Effects of Self-Efficacy and Outcome Expectations on Motor Imagery-Induced Thermal and Mechanical Hypoalgesia: A Single-Blind Randomised Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Randomisation
2.4. Blinding
2.5. Intervention
2.6. Outcome Measures
2.6.1. Primary Outcomes
Heat Pain Threshold (HPT)
Pressure Pain Threshold (PPT)
2.6.2. Secondary Outcome Measures
Cold and Warm Detection Threshold
Autonomic Nervous System Measurement
VAS on Fatigue
2.6.3. Baseline Outcomes
Mental Imagery Ability
Mental Chronometry
Physical Activity Level
2.7. Procedures
2.8. Sample Size Calculation
3. Results
3.1. Primary Outcomes
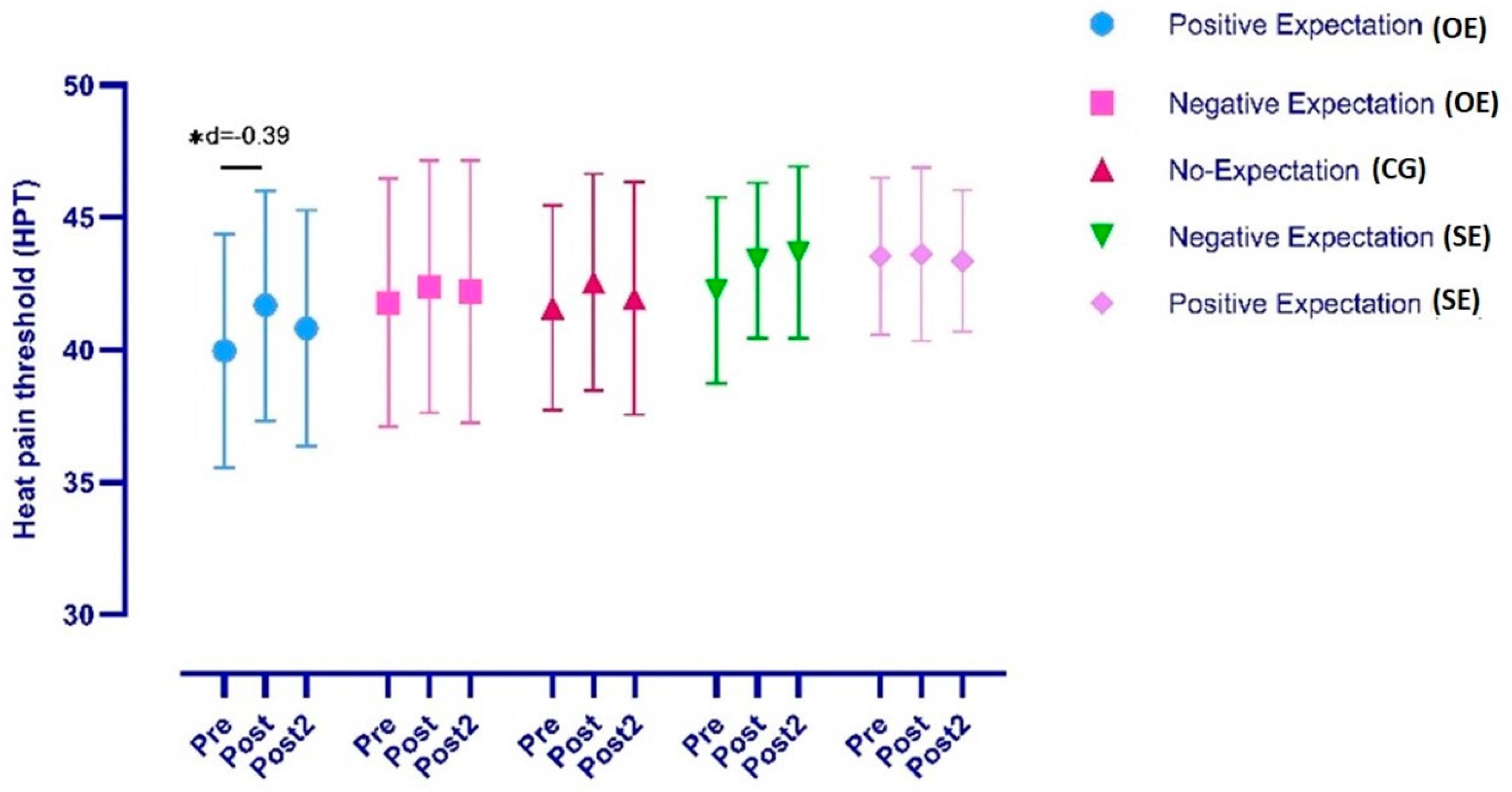
3.1.1. Heat Pain Threshold
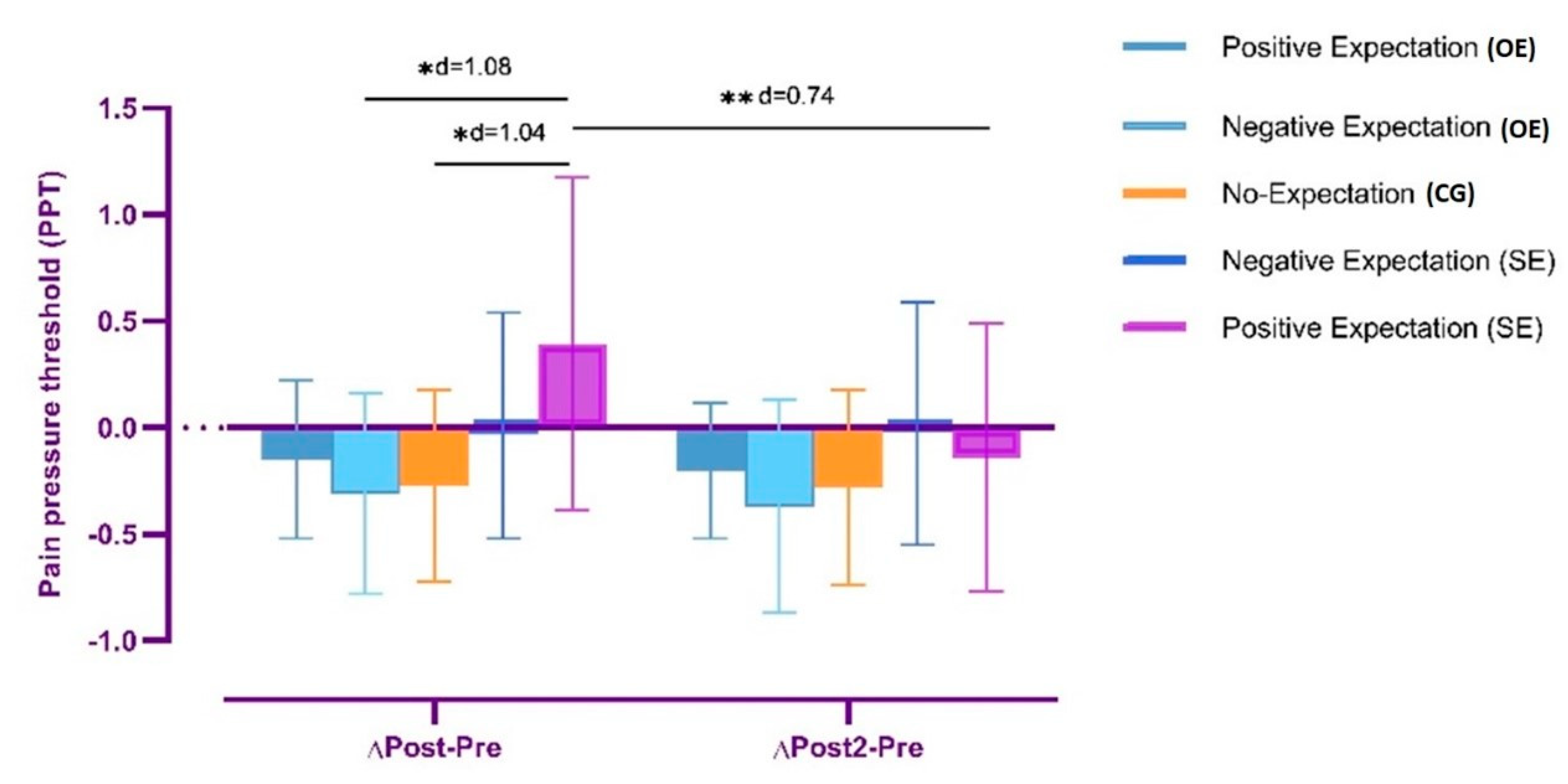
3.1.2. Change in Pain Pressure Threshold
3.1.3. Distal Pain Pressure Threshold
3.2. Secondary Outcomes
3.2.1. Cold Detection Threshold
3.2.2. Warm Detection Threshold
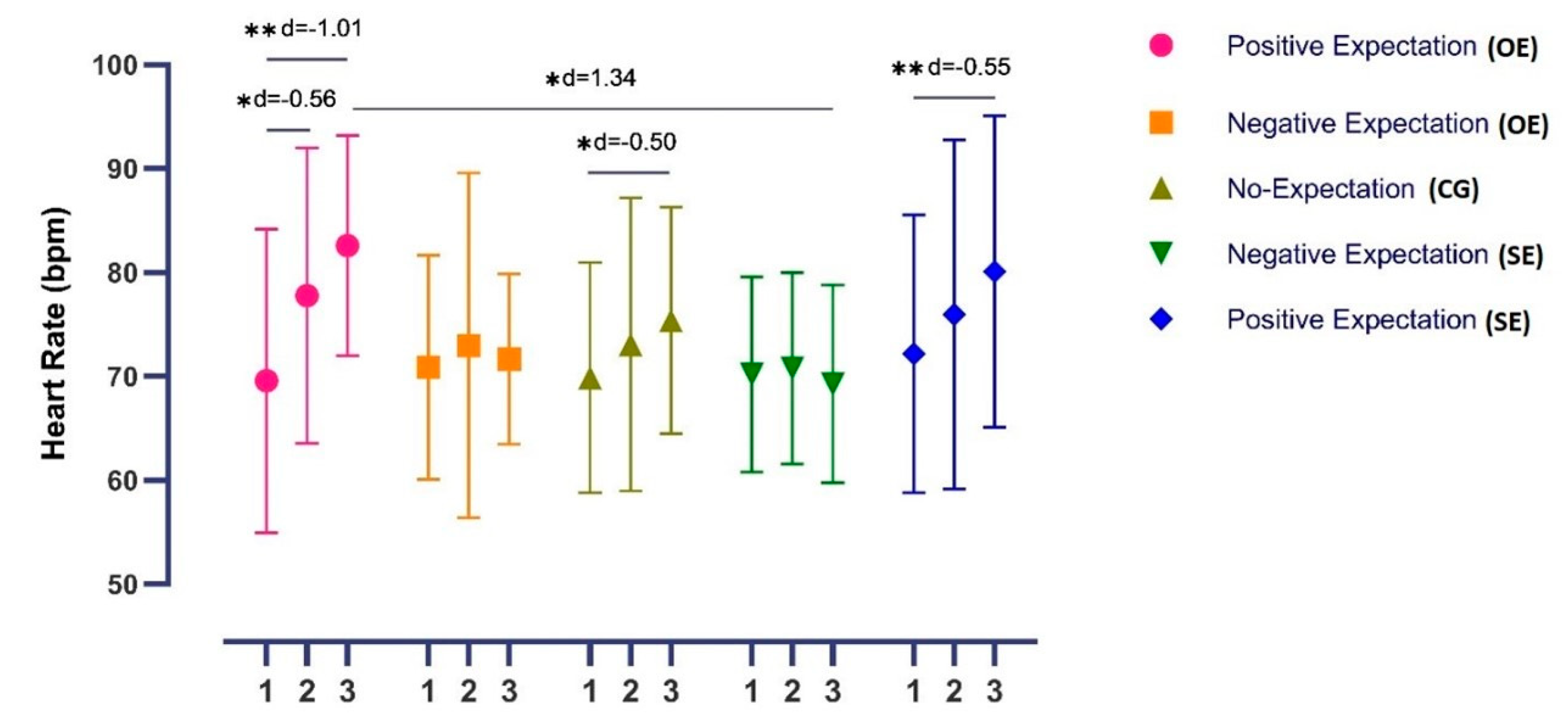
3.2.3. Heart Rate
3.2.4. Fatigue Visual Analogue Scale
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suso-Martí, L.; León-Hernández, J.V.; La Touche, R.; Paris-Alemany, A.; Cuenca-Martínez, F. Motor Imagery and Action Observation of Specific Neck Therapeutic Exercises Induced Hypoalgesia in Patients with Chronic Neck Pain: A Randomized Single-Blind Placebo Trial. J. Clin. Med. 2019, 8, 1019. [Google Scholar] [CrossRef] [PubMed]
- La Touche, R.; Fernández Pérez, J.J.; Martínez García, S.; Cuenca-Martínez, F.; López-de-Uralde-Villanueva, I.; Suso-Martí, L. Hypoalgesic Effects of Aerobic and Isometric Motor Imagery and Action Observation Exercises on Asymptomatic Participants: A Randomized Controlled Pilot Trial. Pain Med. 2020, 21, 2186–2199. [Google Scholar] [CrossRef] [PubMed]
- Suso-Martí, L.; La Touche, R.; Angulo-Díaz-Parreño, S.; Cuenca-Martínez, F. Effectiveness of motor imagery and action observation training on musculoskeletal pain intensity: A systematic review and meta-analysis. Eur. J. Pain 2020, 24, 886–901. [Google Scholar] [CrossRef] [PubMed]
- Beinert, K.; Sofsky, M.; Trojan, J. Train the brain! Immediate sensorimotor effects of mentally-performed flexor exercises in patients with neck pain. A pilot study. Eur. J. Phys. Rehabil. Med. 2019, 55, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Aono, S.; Shiro, Y.; Ushida, T. Effects of Virtual Reality-Based Exercise Imagery on Pain in Healthy Individuals. Biomed. Res. Int. 2019, 2019, 1–9. [Google Scholar] [CrossRef]
- Peerdeman, K.J.; van Laarhoven, A.I.M.; Bartels, D.J.P.; Peters, M.L.; Evers, A.W.M. Placebo-like analgesia via response imagery. Eur. J. Pain 2017, 21, 1366–1377. [Google Scholar] [CrossRef] [PubMed]
- Larsen, D.B.; Graven-Nielsen, T.; Boudreau, S.A. Pain-induced reduction in corticomotor excitability is counteracted by combined action-observation and motor imagery. J. Pain 2019, 11, 1307–1316. [Google Scholar] [CrossRef] [PubMed]
- Bialosky, J.E.; Bishop, M.D.; Robinson, M.E.; Barabas, J.A.; George, S.Z. The influence of expectation on spinal manipulation induced hypoalgesia: An experimental study in normal subjects. BMC Musculoskelet. Disord. 2008, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Vaegter, H.B.; Jones, M.D. Exercise-induced hypoalgesia after acute and regular exercise: Experimental and clinical manifestations and possible mechanisms in individuals with and without pain. PAIN Rep. 2020, 5, e823. [Google Scholar] [CrossRef]
- Bandura, A. Self-efficacy: Toward a Unifying Theory of Behavioral Change. Psychol. Rev. 1977, 84, 191–215. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, F.; Pollo, A.; Lopiano, L.; Lanotte, M.; Vighetti, S.; Rainero, I. Conscious expectation and unconscious conditioning in analgesic, motor, and hormonal placebo/nocebo responses. J. Neurosci. 2003, 23, 4315–4323. [Google Scholar] [CrossRef]
- Moher, D.; Schulz, K.F.; Altman, D.G. CONSORT GROUP (Consolidated Standards of Reporting Trials) The CONSORT statement: Revised recommendations for improving the quality of reports of parallel-group randomized trials. Ann. Intern. Med. 2001, 134, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Cuenca-Martínez, F.; Suso-Martí, L.; Grande-Alonso, M.; Paris-Alemany, A.; La Touche, R. Combining motor imagery with action observation training does not lead to a greater autonomic nervous system response than motor imagery alone during simple and functional movements: A randomized controlled trial. PeerJ 2018, 6, e5142. [Google Scholar] [CrossRef]
- Decety, J.; Jeannerod, M.; Durozard, D.; Baverel, G. Central activation of autonomic effectors during mental simulation of motor actions in man. J. Physiol. 1993, 461, 549–563. [Google Scholar] [CrossRef]
- Cuenca-Martínez, F.; Suso-Martí, L.; León-Hernández, J.V.; Touche, R. The role of movement representation techniques in the motor learning process: A neurophysiological hypothesis and a narrative review. Brain Sci. 2020, 10, 27. [Google Scholar] [CrossRef]
- Guillot, A.; Collet, C. Duration of Mentally Simulated Movement: A Review. J. Mot. Behav. 2005, 37, 10–20. [Google Scholar] [CrossRef]
- Wang, Y.; Mo, X.; Zhang, J.; Fan, Y.; Wang, K.; Peter, S. Quantitative sensory testing (QST) in the orofacial region of healthy Chinese: Influence of site, gender and age. Acta Odontol. Scand. 2018, 76, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Rolke, R.; Baron, R.; Maier, C.; Tölle, T.R.; Treede, R.D.; Beyer, A.; Binder, A.; Birbaumer, N.; Birklein, F.; Bötefür, I.C.; et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): Standardized protocol and reference values. Pain 2006, 123, 231–243. [Google Scholar] [CrossRef]
- Antonaci, F.; Sand, T.; Lucas, G.A. Pressure algometry in healthy subjects: Inter-examiner variability. Scand. J. Rehabil. Med. 1998, 30, 3–8. [Google Scholar] [CrossRef]
- Chesterton, L.S.; Sim, J.; Wright, C.C.; Foster, N.E. Interrater reliability of algometry in measuring pressure pain thresholds in healthy humans, using multiple raters. Clin. J. Pain 2007, 23, 760–766. [Google Scholar] [CrossRef]
- Takala, E.P. Pressure pain threshold on upper trapezius and levator scapulae muscles. Repeatability and relation to subjective symptoms in a working population. Scand. J. Rehabil. Med. 1990, 22, 63–68. [Google Scholar] [PubMed]
- Lee, K.A.; Hicks, G.; Nino-Murcia, G. Validity and reliability of a scale to assess fatigue. Psychiatry Res. 1991, 36, 291–298. [Google Scholar] [CrossRef]
- Campos, A.; Gonzalez, M.A. Spanish Version of the Revised Movement Image Questionnaire (Miq-R): Psychometric Properties and Validation. Rev. Psicol. Del. Deport. 2010, 19, 265–275. [Google Scholar]
- Malouin, F.; Richards, C.L.; Durand, A.; Doyon, J. Reliability of Mental Chronometry for Assessing Motor Imagery Ability After Stroke. Arch. Phys. Med. Rehabil. 2008, 89, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Roman-Viñas, B.; Serra-Majem, L.; Hagströmer, M.; Ribas-Barba, L.; Sjöström, M.; Segura-Cardona, R. International Physical Activity Questionnaire: Reliability and validity in a Spanish population. Eur. J. Sport Sci. 2010, 10, 297–304. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Westbrook, A.; Braver, T.S. Cognitive effort: A neuroeconomic approach. Cogn. Affect. Behav. Neurosci. 2015, 15, 395–415. [Google Scholar] [CrossRef] [PubMed]
- Kool, W.; Botvinick, M. A labor/leisure tradeoff in cognitive control. J. Exp. Psychol. Gen. 2014, 143, 131–141. [Google Scholar] [CrossRef]
- Locke, H.S.; Braver, T.S. Motivational influences on cognitive control: Behavior, brain activation, and individual differences. Cogn. Affect. Behav. Neurosci. 2008, 8, 99–112. [Google Scholar] [CrossRef]
- Pantoja, J.; Ribeiro, S.; Wiest, M.; Soares, E.; Gervasoni, D.; Lemos, N.A.M.; Nicolelis, M.A.L. Neuronal activity in the primary somatosensory thalamocortical loop is modulated by reward contingency during tactile discrimination. J. Neurosci. 2007, 27, 10608–10620. [Google Scholar] [CrossRef]
- Padmala, S.; Pessoa, L. Reward reduces conflict by enhancing attentional control and biasing visual cortical processing. J. Cogn. Neurosci. 2011, 23, 3419–3432. [Google Scholar] [CrossRef] [PubMed]
- Frömer, R.; Lin, H.; Dean Wolf, C.K.; Inzlicht, M.; Shenhav, A. Expectations of reward and efficacy guide cognitive control allocation. Nat. Commun. 2021, 12, 1030. [Google Scholar] [CrossRef] [PubMed]
- Beyer, L.; Weiss, T.; Hansen, E.; Wolf, A.; Seidel, A. Dynamics of central nervous activation during motor imagination. Int. J. Psychophysiol. 1990, 9, 75–80. [Google Scholar] [CrossRef]
- Papadelis, C.; Kourtidou-Papadeli, C.; Bamidis, P.; Albani, M. Effects of imagery training on cognitive performance and use of physiological measures as an assessment tool of mental effort. Brain Cogn. 2007, 64, 74–85. [Google Scholar] [CrossRef]
- Cuenca-Martínez, F.; Suso-Martí, L.; León-Hernández, J.V.; La Touche, R. Effects of movement representation techniques on motor learning of thumb-opposition tasks. Sci. Rep. 2020, 10, 12267. [Google Scholar] [CrossRef]
- Büchel, C.; Geuter, S.; Sprenger, C.; Eippert, F. Placebo analgesia: A predictive coding perspective. Neuron 2014, 81, 1223–1239. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.J.; Stohler, C.S.; Egnatuk, C.M.; Wang, H.; Koeppe, R.A.; Zubieta, J.-K. Placebo and nocebo effects are defined by opposite opioid and dopaminergic responses. Arch. Gen. Psychiatry 2008, 65, 220–231. [Google Scholar] [CrossRef]
- Hétu, S.; Grégoire, M.; Saimpont, A.; Coll, M.-P.; Eugène, F.; Michon, P.-E.; Jackson, P.L. The neural network of motor imagery: An ALE meta-analysis. Neurosci. Biobehav. Rev. 2013, 37, 930–949. [Google Scholar] [CrossRef]
- La Touche, R.; Herranz-Gómez, A.; Destenay, L.; Gey-Seedorf, I.; Cuenca-Martínez, F.; Paris-Alemany, A.; Suso-Martí, L. Effect of brain training through visual mirror feedback, action observation and motor imagery on orofacial sensorimotor variables: A single-blind randomized controlled trial. J. Oral Rehabil. 2020, 47, 620–635. [Google Scholar] [CrossRef] [PubMed]
- Morales Tejera, D.; Fernandez-Carnero, J.; Suso-Martí, L.; Cano-de-la-Cuerda, R.; Lerín-Calvo, A.; Remón-Ramiro, L.; La Touche, R. Comparative study of observed actions, motor imagery and control therapeutic exercise on the conditioned pain modulation in the cervical spine: A randomized controlled trial. Somatosens. Mot. Res. 2020, 37, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Handwerker, H.O.; Kobal, G. Psychophysiology of experimentally induced pain. Physiol. Rev. 1993, 73, 639–671. [Google Scholar] [CrossRef] [PubMed]
- Normandin, A.; Luccarini, P.; Molat, J.L.; Gendron, L.; Dallel, R. Spinal μ and δ Opioids Inhibit Both Thermal and Mechanical Pain in Rats. J. Neurosci. 2013, 33, 11703. [Google Scholar] [CrossRef]
- Staahl, C.; Olesen, A.E.; Andresen, T.; Arendt-Nielsen, L.; Drewes, A.M. Assessing analgesic actions of opioids by experimental pain models in healthy volunteers—An updated review. Br. J. Clin. Pharmacol. 2009, 68, 149. [Google Scholar] [CrossRef] [PubMed]
- von Helmholtz, H.L.F. Handbuch der Physiologischen Optik; Leopold Voss: Leipzig, Germany, 1867. [Google Scholar]
- Kersten, D.; Mamassian, P.; Yuille, A. Object perception as Bayesian inference. Annu. Rev. Psychol. 2004, 55, 271–304. [Google Scholar] [CrossRef] [PubMed]
- Aitken, F.; Turner, G.; Kok, P. Prior Expectations of Motion Direction Modulate Early Sensory Processing. J. Neurosci. 2020, 40, 6389–6397. [Google Scholar] [CrossRef] [PubMed]
- Kok, P.; Brouwer, G.J.; van Gerven, M.A.J.; de Lange, F.P. Prior expectations bias sensory representations in visual cortex. J. Neurosci. 2013, 33, 16275–16284. [Google Scholar] [CrossRef]
- Dijkstra, N.; Ambrogioni, L.; Vidaurre, D.; van Gerven, M. Neural dynamics of perceptual inference and its reversal during imagery. eLife 2020, 9, e53588. [Google Scholar] [CrossRef]
- Lim, M.; O’Grady, C.; Cane, D.; Goyal, A.; Lynch, M.; Beyea, S.; Hashmi, J.A. Threat Prediction from Schemas as a Source of Bias in Pain Perception. J. Neurosci. 2020, 40, 1538–1548. [Google Scholar] [CrossRef]
- Díaz-Sáez, M.C.; La Touche, R.; Cuenca-Martínez, F. Comparative analysis of the autonomic nervous system response during movement representation in healthy individuals and patients with chronic low back pain: A prospective cohort study. Somatosens. Mot. Res. 2021, 38, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Collet, C.; Di Rienzo, F.; El Hoyek, N.; Guillot, A. Autonomic nervous system correlates in movement observation and motor imagery. Front. Hum. Neurosci. 2013, 7, 415. [Google Scholar] [CrossRef]
| Measures | OE+ (n = 15) | OE− (n = 15) | CG (n = 15) | SE− (n = 15) | SE+ (n = 15) | p-Value |
|---|---|---|---|---|---|---|
| Age (years) | 29.53 ± 11.46 | 25.73 ± 8.33 | 26.13 ± 8.09 | 21.93 ± 1.87 | 23.87 ± 7.08 | 0.124 |
| BMI (kg/m2) | 23.37 ± 2.72 | 22.06 ± 1.86 | 23.20 ± 2.91 | 21.20 ± 1.81 | 21.98 ± 1.97 | 0.065 |
| MIQR-total | 48.40 ± 5.55 | 45.93 ± 7.23 | 45.53 ± 8.87 | 44.33 ± 7.74 | 44.13 ± 6.03 | 0.499 |
| MIQR-K | 23.93 ± 3.19 | 22.93 ± 4.44 | 21.73 ± 5.89 | 21.60 ± 5.04 | 21.13 ± 4.12 | 0.460 |
| MIQR-V | 24.47 ± 3.50 | 23.00 ± 3.91 | 23.80 ± 4.64 | 22.73 ± 4.48 | 23.00 ± 3.46 | 0.753 |
| Synchronisation | 0.95 ± 0.18 | 0.88 ± 0.12 | 1.51 ± 1.96 | 1.15 ± 0.18 | 1.15 ± 0.32 | 0.360 |
| IPAQ-Total | 3656.70 ± 3782.29 | 2630.23 ± 2300.34 | 2570.80 ± 1410.93 | 5415.66 ± 1200.61 | 2441.1 ± 1356.60 | 0.590 |
| Cold detection threshold (°C) | 30.58 ± 1.11 | 30.34 ± 1.30 | 30.65 ± 0.84 | 23.53 ± 1.70 | 29.85 ± 1.97 | 0.164 |
| Warm detection threshold (°C) | 34.33 ± 2.34 | 34.24 ± 1.12 | 33.69 ± 0.71 | 34.51 ± 0.73 | 34.47 ± 0.88 | 0.435 |
| Heat pain threshold (°C) | 39.97 ± 4.42 | 41.79 ± 4.69 | 41.62 ± 3.85 | 42.25 ± 3.51 | 43.55 ± 2.96 | 0.183 |
| Quadriceps femoris pressure pain threshold (kg/cm2) | 2.14 ± 1.60 | 2.18 ± 1.41 | 2.10 ± 0.98 | 3.85 ± 1.28 | 3.28 ± 0.89 | <0.001 * |
| Upper trapezius pressure pain threshold (kg/cm2) | 1.70 ± 1.24 | 1.75 ± 1.07 | 1.83 ± 0.91 | 2.06 ± 0.69 | 1.95 ± 0.59 | 0.838 |
| Gender | ||||||
| Male | 8 (53.33) | 8 (53.33) | 4 (26.66) | 6 (40.00) | 6 (40.00) | 0.54 |
| Female | 7(46.67) | 7 (46.67) | 11 (73.34) | 9 (60.00) | 9 (60.00) | |
| Education level | ||||||
| Secondary | 4 (26.66) | 0 (0.00) | 1 (6.66) | 3 (20.00) | 1 (6.66) | 0.14 |
| College | 11 (73.34) | 15 (100.00) | 14 (93.34) | 12 (80.00) | 14 (93.34) | |
| Working status | ||||||
| Student | 7(46.67) | 8 (53.33) | 5 (33.33) | 11 (73.34) | 11 (73.34) | 0.17 |
| Employee | 6 (40.00) | 7(46.67) | 8 (53.33) | 4 (26.66) | 4 (26.66) | |
| Unemployed | 2 (13.33) | 0 (0.00) | 2 (13.33) | 0 (0.00) | 0 (0.00) |
| Measure | Group | Pre | Post | Post 10 min | Mean Difference (95%CI); Effect Size (d) (a) Pre vs. Post (b) Pre vs. Post 10 min (c) Post vs. Post 10 min |
|---|---|---|---|---|---|
| Cold Detection Threshold (°C) | OE+ | 30.58 ± 1.11 | 29.94 ± 0.67 | 30.01 ± 0.93 | (a) 0.63 (−0.84 to 1.452); d = 0.69 (b) 0.56 (−0.42 to 1.54); d = 0.55 (c) −0.70 (−0.72 to 0.587); d = −0.08 |
| OE− | 30.34 ± 1.30 | 29.11 ± 2.17 | 29.18 ± 1.84 | (a) 1.23 * (0.41 to 2.051); d = 0.68 (b) 1.15 * (0.17 to 2.14); d = 0.72 (c) −0.07 (−0.733 to 0.581); d = −0.03 | |
| CG | 30.65 ± 0.84 | 29.30 ± 2.14 | 29.29 ± 2.08 | (a) 1.34 * (0.53 to 2.16); d = 0.83 (b) 1.35 * (0.37 to 2.34); d = 0.85 (c) 0.00 (−0.64 to 0.66); d = 0.00 | |
| SE+ | 29.84 ± 1.97 | 30.15 ± 1.49 | 29.59 ± 1.42 | (a) −0.30 (−0.51 to 1.12); d = −0.17 (b) 0.25 (−0.72 to 1.24); d = 0.14 (c) 0.56 (−0.09 to 1.22); d = 0.38 | |
| SE− | 29.53 ± 1.70 | 28.48 ± 1.99 | 28.54 ± 2.12 | (a) 1.05 * (0.23 to 1.87); d = 0.56 (b) 0.98 * (0.00 to 1.96); d = 0.51 (c) −0.06 (−0.72 to 0.59); d = −0.02 | |
| Warm Detection Threshold (°C) | OE+ | 34.33 ± 2.33 | 34.72 ± 1.43 | 34.57 ± 1.20 | (a) −0.38 (−1.20 to 0.43); d = −0.20 (b) −0.23 (−1.20 to 0.73); d = −0.12 (c) 0.14 (−0.45 to 0.75); d = 0.11 |
| OE− | 34.24 ± 1.12 | 34.87 ± 2.00 | 35.94 ± 2.18 | (a) −0.63 (−1.45 to 0.19); d = −0.38 (b) −1.70 * (−2.66 to −0.73); d = −0.98 (c) −1.06 * (−1.67 to −0.46); d = −0.51 | |
| CG | 33.69 ± 0.71 | 34.37 ± 0.70 | 34.80 ± 1.32 | (a) −0.68 (−1.51 to 0.13; d = −0.96 (b) −1.11 * (−2.084 to −0.15); d = −1.04 (c) −0.43 (−1.03 to 0.17); d = −0.40 | |
| SE+ | 34.47 ± 0.88 | 35.09 ± 0.76 | 35.20 ± 0.74 | (a) −0.61 (−1.43 to 0.20); d = −0.75 (b) −0.72 (−1.69 to 0.23); d = −0.89 (c) −0.11 (−0.72 to 0.49); d = −0.14 | |
| SE− | 34.51 ± 0.73 | 35.48 ± 1.65 | 36.01 ± 2.49 | (a) −0.97 * (−1.79 to −0.15); d = −0.76 (b) −1.50 * (−2.47 to −0.54); d = −0.81 (c) −0.53 (−1.14 to 0.075); d = −0.25 | |
| Upper Trapezius Pain Pressure Threshold (Kg/cm2) | OE+ | 1.71 ± 1.25 | 1.71 ± 1.25 | 1.76 ± 1.44 | (a) −0.003 (−0.315 to 0.309); d = −0.003 (b) 0.057 (−0.408 to 0.294); d = −0.044 (c) −0.053 (−0.256 to 0.149); d = −0.037 |
| OE− | 1.75 ± 1.07 | 1.62 ± 1.00 | 1.69 ± 1.027 | (a) 0.134 (−0.178 to 0.446); d = 0.125 (b) 0.069 (−0.286 to 0.420); d = 0.066 (c) −0.065 (−0.267 to 0.138); d = −0.059 | |
| CG | 1.84 ± 0.92 | 1.78 ± 0.858 | 1.80 ± 0.91 | (a) 0.061 (−0.251 to 0.373); d = 0.068 (b) 0.032 (−0.319 to 0.383) d = 0.032 (c) −0.029 (−0.231 to 0.174) d = −0.034 | |
| SE+ | 1.95 ± 0.59 | 2.05 ± 0.59 | 1.89 ± 0.56 | (a) −0.103 (0.−4.15 to 0.209); d = −0.16 (b) 0.052 (−0.299 to 0.403); d = 0.104 (c) 0.155 (−0.48 to 0.357); d = 0.027 | |
| SE− | 2.06 ± 0.69 | 2.11 ± 0.70 | 2.09 ± 0.69 | (a) −0.57 (−0.369 to 0.255); d = −0.072 (b) −0.35 (−0.386 to 0.316); d = −0.043 (c) 0.23 (−0.180 to 0.225); d = 0.028 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuenca-Martínez, F.; Bocos-Corredor, E.; Espinosa-Giménez, Á.; Barrero-Santiago, L.; Nefa-Díaz, N.; Canchal-Crespo, D.; Varangot-Reille, C.; Herranz-Gómez, A.; Suso-Martí, L.; Sempere-Rubio, N.; et al. Effects of Self-Efficacy and Outcome Expectations on Motor Imagery-Induced Thermal and Mechanical Hypoalgesia: A Single-Blind Randomised Controlled Trial. Int. J. Environ. Res. Public Health 2022, 19, 11878. https://doi.org/10.3390/ijerph191911878
Cuenca-Martínez F, Bocos-Corredor E, Espinosa-Giménez Á, Barrero-Santiago L, Nefa-Díaz N, Canchal-Crespo D, Varangot-Reille C, Herranz-Gómez A, Suso-Martí L, Sempere-Rubio N, et al. Effects of Self-Efficacy and Outcome Expectations on Motor Imagery-Induced Thermal and Mechanical Hypoalgesia: A Single-Blind Randomised Controlled Trial. International Journal of Environmental Research and Public Health. 2022; 19(19):11878. https://doi.org/10.3390/ijerph191911878
Chicago/Turabian StyleCuenca-Martínez, Ferran, Elena Bocos-Corredor, África Espinosa-Giménez, Laura Barrero-Santiago, Naira Nefa-Díaz, David Canchal-Crespo, Clovis Varangot-Reille, Aida Herranz-Gómez, Luis Suso-Martí, Núria Sempere-Rubio, and et al. 2022. "Effects of Self-Efficacy and Outcome Expectations on Motor Imagery-Induced Thermal and Mechanical Hypoalgesia: A Single-Blind Randomised Controlled Trial" International Journal of Environmental Research and Public Health 19, no. 19: 11878. https://doi.org/10.3390/ijerph191911878
APA StyleCuenca-Martínez, F., Bocos-Corredor, E., Espinosa-Giménez, Á., Barrero-Santiago, L., Nefa-Díaz, N., Canchal-Crespo, D., Varangot-Reille, C., Herranz-Gómez, A., Suso-Martí, L., Sempere-Rubio, N., & La Touche, R. (2022). Effects of Self-Efficacy and Outcome Expectations on Motor Imagery-Induced Thermal and Mechanical Hypoalgesia: A Single-Blind Randomised Controlled Trial. International Journal of Environmental Research and Public Health, 19(19), 11878. https://doi.org/10.3390/ijerph191911878









