Biodiversity Effects on Human Mental Health via Microbiota Alterations
Abstract
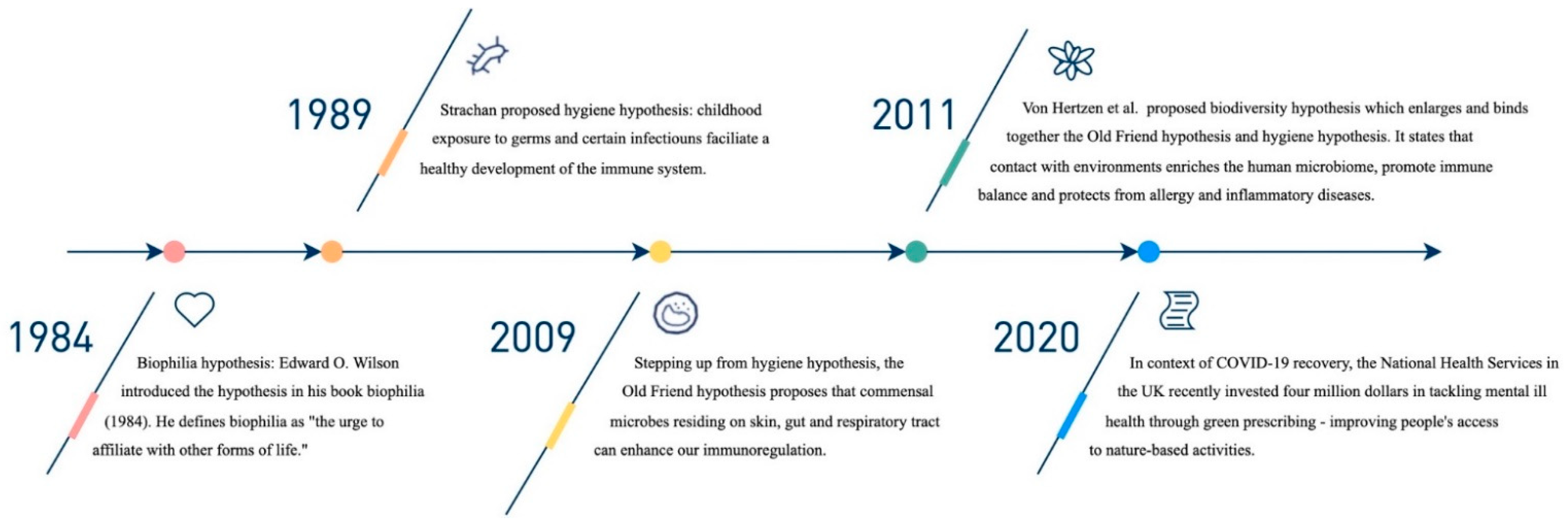
1. Introduction
2. Materials and Methods
3. Results
3.1. Epidemiological Evidence of Ecological Biodiversity Effect on Neuropsychiatric Disorders
3.1.1. Areas of Agreement
3.1.2. Areas of Controversy
3.1.3. New Research Frontiers
3.2. Environmental Microbes and Human Microbiota
3.2.1. Areas of Agreement
3.2.2. Areas of Controversy
3.2.3. New Research Frontiers
3.3. Microbiota in Neuropsychiatric Disorders
3.3.1. Areas of Agreement
3.3.2. New Research Frontiers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The Lancet Global Health. Mental health matters. Lancet Glob. Health 2020, 8, e1352. [Google Scholar] [CrossRef]
- Strachan, D.P. Hay fever, hygiene, and household size. BMJ Br. Med. J. 1989, 299, 1259–1260. [Google Scholar] [CrossRef]
- Okada, H.; Kuhn, C.; Feillet, H.; Bach, J.F. The ‘hygiene hypothesis’ for autoimmune and allergic diseases: An update. Clin. Exp. Immunol. 2010, 160, 1–9. [Google Scholar] [CrossRef]
- Rook, G.A. Regulation of the immune system by biodiversity from the natural environment: An ecosystem service essential to health. Proc. Natl. Acad. Sci. USA 2013, 110, 18360–18367. [Google Scholar] [CrossRef]
- Von Hertzen, L.; Beutler, B.; Bienenstock, J.; Blaser, M.; Cani, P.D.; Eriksson, J.; Färkkilä, M.; Haahtela, T.; Hanski, I.; Jenmalm, M.C.; et al. Helsinki alert of biodiversity and health. Ann. Med. 2015, 47, 218–225. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The human microbiome project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef]
- Correale, J.; Farez, M. Association between parasite infection and immune responses in multiple sclerosis. Ann. Neurol. 2007, 61, 97–108. [Google Scholar] [CrossRef]
- Bruce-Keller, A.J.; Salbaum, J.M.; Berthoud, H.-R. Harnessing Gut Microbes for Mental Health: Getting From Here to There. Biol. Psychiatry 2018, 83, 214–223. [Google Scholar] [CrossRef]
- NIH Human Microbiome Project—Home 2007. Available online: https://www.hmpdacc.org/ (accessed on 1 August 2022).
- Rothschild, D.; Weissbrod, O.; Barkan, E.; Kurilshikov, A.; Korem, T.; Zeevi, D.; Costea, P.I.; Godneva, A.; Kalka, I.N.; Bar, N.; et al. Environment dominates over host genetics in shaping human gut microbiota. Nature 2018, 555, 210–215. [Google Scholar] [CrossRef]
- Das, B.; Nair, G.B. Homeostasis and dysbiosis of the gut microbiome in health and disease. J. Biosci. 2019, 44, 117. [Google Scholar] [CrossRef]
- Wen, L.; Duffy, A. Factors Influencing the Gut Microbiota, Inflammation, and Type 2 Diabetes. J. Nutr. 2017, 147, 1468S–1475S. [Google Scholar] [CrossRef]
- Böbel, T.S.; Hackl, S.B.; Langgartner, D.; Jarczok, M.N.; Rohleder, N.; Rook, G.A.; Lowry, C.A.; Gündel, H.; Waller, C.; Reber, S.O. Less immune activation following social stress in rural vs. urban participants raised with regular or no animal contact, respectively. Proc. Natl. Acad. Sci. USA 2018, 115, 5259–5264. [Google Scholar] [CrossRef]
- Marselle, M.R.; Bowler, D.E.; Watzema, J.; Eichenberg, D.; Kirsten, T.; Bonn, A. Urban street tree biodiversity and antidepressant prescriptions. Sci. Rep. 2020, 10, 22445. [Google Scholar] [CrossRef]
- Methorst, J.; Bonn, A.; Marselle, M.; Böhning-Gaese, K.; Rehdanz, K. Species richness is positively related to mental health—A study for Germany. Landsc. Urban Plan. 2021, 211, 104084. [Google Scholar] [CrossRef]
- Gill, S.C.; Butterworth, P.; Rodgers, B.; Mackinnon, A. Validity of the mental health component scale of the 12-item Short-Form Health Survey (MCS-12) as measure of common mental disorders in the general population. Psychiatry Res. 2007, 152, 63–71. [Google Scholar] [CrossRef]
- Boone-Heinonen, J.; Gordon-Larsen, P.; Guilkey, D.K.; Jacobs, D.R., Jr.; Popkin, B.M. Environment and Physical Activity Dynamics: The Role of Residential Self-selection. Psychol. Sport Exerc. 2011, 12, 54–60. [Google Scholar] [CrossRef]
- Ritz, B.R. A long way from Steubenville: Environmental Epidemiology in a rapidly changing world. Am. J. Epidemiol. 2022; kwac031. [Google Scholar] [CrossRef]
- Craig, M.D.; Roberts, J.D. Evaluation of the impact of time of day, weather, vegetation density and bird movements on outcomes of area searches for birds in eucalypt forests of south-western Australia. Wildl. Res. 2001, 28, 33–39. [Google Scholar] [CrossRef]
- Peen, J.; Schoevers, R.A.; Beekman, A.T.; Dekker, J. The current status of urban-rural differences in psychiatric disorders. Acta Psychiatr. Scand. 2010, 121, 84–93. [Google Scholar] [CrossRef]
- Vassos, E.; Pedersen, C.B.; Murray, R.M.; Collier, D.A.; Lewis, C.M. Meta-Analysis of the Association of Urbanicity With Schizophrenia. Schizophr. Bull. 2012, 38, 1118–1123. [Google Scholar] [CrossRef]
- Lai, P.S.; Allen, J.G.; Hutchinson, D.S.; Ajami, N.J.; Petrosino, J.F.; Winters, T.; Hug, C.; Wartenberg, G.R.; Vallarino, J.; Christiani, D.C. Impact of environmental microbiota on human microbiota of workers in academic mouse research facilities: An observational study. PLoS ONE 2017, 12, e0180969. [Google Scholar] [CrossRef]
- Selway, C.A.; Mills, J.G.; Weinstein, P.; Skelly, C.; Yadav, S.; Lowe, A.; Breed, M.F.; Weyrich, L.S. Transfer of environmental microbes to the skin and respiratory tract of humans after urban green space exposure. Environ. Int. 2020, 145, 106084. [Google Scholar] [CrossRef]
- Fierer, N.; Jackson, R.B. The diversity and biogeography of soil bacterial communities. Proc. Natl. Acad. Sci. USA 2006, 103, 626–631. [Google Scholar] [CrossRef]
- Kernaghan, G.; Widden, P.; Bergeron, Y.; Légaré, S.; Paré, D. Biotic and abiotic factors affecting ectomycorrhizal diversity in boreal mixed-woods. Oikos 2003, 102, 497–504. [Google Scholar] [CrossRef]
- Hanski, I.; von Hertzen, L.; Fyhrquist, N.; Koskinen, K.; Torppa, K.; Laatikainen, T.; Karisola, P.; Auvinen, P.; Paulin, L.; Mäkelä, M.J.; et al. Environmental biodiversity, human microbiota, and allergy are interrelated. Proc. Natl. Acad. Sci. USA 2012, 109, 8334–8339. [Google Scholar] [CrossRef]
- Jin, J.-S.; Touyama, M.; Yamada, S.; Yamazaki, T.; Benno, Y. Alteration of a Human Intestinal Microbiota under Extreme Life Environment in the Antarctica. Biol. Pharm. Bull. 2014, 37, 1899–1906. [Google Scholar] [CrossRef][Green Version]
- Pearson, A.L.; Pechal, J.; Lin, Z.; Benbow, M.E.; Schmidt, C.; Mavoa, S. Associations detected between measures of neighborhood environmental conditions and human microbiome diversity. Sci. Total Environ. 2020, 745, 141029. [Google Scholar] [CrossRef]
- Sun, J.; Liao, X.-P.; D’Souza, A.W.; Boolchandani, M.; Li, S.; Cheng, K.; Martínez, J.L.; Li, L.; Feng, Y.-J.; Fang, L.-X.; et al. Environmental remodeling of human gut microbiota and antibiotic resistome in livestock farms. Nat. Commun. 2020, 11, 1427. [Google Scholar] [CrossRef]
- Ursell, L.K.; Metcalf, J.L.; Parfrey, L.W.; Knight, R. Defining the human microbiome. Nutr. Rev. 2012, 70 (Suppl. 1), S38–S44. [Google Scholar] [CrossRef]
- Liddicoat, C.; Sydnor, H.; Cando-Dumancela, C.; Dresken, R.; Liu, J.; Gellie, N.J.C.; Mills, J.G.; Young, J.M.; Weyrich, L.S.; Hutchinson, M.R.; et al. Naturally-diverse airborne environmental microbial exposures modulate the gut microbiome and may provide anxiolytic benefits in mice. Sci. Total Environ. 2020, 701, 134684. [Google Scholar] [CrossRef]
- Mutlu, E.A.; Comba, I.Y.; Cho, T.; Engen, P.A.; Yazıcı, C.; Soberanes, S.; Hamanaka, R.B.; Niğdelioğlu, R.; Meliton, A.Y.; Ghio, A.J.; et al. Inhalational exposure to particulate matter air pollution alters the composition of the gut microbiome. Environ. Pollut. 2018, 240, 817–830. [Google Scholar] [CrossRef]
- Bajinka, O.; Simbilyabo, L.; Tan, Y.; Jabang, J.; Saleem, S.A. Lung-brain axis. Crit. Rev. Microbiol. 2021, 48, 257–269. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Dinan, T.G.; Cryan, J.F. Gut instincts: Microbiota as a key regulator of brain development, ageing and neurodegeneration: Microbiota-gut-brain axis across the lifespan. J. Physiol. 2017, 595, 489–503. [Google Scholar] [CrossRef]
- Maiuolo, J.; Gliozzi, M.; Musolino, V.; Carresi, C.; Scarano, F.; Nucera, S.; Scicchitano, M.; Oppedisano, F.; Bosco, F.; Ruga, S.; et al. The Contribution of Gut Microbiota–Brain Axis in the Development of Brain Disorders. Front. Neurosci. 2021, 15, 616883. [Google Scholar] [CrossRef]
- Mason, B.L. Feeding Systems and the Gut Microbiome: Gut-Brain Interactions With Relevance to Psychiatric Conditions. Psychosomatics 2017, 58, 574–580. [Google Scholar] [CrossRef]
- Pusceddu, M.M.; Del Bas, J.M. The role of the gut microbiota in the pathophysiology of mental and neurological disorders. Psychiatr. Genet. 2020, 30, 87–100. [Google Scholar] [CrossRef]
- Rogers, G.B.; Keating, D.J.; Young, R.L.; Wong, M.L.; Licinio, J.; Wesselingh, S. From gut dysbiosis to altered brain function and mental illness: Mechanisms and pathways. Mol. Psychiatry 2016, 21, 738–748. [Google Scholar] [CrossRef]
- Zhu, S.; Jiang, Y.; Xu, K.; Cui, M.; Ye, W.; Zhao, G.; Jin, L.; Chen, X. The progress of gut microbiome research related to brain disorders. J. Neuroinflamm. 2020, 17, 25. [Google Scholar] [CrossRef]
- Bell, J.S.; Spencer, J.I.; Yates, R.L.; Yee, S.A.; Jacobs, B.M.; DeLuca, G.C. Invited Review: From nose to gut—The role of the microbiome in neurological disease. Neuropathol. Appl. Neurobiol. 2019, 45, 195–215. [Google Scholar] [CrossRef]
- Webster, H.J.; Emami-Khoyi, A.; van Dyk, J.C.; Teske, P.R.; Jansen van Vuuren, B. Environmental DNA Metabarcoding as a Means of Estimating Species Diversity in an Urban Aquatic Ecosystem. Animals 2020, 10, 2064. [Google Scholar] [CrossRef]
- Deiner, K.; Bik, H.M.; Mächler, E.; Seymour, M.; Lacoursière-Roussel, A.; Altermatt, F.; Creer, S.; Bista, I.; Lodge, D.M.; de Vere, N.; et al. Environmental DNA metabarcoding: Transforming how we survey animal and plant communities. Mol. Ecol. 2017, 26, 5872–5895. [Google Scholar] [CrossRef]
- Robinson, J.M.; Cando-Dumancela, C.; Liddicoat, C.; Weinstein, P.; Cameron, R.; Breed, M.F. Vertical Stratification in Urban Green Space Aerobiomes. Environ. Health Perspect. 2020, 128, 117008. [Google Scholar] [CrossRef]
- Turnbull, R. Healthy, happy places—a more integrated approach to creating health and well-being through the built environment? Br. Med. Bull. 2021, 140, 62–75. [Google Scholar] [CrossRef]
- Thompson Coon, J.; Boddy, K.; Stein, K.; Whear, R.; Barton, J.; Depledge, M.H. Does participating in physical activity in outdoor natural environments have a greater effect on physical and mental wellbeing than physical activity indoors? A systematic review. Environ. Sci. Technol. 2011, 45, 1761–1772. [Google Scholar] [CrossRef]
| Microbiota Alterations | Interventional Outcomes | |||
|---|---|---|---|---|
| Anxiety |   | Symptoms ↓ with Lactobacillus and Bifidobacteria probiotic [8,37,38,40] Symptoms ↓ with Lactobacillus, Bifidobacterium and Streptococcus genera [38] | ||
| Depression |   | ↑ Firmicutes and ↓ Bacteroidetes, Proteobacteria and Actinobacteria [35] ↓ Bacteroides, Coprococcus and Dialister genera [38] |     | ↓ Depression and anxiety symptoms with Lactobacillus rhamnosus and Lactobacillus helveticus [36] Symptoms ↓ with probiotic Lactobacillus rhamnosus [8,36] Symptoms ↓ with Lactobacillus acidophilus and casei and Bifidobacterium bifidum probiotics [34] Symptoms ↓ with prebiotics + probiotics [34] |
| Bipolar |  | ↓ Faecalibacterium and Ruminococcaceae genera [38] |   | ↓ Hospitalisation frequency after mania episodes with probiotics Lactobacillus rhamnosus strain GG and Bifidobacterium animalis subsp. lactis) [38] ↑ Incidence of mania with antibiotic use [38] |
| Autism |     | ↑ Bacteroidetes/Firmicutes ratio [35] ↑ Microbial metabolites (SCFAs) and ↓ microbiota metabolite 5-aminovaleric acid [38] ↑ Faecalibacterium, Lactobacillus, Bacteroides, Prevotella, and Alistipes genera [36,40] ↓ Prevotella, Bacteroides, Bifidobacterium, and Escherichia genera, and Akkermansia muciniphila [36,38,40] |      | Symptoms ↓ with Bacteroides fragilis in mice [35,38,40] Symptoms ↓ Faecal Microbiota Transplantation [8,38] Symptoms ↓ with Lactobacillus reuteri [38] Symptoms ↓ with microbiota metabolite 5-aminovaleric acid [38] Symptoms ↓ with prebiotic Bimuno® galactooligosaccharide [38] |
| Dementia and cognitive impairment |       | ↓ Prevotellaceae in Parkinson’s disease (PD) [35,38,40] ↓ Faecalibacterium prausnitzii, Lactobacillaceae, Enterococcaceae, and Bacteroidetes Prevotellaceae; ↑ Akkermansia muciniphila, Bifidobacterium and Enterobacteriaceae; ↓ SCFAs such as acetate, propionate, and butyrate in PD [38] ↑ lactobacillus levels; ↓ Clostridium coccoides group, Clostridium leptum subgroup and Bacteroides fragilis; ↓ hydrogen-producing fecal bacteria in PD [38] ↓ butyrate-producing and anti-inflammatory bacterial genera such as Blautia, Coprococcus, and Roseburia in PD [40] ↑ Oscillospira and Bacteroides, Ralstonia in PD [40] ↑ Helicobacter pylori in Alzheimer’s disease (AD) [40] |       | Bifidobacterium longum 1714 ↑ cognition [35] Antibiotics ↓ Aβ plaque pathology [38] Probiotic ↑ spatial memory in mice with Ab plaque [38] Three months of doxycycline and rifampin ↓Alzheimer’s disease severity [38] Probiotic ↑ cognitive function in Alzheimer’s disease (AD) [38,40] Symptoms ↓ with Lactobacillus and Bifidobacterium species via fermented milk in Alzheimer’s disease [40] |
| Multiple sclerosis |    | ↑ Escherichia, Shigella, Clostridium, Eubacterium rectal, Corynebacterium, Firmicutes, Psuedomonas, Haemophilus, Blautia, and Dorea and ↓ Parabacteroides, Adlercreutzia and Prevotella genera [36,40] ↓ Metabolites (lipid 654) of Bacteroidetes [36] ↓ Firmicutes and Bacteroidetes [40] |   | Probiotics ↓ inflammatory cells in patients [36] ↓ Faecalibacterium and Fusobacterium [36,40] |
| Visceral pain |     | ↓ pain with Lactobacillus [37,40] ↓ pain with Bifidobacterium infantis 35624 and Lactobacillus farciminis [40] ↓ pain with Lactobacillus salivarius UCC4331 [40] ↓ pain with Bifidobacterium infantis 35624 [40] | ||
| Migraine |  | ↑ Helicobacter pylori [40] | ||
| Anorexia nervosa |   | ↓ Roseburia spp., ↑ Methanobacterbrevi smithii, Verrucomicrobia and Bifidobacteria [37] ↑ metabolites ClpB of Escherichia coli [37] | ||
| Schizophrenia |  | Gut microbiota was implicated in the reductive metabolism of psychotropic medications [39] |   | ↓ Ampicillin [35] No differences were found in schizophrenia symptoms in the PANSS score between probiotic and placebo supplementation [38] |
| Legend |  Observations made in human; Observations made in human;  Observations made from animal studies; PD: Parkinson’s disease; AD: Alzheimer’s disease; SCFAs: Short-chain fatty acids Observations made from animal studies; PD: Parkinson’s disease; AD: Alzheimer’s disease; SCFAs: Short-chain fatty acids | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, Y.S.; Osborne, N.J. Biodiversity Effects on Human Mental Health via Microbiota Alterations. Int. J. Environ. Res. Public Health 2022, 19, 11882. https://doi.org/10.3390/ijerph191911882
Wong YS, Osborne NJ. Biodiversity Effects on Human Mental Health via Microbiota Alterations. International Journal of Environmental Research and Public Health. 2022; 19(19):11882. https://doi.org/10.3390/ijerph191911882
Chicago/Turabian StyleWong, Yee Sang, and Nicholas John Osborne. 2022. "Biodiversity Effects on Human Mental Health via Microbiota Alterations" International Journal of Environmental Research and Public Health 19, no. 19: 11882. https://doi.org/10.3390/ijerph191911882
APA StyleWong, Y. S., & Osborne, N. J. (2022). Biodiversity Effects on Human Mental Health via Microbiota Alterations. International Journal of Environmental Research and Public Health, 19(19), 11882. https://doi.org/10.3390/ijerph191911882






