Does Dominant Somatotype Differentiate Performance of Jumping and Sprinting Variables in Young Healthy Adults?
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Experimental Design
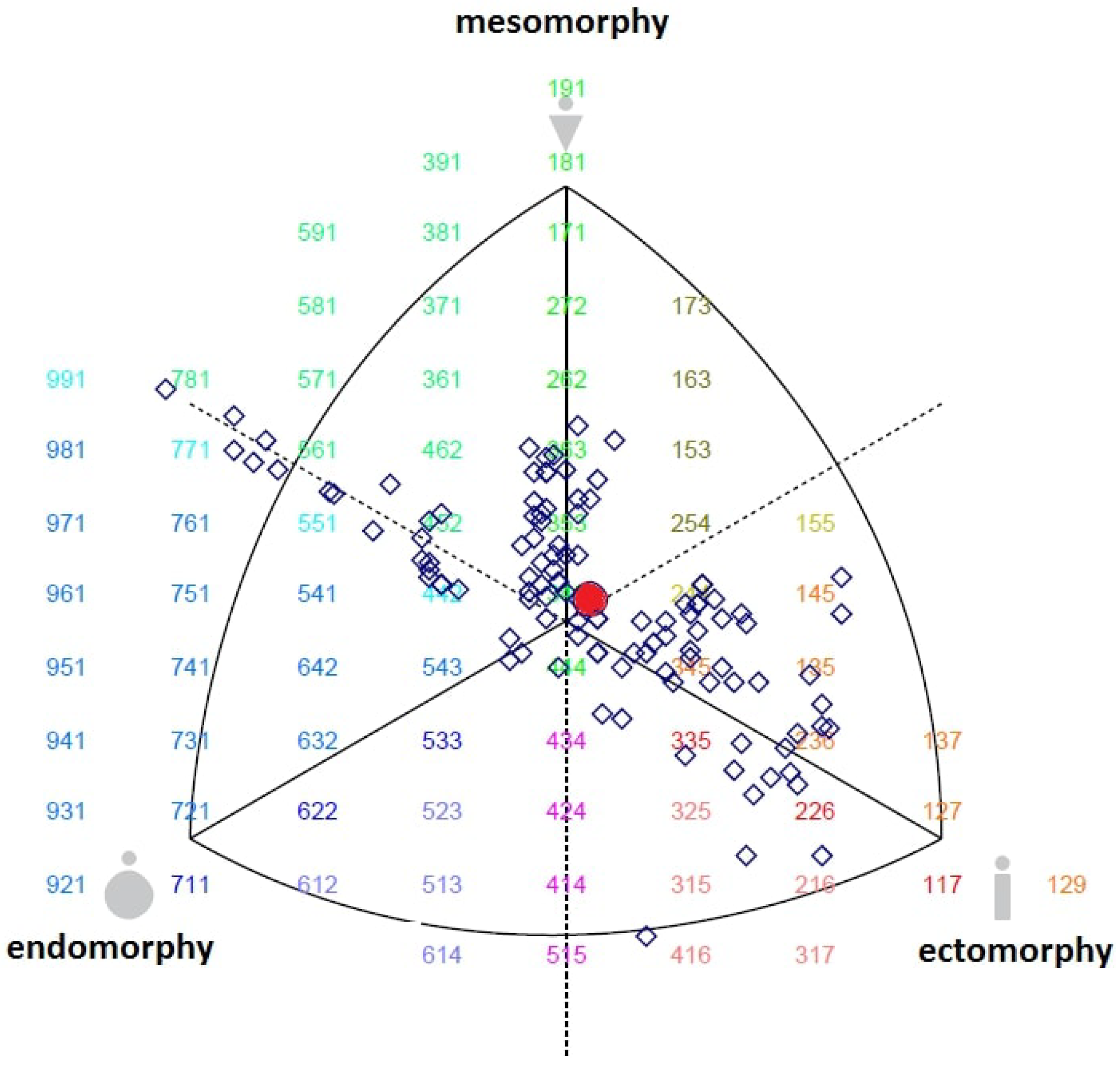
2.3. Anthropometric Measurements and Somatotype Classification
2.4. Jump Test Procedure
2.5. Determining the Absolute and Relative Peak Power
2.6. Sprint Test Procedure
2.7. Determining the Velocity and Sprint Momentum
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carter, J.L.; Heath, B.H. Somatotyping: Development and Applications; Cambridge University Press: Cambridge, UK, 1990. [Google Scholar]
- Carter, J.E.L.; Ackland, T.R.; Kerr, D.A.; Stapff, A.B. Somatotype and size of elite female basketball players. J. Sports Sci. 2005, 23, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Heath, B.H.; Carter, J.L. A modified somatotype method. Am. J. Phys. Anthropol. 1967, 27, 57–74. [Google Scholar] [CrossRef] [PubMed]
- Fidelix, Y.L.; Berria, J.; Ferrari, E.P.; Ortiz, J.G.; Cetolin, T.; Petroski, E.L. Somatotype of competitive youth soccer players from Brazil. J. Hum. Kinet. 2014, 42, 259. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guimarães Almeida, L.; Numata Filho, E.S.; dos Santos, G.A.; Carneiro Cardoso, J.T.; Rodrigues Moreira, S. Anthropometric Profile and Functional Performance of Capoeira Competitors in the World Games. Int. J. Morphol. 2021, 39, 969–976. [Google Scholar] [CrossRef]
- Laubach, L.L.; McConville, J.T. The relationship of strength to body size and typology. Med. Sci. Sports Exerc. 1969, 1, 189–194. [Google Scholar] [CrossRef]
- Marta, C.; Marinho, D.A.; Costa, A.M.; Barbosa, T.M.; Marques, M.C. Somatotype is more interactive with strength than fat mass and physical activity in peripubertal children. J. Hum. Kinet. 2011, 29A, 83–91. [Google Scholar] [CrossRef]
- Lewandowska, J.; Buśko, K.; Pastuszak, A.; Boguszewska, K. Somatotype variables related to muscle torque and power in judoists. J. Hum. Kinet. 2011, 30, 21. [Google Scholar] [CrossRef]
- Kandel, M.; Baeyens, J.P.; Clarys, P. Somatotype, training and performance in Ironman athletes. Eur. J. Sport Sci. 2014, 14, 301–308. [Google Scholar] [CrossRef]
- Allen, S.V.; Hopkins, W.G. Age of peak competitive performance of elite athletes: A systematic review. Sports Med. 2015, 45, 1431–1441. [Google Scholar] [CrossRef]
- Ryan-Stewart, H.; Faulkner, J.; Jobson, S. The influence of somatotype on anaerobic performance. PLoS ONE 2018, 13, e0197761. [Google Scholar] [CrossRef]
- Cinarli, F.S.; Kafkas, M.E. The effect of somatotype characters on selected physical performance parameters. J. Phys. Educ. Stud. 2019, 23, 279–287. [Google Scholar] [CrossRef]
- Marta, C.C.; Marinho, D.A.; Barbosa, T.M.; Carneiro, A.L.; Izquierdo, M.; Marques, M.C. Effects of body fat and dominant somatotype on explosive strength and aerobic capacity trainability in prepubescent children. J. Strength Cond. Res. 2013, 27, 3233–3244. [Google Scholar] [CrossRef]
- Kutseryb, T.; Vovkanych, L.; Hrynkiv, M.; Majevska, S.; Muzyka, F. Peculiarities of the somatotype of athletes with different directions of the training process. J. Phys. Educ. Sport 2017, 17, 431. [Google Scholar]
- Keogh, J.W.; Hume, P.A.; Pearson, S.N.; Mellow, P. Anthropometric dimensions of male powerlifters of varying body mass. J. Sports Sci. 2007, 25, 1365–1376. [Google Scholar] [CrossRef] [PubMed]
- Perez-Gomez, J.; Rodriguez, G.V.; Ara, I.; Olmedillas, H.; Chavarren, J.; González-Henriquez, J.J.; Calbet, J.A. Role of muscle mass on sprint performance: Gender differences? Eur. J. Appl. Physiol. 2008, 102, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Moncef, C.; Said, M.; Olfa, N.; Dagbaji, G. Influence of morphological characteristics on physical and physiological performances of tunisian elite male handball players. Asian J. Sports Med. 2012, 3, 74. [Google Scholar] [CrossRef]
- Barbieri, D.; Zaccagni, L.; Babić, V.; Rakovac, M.; Mišigoj-Duraković, M.; Gualdi-Russo, E. Body composition and size in sprint athletes. J. Sports Med. Phys. Fit. 2017, 57, 1142–1146. [Google Scholar] [CrossRef]
- Stewart, A.; Marfell-Jones, M.; Olds, T.; de Ridder, H. International Standards for Anthropometric Assessment; International Society for the Advancement of Kinanthropometry: Melbourne, Australia, 2011. [Google Scholar]
- Wilczyński, B.; Hinca, J.; Ślęzak, D.; Zorena, K. The relationship between dynamic balance and jumping tests among adolescent amateur rugby players: A preliminary study. Int. J. Environ. Res. Public. Health 2021, 18, 312. [Google Scholar] [CrossRef]
- Sayers, S.P.; Harackiewicz, D.V.; Harman, E.A.; Frykman, P.N.; Rosenstein, M.T. Cross-validation of three jump power equations. Med. Sci. Sports. Exerc. 1999, 31, 572–577. [Google Scholar] [CrossRef]
- Jalilvand, F.; Banoocy, N.K.; Rumpf, M.C.; Lockie, R.G. Relationship between body mass, peak power, and power-to-body mass ratio on sprint velocity and momentum in high-school football players. J. Strength Cond. Res. 2019, 33, 1871–1877. [Google Scholar] [CrossRef]
- Gabbett, T.J.; Kelly, J.N.; Sheppard, J.M. Speed, change of direction speed, and reactive agility of rugby league players. J. Strength Cond. Res. 2008, 22, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Mann, J.B.; Mayhew, J.L.; Dos Santos, M.L.; Dawes, J.J.; Signorile, J.F. Momentum, Rather Than Velocity, Is a More Effective Measure of Improvements in Division IA Football Player Performance. J. Strength Cond. Res. 2022, 36, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Earlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Hopkins, W.G. How to interpret changes in an athletic performance test. Sport Sci. 2004, 8, 1–7. [Google Scholar]
- Vila, H.; Manchado, C.; Rodriguez, N.; Abraldes, J.A.; Alcaraz, P.E.; Ferragut, C. Anthropometric profile, vertical jump, and throwing velocity in elite female handball players by playing positions. J. Strength Cond. Res. 2012, 26, 2146–2155. [Google Scholar] [CrossRef] [PubMed]
- Saha, S. Somatotype, body composition and explosive power of athlete and non-athlete. J. Sports Med. Doping Stud. 2014, 4, 2. [Google Scholar] [CrossRef]
- Esco, M.R.; Fedewa, M.V.; Cicone, Z.S.; Sinelnikov, O.A.; Sekulic, D.; Holmes, C.J. Field-based performance tests are related to body fat percentage and fat-free mass, but not body mass index, in youth soccer players. Sports 2018, 6, 105. [Google Scholar] [CrossRef]
- Nikolaidis, P.T. Body mass index and body fat percentage are associated with decreased physical fitness in adolescent and adult female volleyball players. J. Res. Med. Sci. 2013, 18, 22. [Google Scholar]
- Zary, J.C.; Reis, V.M.; Rouboa, A.; Silva, A.J.; Fernandes, P.R. The somatotype and dermatoglyphic profiles of adult, junior and juvenile male Brazilian top-level volleyball players. Sci. Sports 2010, 25, 146–152. [Google Scholar] [CrossRef]
- Martín-Matillas, M.; Valadés, D.; Hernández-Hernández, E.; Olea-Serrano, F.; Sjöström, M.; Delgado-Fernández, M.; Ortega, F.B. Anthropometric, body composition and somatotype characteristics of elite female volleyball players from the highest Spanish league. J. Sports Sci. 2014, 32, 137–148. [Google Scholar] [CrossRef]
- Gualdi-Russo, E.; Zaccagni, L. Somatotype, role and performance in elite volleyball players. J Sports Med. Phys. Fit. 2001, 41, 256. [Google Scholar]
- Aerenhouts, D.; Delecluse, C.; Hagman, F.; Taeymans, J.; Debaere, S.; Van Gheluwe, B.; Clarys, P. Comparison of anthropometric characteristics and sprint start performance between elite adolescent and adult sprint athletes. Eur. J. Sport Sci. 2012, 12, 9–15. [Google Scholar] [CrossRef]
- Rahmawati, N.T.; Budiharjo, S.; Ashizawa, K. Somatotypes of young male athletes and non-athlete students in Yogyakarta, Indonesia. Anthropol. Sci. 2007, 115, 1–7. [Google Scholar] [CrossRef]
- Abe, T.; Kawamoto, K.; Dankel, S.J.; Bell, Z.W.; Spitz, R.W.; Wong, V.; Loenneke, J.P. Longitudinal associations between changes in body composition and changes in sprint performance in elite female sprinters. Eur. J. Sport Sci. 2020, 20, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.T.; Cronin, J.B.; Pickering, S.L.; Douglas, L. Do force–time and power–time measures in a loaded jump squat differentiate between speed performance and playing level in elite and elite junior rugby union players? J. Strength Cond. Res. 2011, 25, 2382–2391. [Google Scholar] [CrossRef] [PubMed]
- Barr, M.J.; Sheppard, J.M.; Gabbett, T.J.; Newton, R.U. Long-term training-induced changes in sprinting speed and sprint momentum in elite rugby union players. J. Strength Cond. Res. 2014, 28, 2724–2731. [Google Scholar] [CrossRef]
- Baker, D.G.; Newton, R.U. Comparison of lower body strength, power, acceleration, speed, agility, and sprint momentum to describe and compare playing rank among professional rugby league players. J. Strength Cond. Res. 2008, 22, 153–158. [Google Scholar] [CrossRef]
| Variables | Mean ± SD | Minimum | Maximum | 95% CI |
|---|---|---|---|---|
| Age (y) | 21.82 ± 3.18 | 19 | 25 | 21.23–22.42 |
| Height (m) | 1.73 ± 0.08 | 1.55 | 1.93 | 1.71–1.74 |
| Body mass (kg) | 63.3 ± 10.49 | 39.5 | 89.5 | 61.33–65.26 |
| Skinfolds (mm) | ||||
| Triceps | 11.57 ± 4.89 | 4.3 | 25 | 10.66–12.49 |
| Supscapula | 12.28 ± 4.52 | 6 | 32 | 11.44–13.13 |
| Suprailiacus | 7.06 ± 3.17 | 2.9 | 19 | 6.47–7.66 |
| Medial calf | 9.36 ± 4.35 | 2 | 26 | 8.54–10.17 |
| Girth (cm) | ||||
| Upper arm | 29.6 ± 3.73 | 22 | 37 | 28.91–30.30 |
| Medial calf | 37.81 ± 2.90 | 26 | 42.7 | 32.12–43.49 |
| Breadth (cm) | ||||
| Humerus | 6.3 ± 0.49 | 5.5 | 7.6 | 6.21–6.4 |
| Femur | 9.02 ± 0.84 | 6.1 | 10.7 | 8.87–9.18 |
| Endomorphy | 3.03 ± 1.08 | 1.3 | 6.9 | 2.83–3.23 |
| Mesomorphy | 3.55 ± 1.13 | 0.2 | 6.8 | 3.34–3.76 |
| Ectomorphy | 3.44 ± 1.37 | 0.2 | 6.2 | 3.18–3.7 |
| Variables | Mean ± SD | Minimum | Maximum | 95% CI |
|---|---|---|---|---|
| VJ (cm) | 31.74 ± 7.76 | 15.8 | 53.58 | 30.28–33.19 |
| PAPw (W) | 2583.04 ± 735.24 | 937.33 | 3994.82 | 2445.37–2720.71 |
| P:BM (W·kg−1) | 40.79 ± 8.14 | 22.97 | 65.76 | 39.26–42.31 |
| ST (s) | 4.34 ± 0.54 | 3.74 | 6.69 | 4.24–4.44 |
| SV (m·s−1) | 7 ± 0.76 | 4.48 | 8.02 | 6.85–7.14 |
| Momentum (kg·m·s−1) | 446.47 ± 100.4 | 234.65 | 653.28 | 427.67–465.27 |
| Variables | Balanced Ectomorph (n = 25) | Balanced Mesomorph (n = 22) | Central (n = 24) | Mesomorph-Endomorph (n = 21) | Mesomorphic Ectomorph (n = 20) | F | p | η2 |
|---|---|---|---|---|---|---|---|---|
| Age (year) | 22.08 ± 1.6 | 21.22 ± 1.65 | 20.95 ± 1.45 | 21.76 ± 1.7 | 21.75 ± 1.33 | 2.000 | 0.100 | 0.07 |
| Height (m) | 1.76 ± 0.10 | 1.73 ± 0.07 | 1.73 ± 0.07 | 1.7 ± 0.07 | 1.73 ± 0.08 | 1.556 | 0.191 | 0.05 |
| Weight (kg) | 57.5 ± 8.38 | 67.66 ± 8.29 | 63.41 ± 8.42 | 71.71 ± 10.5 | 56.43 ± 9.33 | 11.198 | <0.001 ** | 0.29 |
| Endomorphy | 2.40 ± 0.57 | 2.75 ± 0.32 | 3.29 ± 0.47 | 4.80 ± 0.70 | 1.95 ± 0.43 | 95,491 | <0.001 ** | 0.78 |
| Mesomorphy | 2.14 ± 0.74 | 4.54 ± 0.46 | 3.38 ± 0.51 | 4.70 ± 0.78 | 3.22 ± 0.44 | 68,615 | <0.001 ** | 0.71 |
| Ectomorphy | 4.8 ± 0.76 | 2.67 ± 0.34 | 3.24 ± 0.36 | 1.62 ± 0.90 | 4.64 ± 0.75 | 93,396 | <0.001 ** | 0.77 |
| Variables | Balanced Ectomorph (n = 25) | Balanced Mesomorph (n = 22) | Central (n = 24) | Mesomorph-Endomorph (n = 21) | Mesomorphic Ectomorph (n = 20) | F | p | η2 |
|---|---|---|---|---|---|---|---|---|
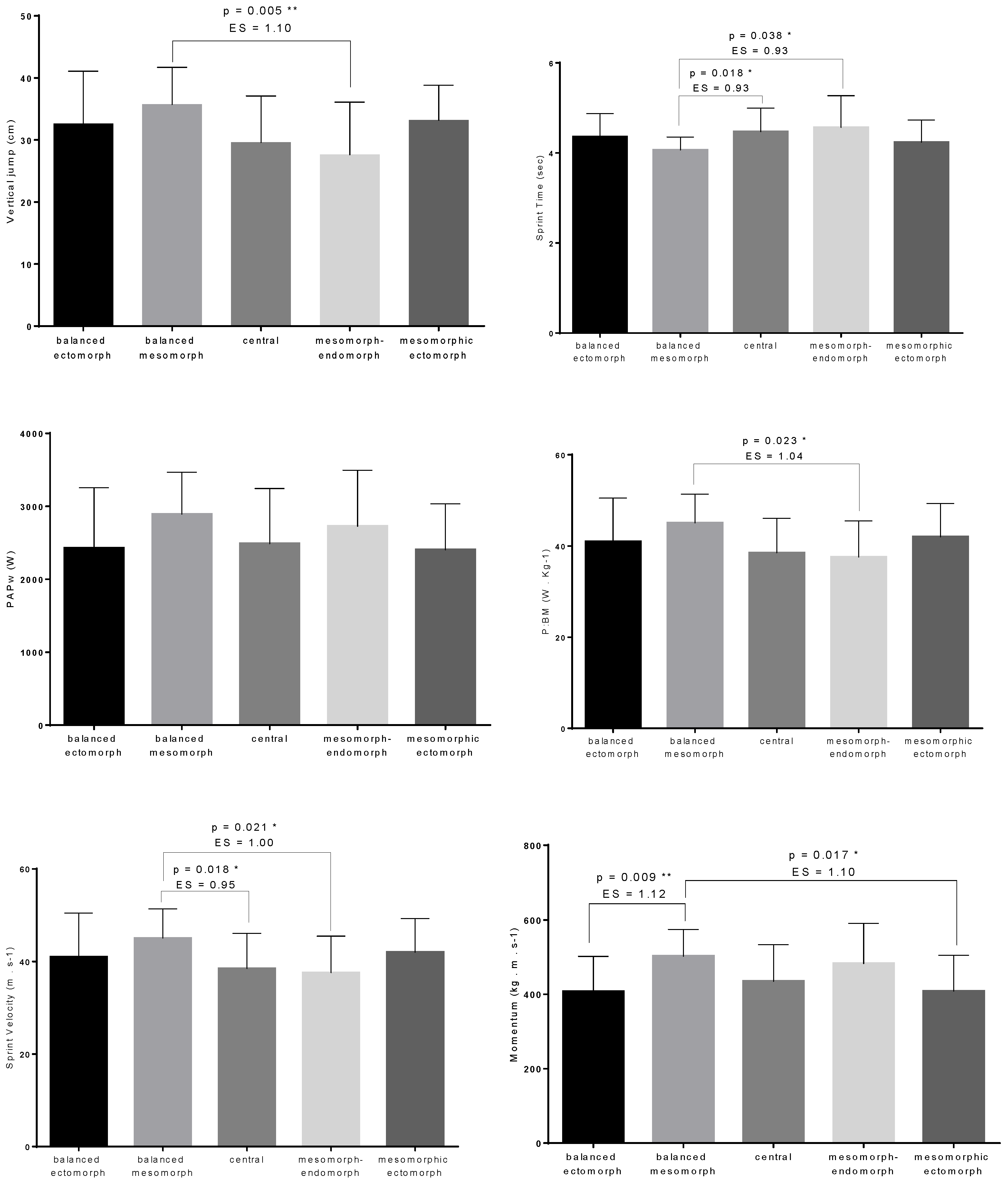
| VJ (cm) | 32.63 ± 8.45 | 35.71 ± 6.01 | 29.58 ± 7.53 | 27.61 ± 8.48 | 33.19 ± 5.64 | 4.043 | 0.004 ** | 0.13 |
| PAPw (W) | 2427.71 ± 828.56 | 2892.09 ± 575.76 | 2486.04 ±757.84 | 2726.33 ± 767.32 | 2403.21 ± 631.35 | 1.915 | 0.113 | 0.06 |
| P:BM (W·Kg−1) | 40.96 ± 9.53 | 45.06 ± 6.32 | 38.49 ± 7.57 | 37.59 ± 7.9 | 42.01 ± 7.29 | 3.134 | 0.018 * | 0.10 |
| ST (s) | 4.26 ± 0.52 | 4.07 ± 0.29 | 4.47 ± 0.53 | 4.57 ± 0.7 | 4.24 ± 0.49 | 3.117 | 0.018 * | 0.10 |
| SV (m·s−1) | 6.97 ± 0.76 | 7.41 ± 0.48 | 6.8 ± 0.77 | 6.69 ± 0.89 | 7.16 ± 0.73 | 3.306 | 0.014 * | 0.11 |
| Momentum (kg·m·s−1) | 407.76 ± 94.41 | 502.47 ± 71.97 | 435.22 ± 98.3 | 482.84 ± 107.9 | 408.62 ± 96.05 | 4.657 | 0.002 ** | 0.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cinarli, F.S.; Buyukcelebi, H.; Esen, O.; Barasinska, M.; Cepicka, L.; Gabrys, T.; Nalbant, U.; Karayigit, R. Does Dominant Somatotype Differentiate Performance of Jumping and Sprinting Variables in Young Healthy Adults? Int. J. Environ. Res. Public Health 2022, 19, 11873. https://doi.org/10.3390/ijerph191911873
Cinarli FS, Buyukcelebi H, Esen O, Barasinska M, Cepicka L, Gabrys T, Nalbant U, Karayigit R. Does Dominant Somatotype Differentiate Performance of Jumping and Sprinting Variables in Young Healthy Adults? International Journal of Environmental Research and Public Health. 2022; 19(19):11873. https://doi.org/10.3390/ijerph191911873
Chicago/Turabian StyleCinarli, Fahri Safa, Hakan Buyukcelebi, Ozcan Esen, Magdalena Barasinska, Ladislav Cepicka, Tomasz Gabrys, Umut Nalbant, and Raci Karayigit. 2022. "Does Dominant Somatotype Differentiate Performance of Jumping and Sprinting Variables in Young Healthy Adults?" International Journal of Environmental Research and Public Health 19, no. 19: 11873. https://doi.org/10.3390/ijerph191911873
APA StyleCinarli, F. S., Buyukcelebi, H., Esen, O., Barasinska, M., Cepicka, L., Gabrys, T., Nalbant, U., & Karayigit, R. (2022). Does Dominant Somatotype Differentiate Performance of Jumping and Sprinting Variables in Young Healthy Adults? International Journal of Environmental Research and Public Health, 19(19), 11873. https://doi.org/10.3390/ijerph191911873









