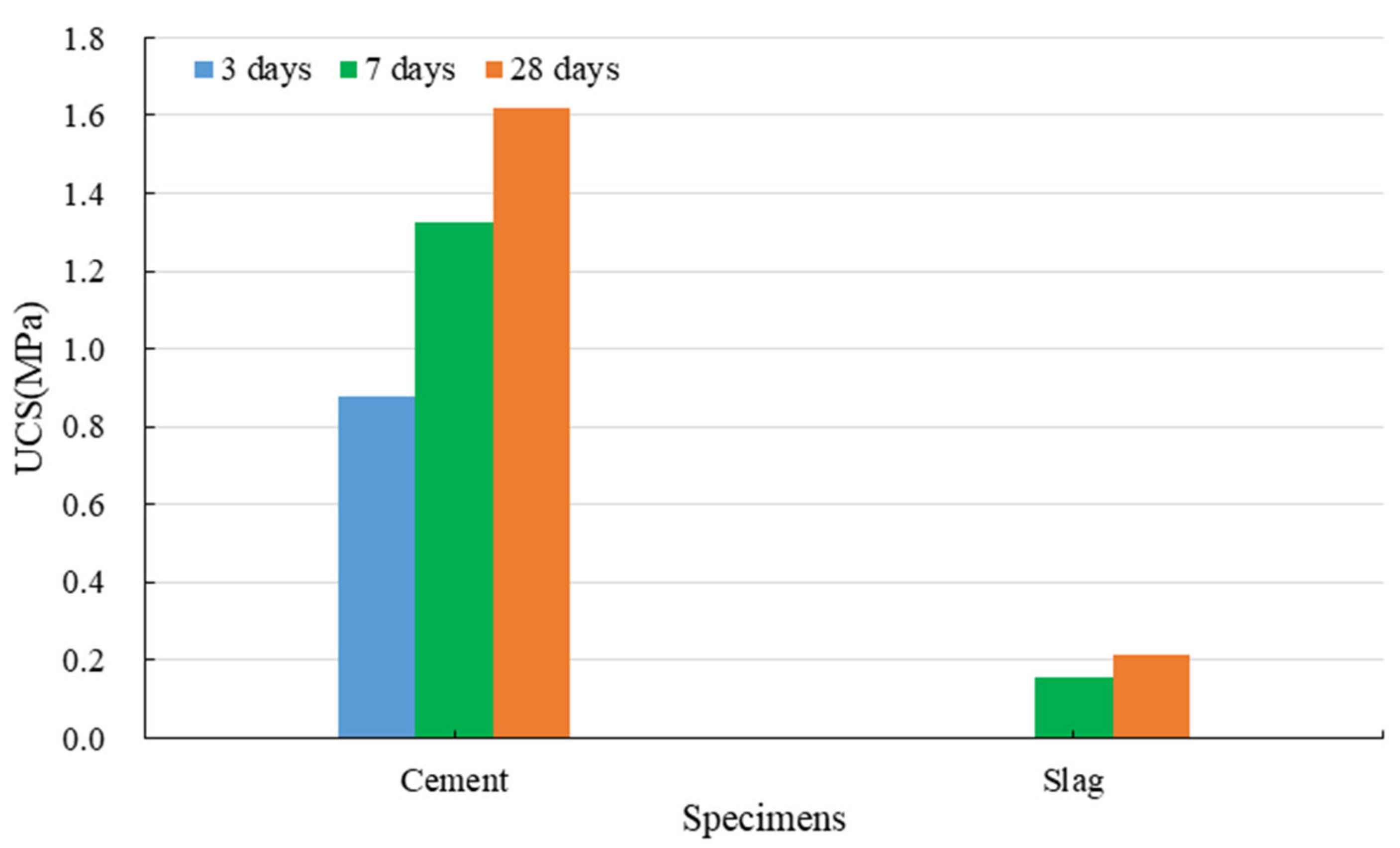
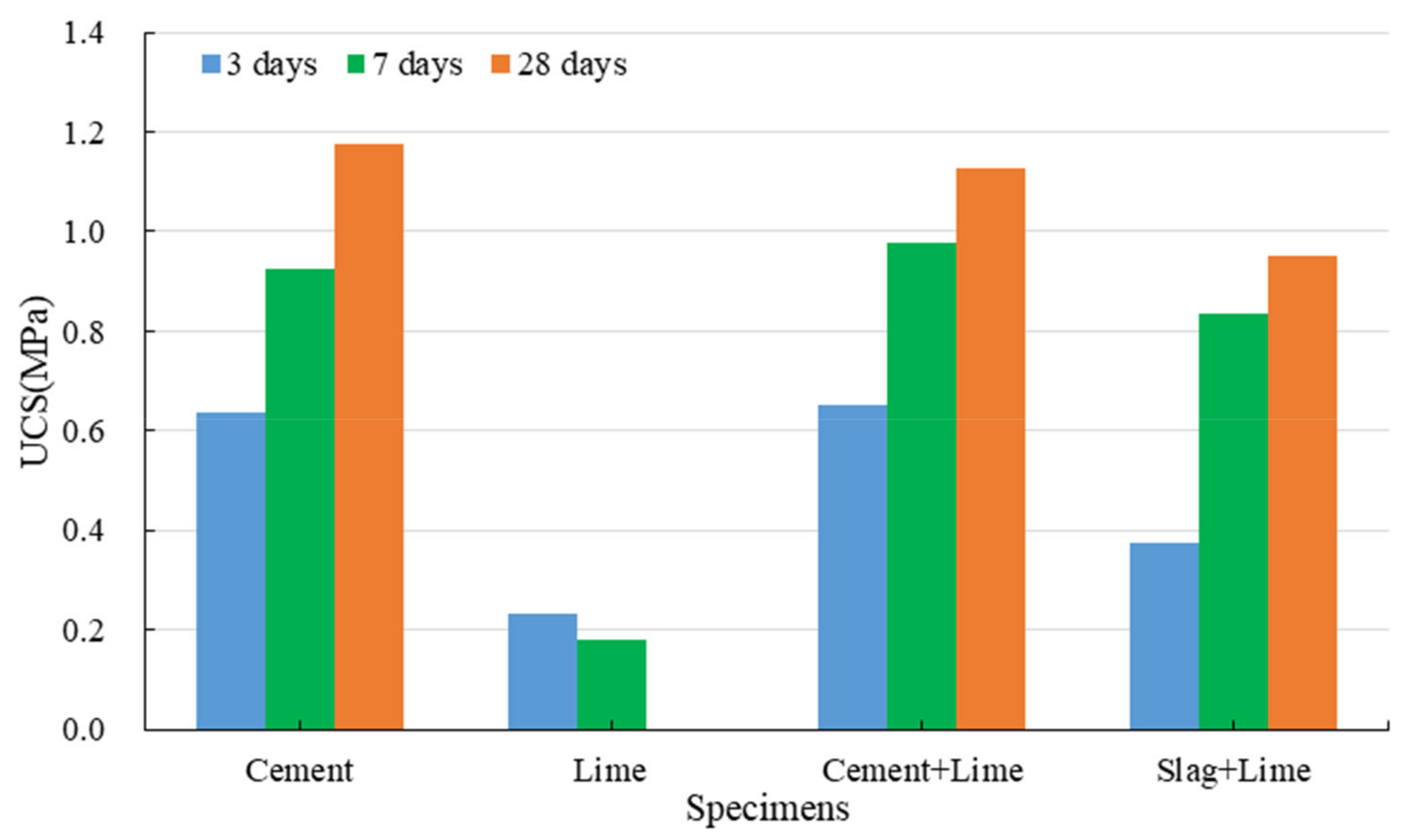
3.1.2. Strength Development of Red Mud-Based CPB with the Binder
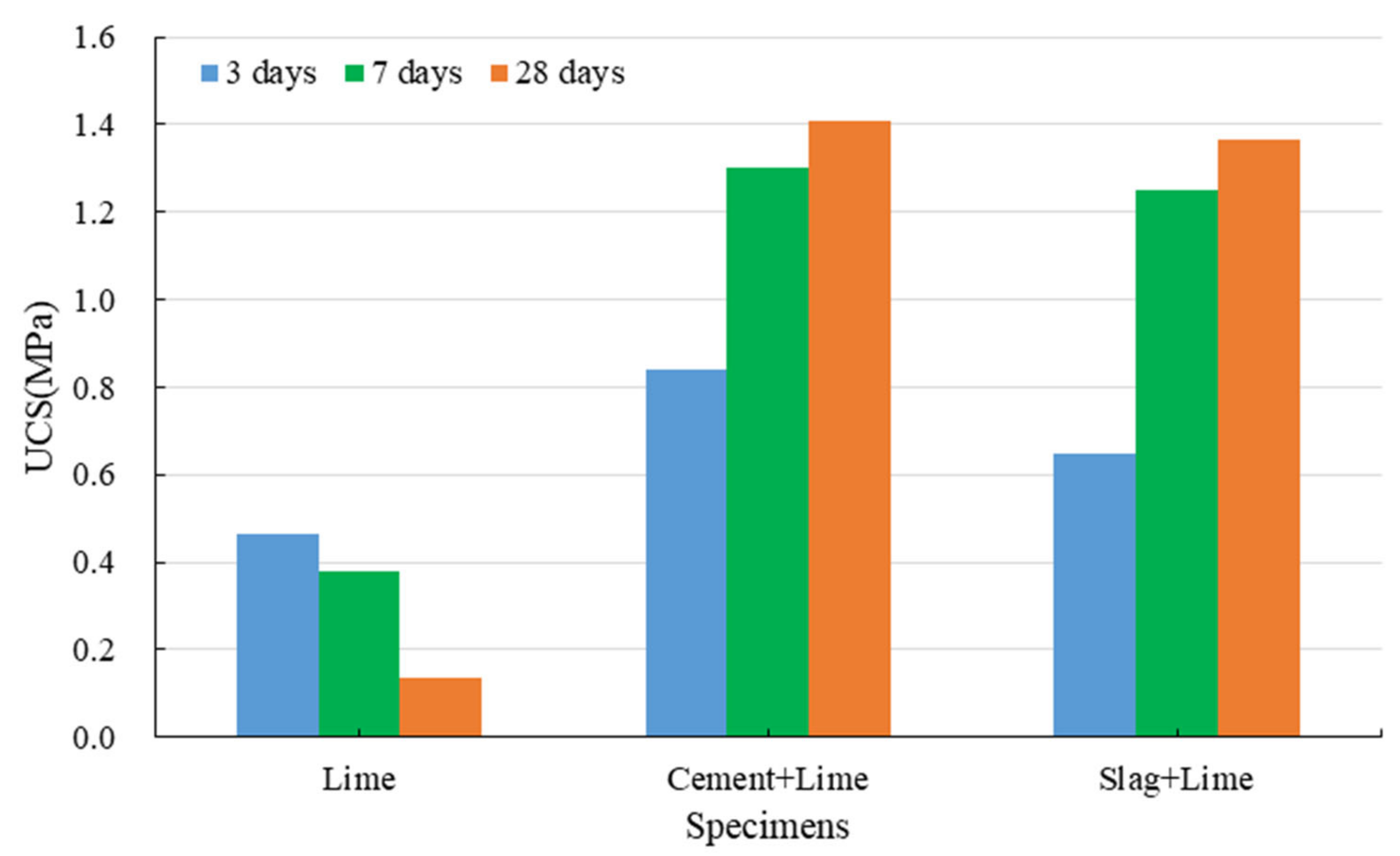
3.1.3. Strength Development of Red Mud-Based CPB with Lime
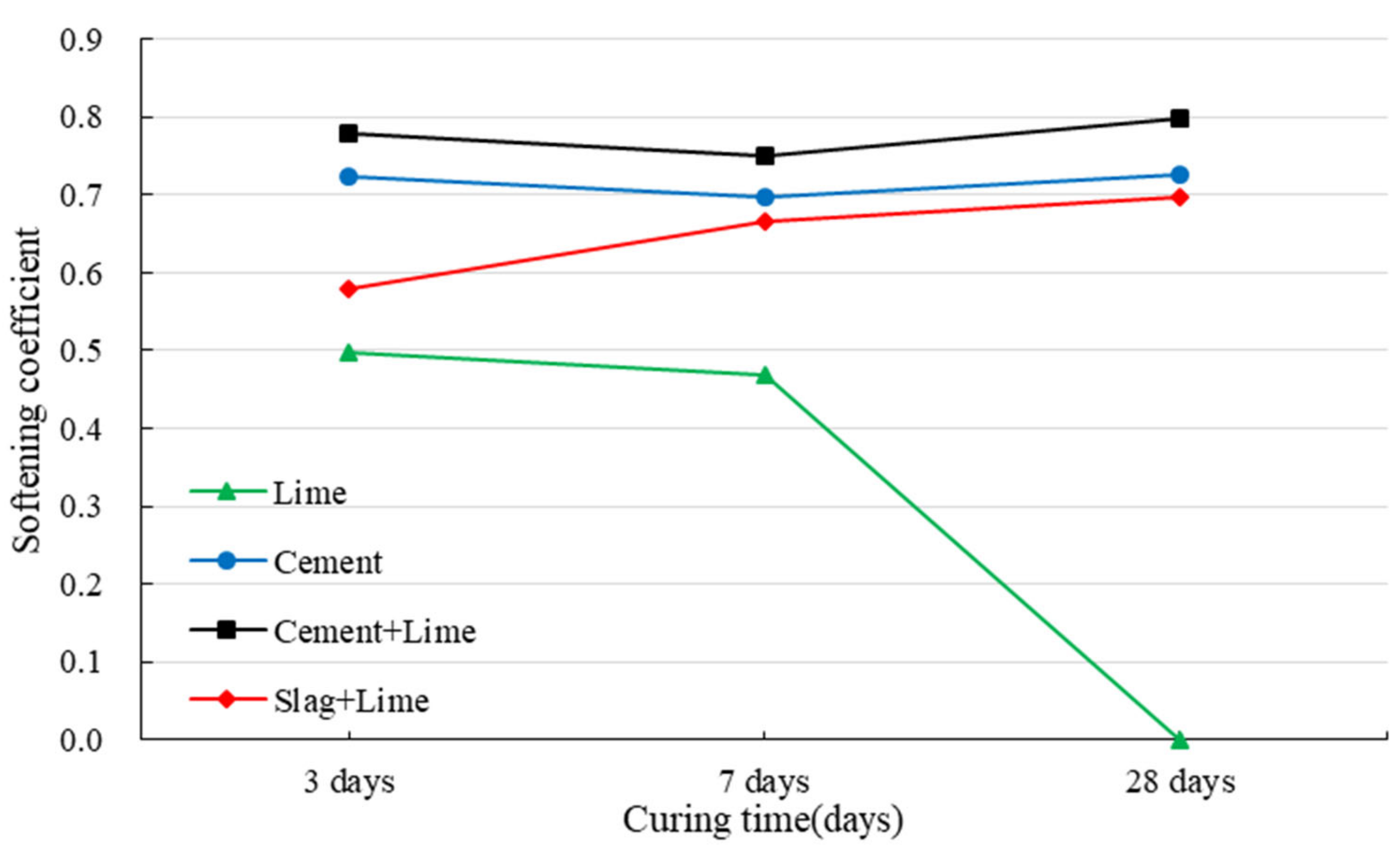
3.2. Strength Development of Red Mud-Based CPB under the Immersion Conditions
3.3. Leaching Experiments
4. Conclusions
- (1)
- Red mud from the Bayer process is obviously not an ideal backfilling aggregate due to the fine particle size distribution and strong alkaline. In addition, the specimens made of red mud show poor water resistance and disintegrate after being immersed in water.
- (2)
- Cement can effectively improve the mechanical properties and water resistance of red mud-based CPB. The specimens with cement have a softening coefficient of 0.71, and those with cement and lime have a softening coefficient of 0.77.
- (3)
- Slag cannot be used alone as a binder. However, red mud-based CPB prepared with slag and lime shows excellent mechanical properties and water resistance.
- (4)
- The addition of binders has a significant inhibitory effect on the leaching of hazardous substances in red mud under the solidification and stabilization effects. The leaching concentration of hexavalent chromium, selenium, fluoride, arsenic, lead, and vanadium is reduced by more than 70%.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Qu, Y.; Wu, S. Engineering geological properties and comprehensive utilization of the solid waste red mud in aluminium industry. Environ. Geol. 2001, 41, 249–256. [Google Scholar] [CrossRef]
- Archambo, M.; Kawatra, S.K. Red mud: Fundamentals and new avenues for utilization. Miner. Process. Extr. Metall. Rev. 2021, 42, 427–450. [Google Scholar] [CrossRef]
- Amritphale, S.S.; Anshul, A.; Chandra, N.; Ramakrishnan, N. A novel process for making radiopaque materials using bauxite-Red mud. J. Eur. Ceram. Soc. 2007, 27, 1945–1951. [Google Scholar] [CrossRef]
- Wang, S.; Jin, H.; Deng, Y.; Xiao, Y. Comprehensive utilization status of red mud in China: A critical review. J. Clean Prod. 2021, 289, 125136. [Google Scholar] [CrossRef]
- Liu, W.; Chen, X.; Li, W.; Yu, Y.; Yan, K. Environmental assessment, management and utilization of red mud in China. J. Clean Prod. 2014, 841, 606–610. [Google Scholar] [CrossRef]
- Khairul, M.A.; Zanganeh, J.; Moghtaderi, B. The composition, recycling and utilisation of Bayer red mud. Resour. Conserv. Recycl. 2019, 141, 483–498. [Google Scholar] [CrossRef]
- Wang, L.; Sun, N.; Tang, H.; Sun, W. A review on comprehensive utilization of red mud and prospect analysis. Minerals 2019, 9, 362. [Google Scholar] [CrossRef]
- Brunori, C.; Cremisini, C.; Massanisso, P.; Pinto, V.; Torricelli, L. Reuse of a treated red mud bauxite waste: Studies on environmental compatibility. J. Hazard. Mater. 2005, 1171, 55–63. [Google Scholar] [CrossRef]
- Zhu, X.; Li, W.; Guan, X. An active dealkalization of red mud with roasting and water leaching. J. Hazard. Mater. 2015, 286, 85–91. [Google Scholar] [CrossRef]
- Ning, G.; Zhang, B.; Liu, C.; Li, S.; Ye, Y.; Jiang, M. Large-scale consumption and zero-waste recycling method of red mud in steel making process. Minerals 2018, 8, 102. [Google Scholar] [CrossRef]
- Kounalakis, P.; Aravossis, K.; Karayianni, C.H.S. Feasibility study for an innovative industrial red mud utilisation method. Waste Manage. Res. 2016, 342, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Li, L. Properties of red mud tailings produced under varying process conditions. J. Environ. Eng. 1998, 1243, 254–264. [Google Scholar] [CrossRef]
- Cheng, X.; Long, D.; Zhang, C.; Gao, X.; Yu, Y.; Mei, K.; Zhang, C.; Guo, X.; Chen, Z. Utilization of red mud, slag and waste drilling fluid for the synthesis of slag-red mud cementitious material. J. Clean Prod. 2019, 238, 1–8. [Google Scholar] [CrossRef]
- Hertel, T.; Van den Bulck, A.; Onisei, S.; Sivakumar, P.P.; Pontikes, Y. Boosting the use of bauxite residue (red mud) in cement-Production of an Fe-rich calcium sulfoaluminate-ferrite clinker and characterisation of the hydration. Cem. Concr. Res. 2021, 145, 106463. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, Y.; Kim, M.; Park, H. Preparation of high porosity bricks by utilizing red mud and mine tailing. J. Clean Prod. 2019, 207, 490–497. [Google Scholar] [CrossRef]
- Hertel, T.; Pontikes, Y. Geopolymers, inorganic polymers, alkali-activated materials and hybrid binders from bauxite residue (red mud)—Putting things in perspective. J. Clean. Prod. 2020, 258, 120610. [Google Scholar] [CrossRef]
- Xu, X.; Song, J.; Li, Y.; Wu, J.; Liu, X.; Zhang, C. The microstructure and properties of ceramic tiles from solid wastes of Bayer red muds. Constr. Build. Mater. 2019, 212, 266–274. [Google Scholar] [CrossRef]
- Zhang, J.; Yao, Z.; Wang, K.; Wang, F.; Jiang, H.; Liang, M.; Wei, J.; Airey, G. Sustainable utilization of bauxite residue (Red Mud) as a road material in pavements: A critical review. Constr. Build. Mater. 2021, 270, 121419. [Google Scholar] [CrossRef]
- Mukiza, E.; Zhang, L.; Liu, X.; Zhang, N. Utilization of red mud in road base and subgrade materials: A review. Resour. Conserv. Recycl. 2019, 141, 187–199. [Google Scholar] [CrossRef]
- Samal, S. Utilization of red mud as a source for metal ions—A review. Materials 2021, 14, 2211. [Google Scholar] [CrossRef]
- Zinoveev, D.; Pasechnik, L.; Fedotov, M.; Dyubanov, V.; Grudinsky, P.; Alpatov, A. Extraction of valuable elements from red mud with a focus on using liquid media—A review. Recycling 2021, 6, 38. [Google Scholar] [CrossRef]
- Grudinsky, P.; Zinoveev, D.; Yurtaeva, A.; Kondratiev, A.; Dyubanov, V.; Petelin, A. Iron recovery from red mud using carbothermic roasting with addition of alkaline salts. J. Sustain. Metall. 2021, 7, 858–873. [Google Scholar] [CrossRef]
- Pasechnik, L.A.; Skachkov, V.M.; Chufarov, A.Y.; Suntsov, A.Y.; Yatsenko, S.P. High purity scandium extraction from red mud by novel simple technology. Hydrometallurgy 2021, 202, 105597. [Google Scholar] [CrossRef]
- Wu, W.; Chen, Z.; Huang, Y.; Li, J.; Chen, D.; Chen, N.; Su, M. Red mud for the efficient adsorption of U (VI) from aqueous solution: Influence of calcination on performance and mechanism. J. Hazard. Mater. 2021, 409, 124925. [Google Scholar] [CrossRef]
- Lyu, F.; Niu, S.; Wang, L.; Liu, R.; Sun, W.; He, D. Efficient removal of Pb (II) ions from aqueous solution by modified red mud. J. Hazard. Mater. 2021, 406, 124678. [Google Scholar] [CrossRef]
- Li, X.; Ji, M.; Nghiem, L.D.; Zhao, Y.; Liu, D.; Yang, Y. A novel red mud adsorbent for phosphorus and diclofenac removal from wastewater. J. Mol. Liq. 2020, 303, 112286. [Google Scholar] [CrossRef]
- Bian, J.; Fall, M.; Haruna, S. Sulphate induced changes in rheological properties of fibre-reinforced cemented paste backfill. Mag. Concr. Res. 2021, 73, 574–583. [Google Scholar] [CrossRef]
- Ghirian, A.; Fall, M. Coupled behavior of cemented paste backfill at early ages. Geotech. Geol. Eng. 2015, 33, 1141–1166. [Google Scholar] [CrossRef]
- Ghirian, A.; Fall, M. Strength evolution and deformation behaviour of cemented paste backfill at early ages: Effect of curing stress, filling strategy and drainage. Int. J. Min. Sci. Technol. 2016, 26, 809–817. [Google Scholar] [CrossRef]
- Ercikdi, B.; Cihangir, F.; Kesimal, A.; Deveci, H.; Alp, I. Utilization of industrial waste products as pozzolanic material in cemented paste backfill of high sulphide mill tailings. J. Hazard. Mater. 2009, 168, 848–856. [Google Scholar] [CrossRef]
- Pokharel, M.; Fall, M. Combined influence of sulphate and temperature on the saturated hydraulic conductivity of hardened cemented paste backfill. Cem. Concr. Compos. 2013, 38, 21–28. [Google Scholar] [CrossRef]
- Xu, W.; Cao, Y.; Liu, B. Strength efficiency evaluation of cemented tailings backfill with different stratified structures. Eng. Struct. J. 2019, 180, 18–28. [Google Scholar] [CrossRef]
- Ercikdi, B.; Yilmaz, T.; Külekci, G. Strength and ultrasonic properties of cemented paste backfill. Ultrasonics 2014, 54, 195–204. [Google Scholar] [CrossRef]
- Belem, T.; Benzaazoua, M. Design and application of underground mine paste backfill technology. Geotech. Geol. Eng. 2008, 26, 147–174. [Google Scholar] [CrossRef]
- Li, X.; Du, J.; Gao, L.; He, S.; Gan, L.; Sun, C.; Shi, Y. Immobilization of phosphogypsum for cemented paste backfill and its environmental effect. J. Clean Prod. 2017, 156, 137–146. [Google Scholar] [CrossRef]
- Jiao, H.; Wu, A.; Wang, H.; Yang, S.; Li, R.; Xiao, Y. The influence of cemented paste backfill on groundwater quality. Prog. Earth Planet. Sci. 2011, 2, 183–188. [Google Scholar] [CrossRef]
- Uibu, M.; Somelar, P.; Raado, L.M.; Irha, N.; Hain, T.; Koroljova, A.; Kuusik, R. Oil shale ash based backfilling concrete—Strength development, mineral transformations and leachability. Constr. Build. Mater. 2016, 102, 620–630. [Google Scholar] [CrossRef]
- Zhu, L.; Ni, W.; Zhang, X.; Huang, X. Performance and microstructure of cemented whole-tailings backfilling materials based on red mud, slag and cement. J. Univ. Sci. Technol. Beijing 2010, 32, 838–842. (In Chinese) [Google Scholar]
- Kesimal, A.; Yilmaz, E.; Ercikdi, B.; Alp, I.; Deveci, H. Effect of properties of tailings and binder on the short-and long-term strength and stability of cemented paste backfill. Mater. Lett. 2005, 59, 3703–3709. [Google Scholar] [CrossRef]
- Cihangir, F.; Ercikdi, B.; Kesimal, A.; Deveci, H.; Erdemir, F. Paste backfill of high-sulphide mill tailings using alkali-activated blast furnace slag: Effect of activator nature, concentration and slag properties. Miner. Eng. 2015, 83, 117–127. [Google Scholar] [CrossRef]
- Ercikdi, B.; Baki, H.; Izki, M. Effect of desliming of sulphide-rich mill tailings on the long-term strength of cemented paste backfill. J. Environ. Manag. 2013, 115, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; He, Y. Structural characteristics of cement-stabilized soil bases with 3D finite element method. Front. Archit. Civ. Eng. China 2009, 3, 428–434. [Google Scholar] [CrossRef]
- Ouellet, S.; Bussiere, B.; Aubertin, M.; Benzaazoua, M. Characterization of cemented paste backfill pore structure using SEM and IA analysis. Bull. Eng. Geol. Environ. 2008, 67, 139–152. [Google Scholar] [CrossRef]
- Rong, H.; Zhou, M.; Hou, H. Pore structure evolution and its effect on strength development of sulfate-containing cemented paste backfill. Minerals 2017, 7, 8. [Google Scholar] [CrossRef]
- Yilmaz, E.; Belem, T.; Bussière, B.; Benzaazoua, M. Relationships between microstructural properties and compressive strength of consolidated and unconsolidated cemented paste backfills. Cem. Concr. Compos. 2011, 33, 702–715. [Google Scholar] [CrossRef]
- Lu, N.; Likos, W.J. Suction stress characteristic curve for unsaturated soil. J. Geotech. Geoenviron. Eng. 2006, 132, 131–142. [Google Scholar] [CrossRef]
- Blight, G.E. Effective stress evaluation for unsaturated soils. J. Soil Mech. Found. Div. 1967, 93, 125–148. [Google Scholar] [CrossRef]
- Gallipoli, D.; Gens, A.; Sharma, R.; Vaunat, J. An elasto-plastic model for unsaturated soil incorporating the effects of suction and degree of saturation on mechanical behaviour. Geotechnique 2003, 53, 123–135. [Google Scholar] [CrossRef]
- Dong, Y.; Lu, N.; Fox, P. Drying-induced consolidation in soil. J. Geotech. Geoenviron. Eng. 2020, 146, 04020092. [Google Scholar] [CrossRef]
- Lu, N. Generalized soil water retention equation for adsorption and capillarity. J. Geotech. Geoenviron. Eng. 2016, 142, 04016051. [Google Scholar] [CrossRef]
- Lu, N.; Godt, J.W.; Wu, D.T. A closed-form equation for effective stress in unsaturated soil. Water Resour. Res. 2010, 46, W05515. [Google Scholar] [CrossRef]
- Wei, C. A theoretical framework for modeling the chemomechanical behavior of unsaturated soils. Vadose Zone J. 2014, 13, 1–21. [Google Scholar] [CrossRef]
- Scrivener, K.; Ouzia, A.; Juilland, P.; Mohamed, A.K. Advances in understanding cement hydration mechanisms. Cem. Concr. Res. 2019, 124, 105823. [Google Scholar] [CrossRef]
- Juilland, P.; Nicoleau, L.; Arvidson, R.S.; Gallucci, E. Advances in dissolution understanding and their implications for cement hydration. RILEM Tech. Lett. 2017, 2, 90. [Google Scholar] [CrossRef]
- Wang, Y.; Fall, M.; Wu, A. Initial temperature-dependence of strength development and self-desiccation in cemented paste backfill that contains sodium silicate. Cem. Concr. Compos. 2016, 67, 101–110. [Google Scholar] [CrossRef]
- Li, W.; Fall, M. Sulphate effect on the early age strength and self-desiccation of cemented paste backfill. Constr. Build. Mater. 2016, 106, 296–304. [Google Scholar] [CrossRef]
- Zhuang, S.; Wang, Q. Inhibition mechanisms of steel slag on the early-age hydration of cement. Cem. Concr. Res. 2021, 140, 106283. [Google Scholar] [CrossRef]
- Manzoor, S.O.; Yousuf, A. Stabilisation of soils with lime: A review. J. Mater. Environ. Sci. 2020, 11, 1538–1551. [Google Scholar]
- Baldovino, J.; Izzo, R.; Moreira, E.B.; Rose, J.L. Optimizing the evolution of strength for lime-stabilized rammed soil. J. Rock Mech. Geotech. Eng. 2019, 11, 882–891. [Google Scholar] [CrossRef]
- Dhar, S.; Hussain, M. The strength and microstructural behavior of lime stabilized subgrade soil in road construction. Int. J. Geotech. Eng. 2019, 15, 471–483. [Google Scholar] [CrossRef]
- Ghobadi, M.H.; Abdilor, Y.; Babazadeh, R. Stabilization of clay soils using lime and effect of pH variations on shear strength parameters. Bull. Eng. Geol. Environ. 2014, 73, 611–619. [Google Scholar] [CrossRef]
- Pavlík, Z.; Benešová, H.; Matiašovský, P.; Pavlíková, M. Study on carbonation process of several types of advanced lime-based plasters. World Acad. Sci. Eng. Technol. 2012, 70, 1005–1009. [Google Scholar]
- Cultrone, G.; Sebastián, E.; Huertas, M.O. Forced and natural carbonation of lime-based mortars with and without additives: Mineralogical and textural changes. Cem. Concr. Res. 2005, 35, 2278–2289. [Google Scholar] [CrossRef]
- Cizer, Ö.; Van Balen, K.; Elsen, J.; Van Gemert, D. Real-time investigation of reaction rate and mineral phase modifications of lime carbonation. Constr. Build. Mater. 2012, 35, 741–751. [Google Scholar] [CrossRef]
- Chen, Q.; He, Y.; Qi, C.; Zhang, Q.; Xiao, C. Lithium slag and fly ash-based binder for cemented fine tailings backfill. J. Environ. Manag. 2019, 248, 109282. [Google Scholar] [CrossRef]
- Feng, Y.; Kero, J.; Yang, Q.; Chen, Q.; Engström, F.; Samuelsson, C.; Qi, C. Mechanical activation of granulated copper slag and its influence on hydration heat and compressive. Materials 2019, 12, 772. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, B.; Feng, Y.; Qi, C.; Chen, Q.; Xiao, C. Hydration development of blended cement paste with granulated copper slag modified with CaO and Al2O3. J. Mater. Res. Technol. 2022, 18, 909–920. [Google Scholar] [CrossRef]
- Norouzian, K.; Abbasi, N.; Koupai, J.A. Evaluation of softening of clayey soil stabilized with sewage sludge ash and lime. Civ. Eng. J. 2018, 4, 743. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, Y.; Huang, S.; Tao, G. Properties and microstructure of cementing material made with waste silty clay soil. Eur. J. Environ. Civ. Eng. 2020, 24, 849–860. [Google Scholar] [CrossRef]
- Kiventerä, J.; Piekkari, K.; Isteri, V.; Ohenoja, K.; Tanskanen, P.; Illikainen, M. Solidification/stabilization of gold mine tailings using calcium sulfoaluminate-belite cement. J. Clean Prod. 2019, 239, 1–8. [Google Scholar] [CrossRef]
- Huang, Y.; Qian, J.; Lu, L.; Zhang, W.; Wang, S.; Wang, W.; Cheng, X. Phosphogypsum as a component of calcium sulfoaluminate cement: Hazardous elements immobilization, radioactivity and performances. J. Clean Prod. 2020, 248, 119287. [Google Scholar] [CrossRef]
- Pan, Y.; Rossabi, J.; Pan, C.; Xie, X. Stabilization/solidification characteristics of organic clay contaminated by lead when using cement. J. Hazard. Mater. 2019, 362, 132–139. [Google Scholar] [CrossRef] [PubMed]


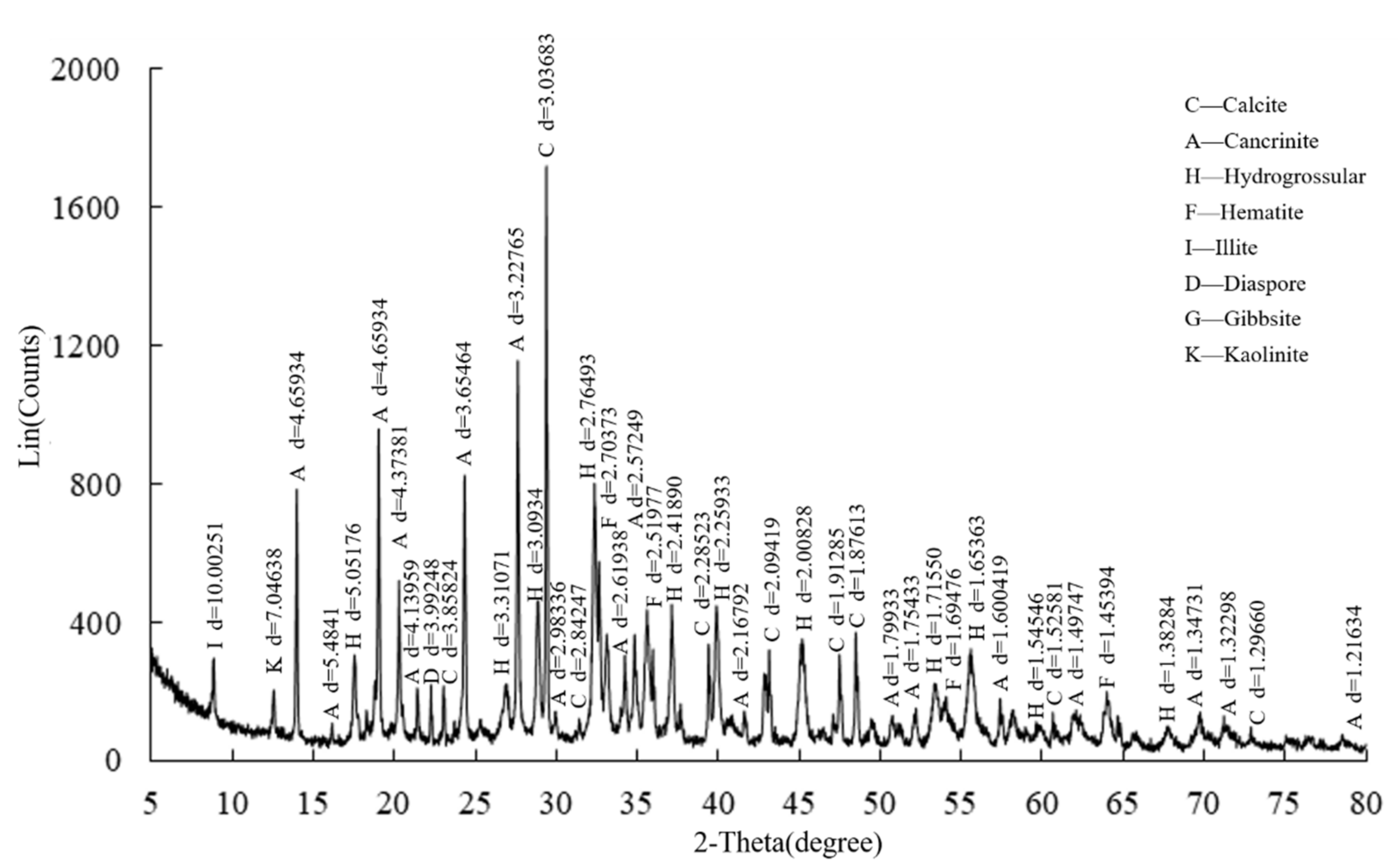


| Specific Gravity | Permeability Coefficient (cm/s) | Median Particle Size (μm) | Specific Surface Area (m2/kg) | Nonuniform Coefficient | Curvature Coefficient | pH |
|---|---|---|---|---|---|---|
| 2.424 | 3.35 × 10−7 | 3.248 | 2940 | 4.741 | 0.946 | 12.1 |
| Element Unit | CaO (wt. %) | SiO2 (wt. %) | Fe2O3 (wt. %) | Al2O3 (wt. %) | MgO (wt. %) | SO3 (wt. %) | LOI |
|---|---|---|---|---|---|---|---|
| Cement | 63.20 | 20.9 | 2.77 | 5.45 | 2.7 | 2.54 | - |
| Slag | 39.25 | 33.40 | 0.31 | 15.15 | 7.67 | 2.38 | 0.11 |
| Specimens | Mn | Cu | Zn | Hg | As | Se |
|---|---|---|---|---|---|---|
| Red mud sample | 0.002 | 0.03 | 0.02 | <0.0001 | 0.007 | 0.008 |
| Sample with cement | <0.001 | <0.01 | <0.01 | <0.0001 | 0.003 | 0.002 |
| Sample with slag | <0.001 | <0.01 | <0.01 | <0.0001 | 0.002 | 0.002 |
| Standards | ≤0.1 | ≤1 | ≤1 | ≤0.001 | ≤0.01 | ≤0.01 |
| Specimens | Cd | Cr6+ | Pb | Be | Sb | Ba |
| Red mud sample | <0.001 | 0.131 | 0.01 | <0.001 | <0.002 | <0.01 |
| Sample with cement | <0.001 | 0.028 | <0.001 | <0.001 | <0.002 | <0.01 |
| Sample with slag | <0.001 | 0.035 | <0.001 | <0.001 | <0.002 | <0.01 |
| Standards | ≤0.005 | ≤0.05 | ≤0.01 | ≤0.002 | ≤0.005 | ≤0.7 |
| Specimens | Ni | Co | Mo | Ag | V | Fluoride |
| Red mud sample | 0.01 | 0.02 | 0.11 | <0.01 | 1.05 | 10.6 |
| Sample with cement | <0.001 | <0.001 | 0.03 | <0.01 | 0.31 | 2.02 |
| Sample with slag | <0.001 | <0.001 | 0.03 | <0.01 | 0.28 | 1.75 |
| Standards | ≤0.02 | ≤0.05 | ≤0.07 | ≤0.05 | - | ≤1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bian, J.; Li, S.; Zhang, Q. Experimental Investigation on Red Mud from the Bayer Process for Cemented Paste Backfill. Int. J. Environ. Res. Public Health 2022, 19, 11926. https://doi.org/10.3390/ijerph191911926
Bian J, Li S, Zhang Q. Experimental Investigation on Red Mud from the Bayer Process for Cemented Paste Backfill. International Journal of Environmental Research and Public Health. 2022; 19(19):11926. https://doi.org/10.3390/ijerph191911926
Chicago/Turabian StyleBian, Jiwei, Shuai Li, and Qinli Zhang. 2022. "Experimental Investigation on Red Mud from the Bayer Process for Cemented Paste Backfill" International Journal of Environmental Research and Public Health 19, no. 19: 11926. https://doi.org/10.3390/ijerph191911926
APA StyleBian, J., Li, S., & Zhang, Q. (2022). Experimental Investigation on Red Mud from the Bayer Process for Cemented Paste Backfill. International Journal of Environmental Research and Public Health, 19(19), 11926. https://doi.org/10.3390/ijerph191911926







