Procalcitonin as a Candidate Biomarker for Malarial Infection and Severe Malaria: A Meta-Analysis
Abstract
:1. Background
2. Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
2.3. Information Sources and Searches
2.4. Study Screening and Selection
2.5. Data Extraction
2.6. Assessment of Risk of Bias
2.7. Data Synthesis and Statistical Analysis
3. Results
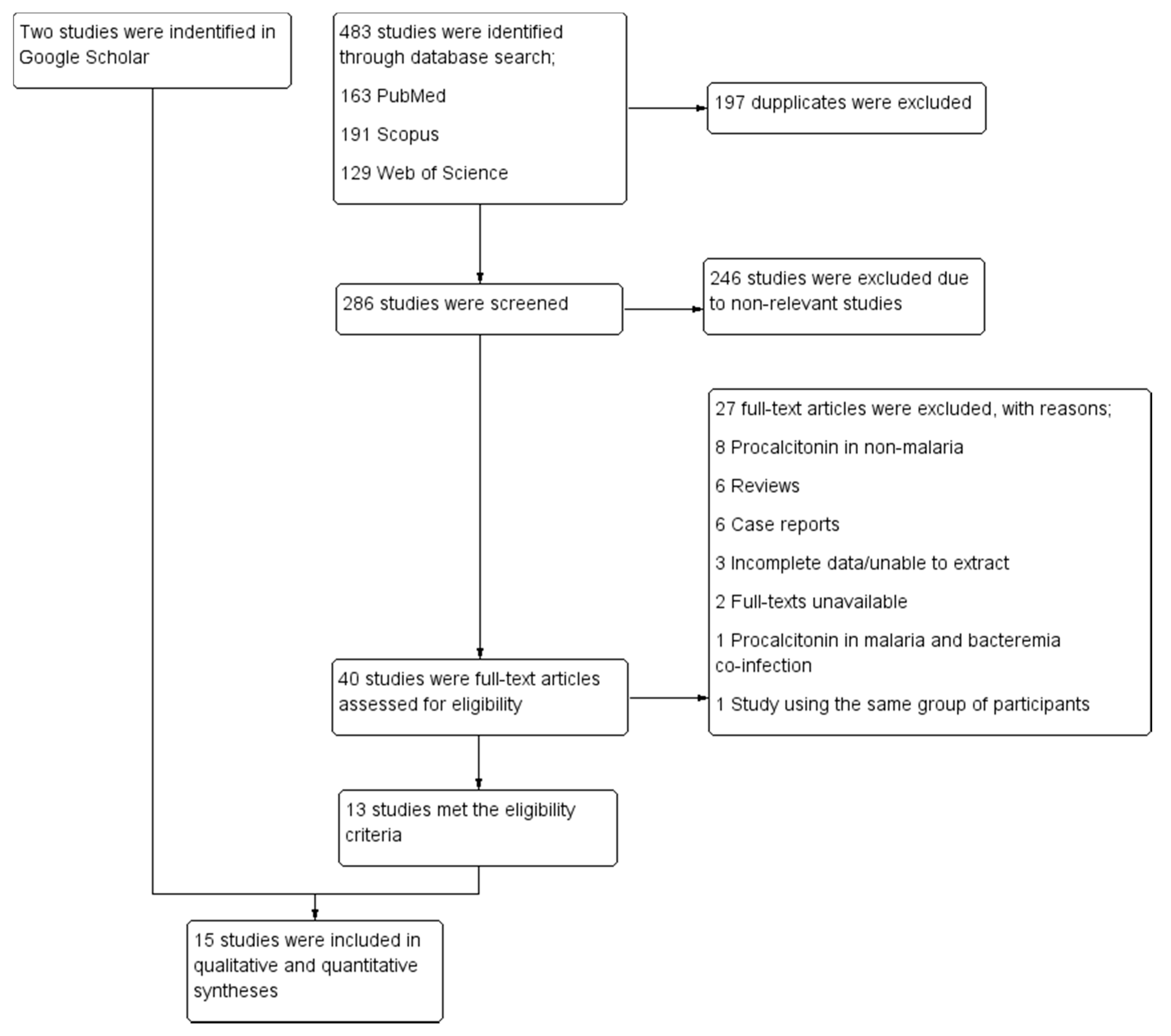
3.1. Search Results
3.2. Characteristics of the Included Studies
3.3. Risk of Bias among the Included Studies
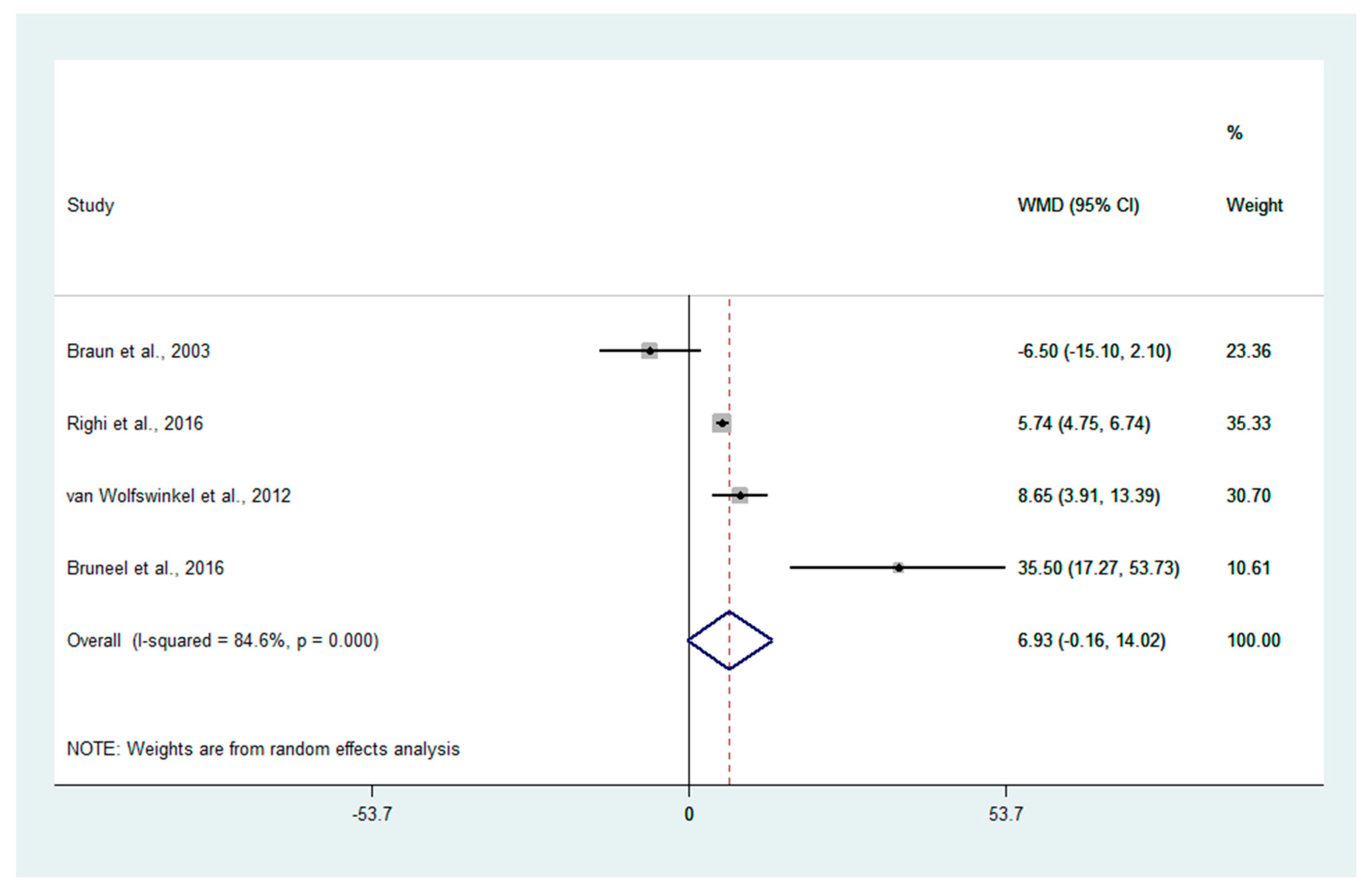
3.4. Mean PCT Levels in Patients with Uncomplicated and Severe Malaria
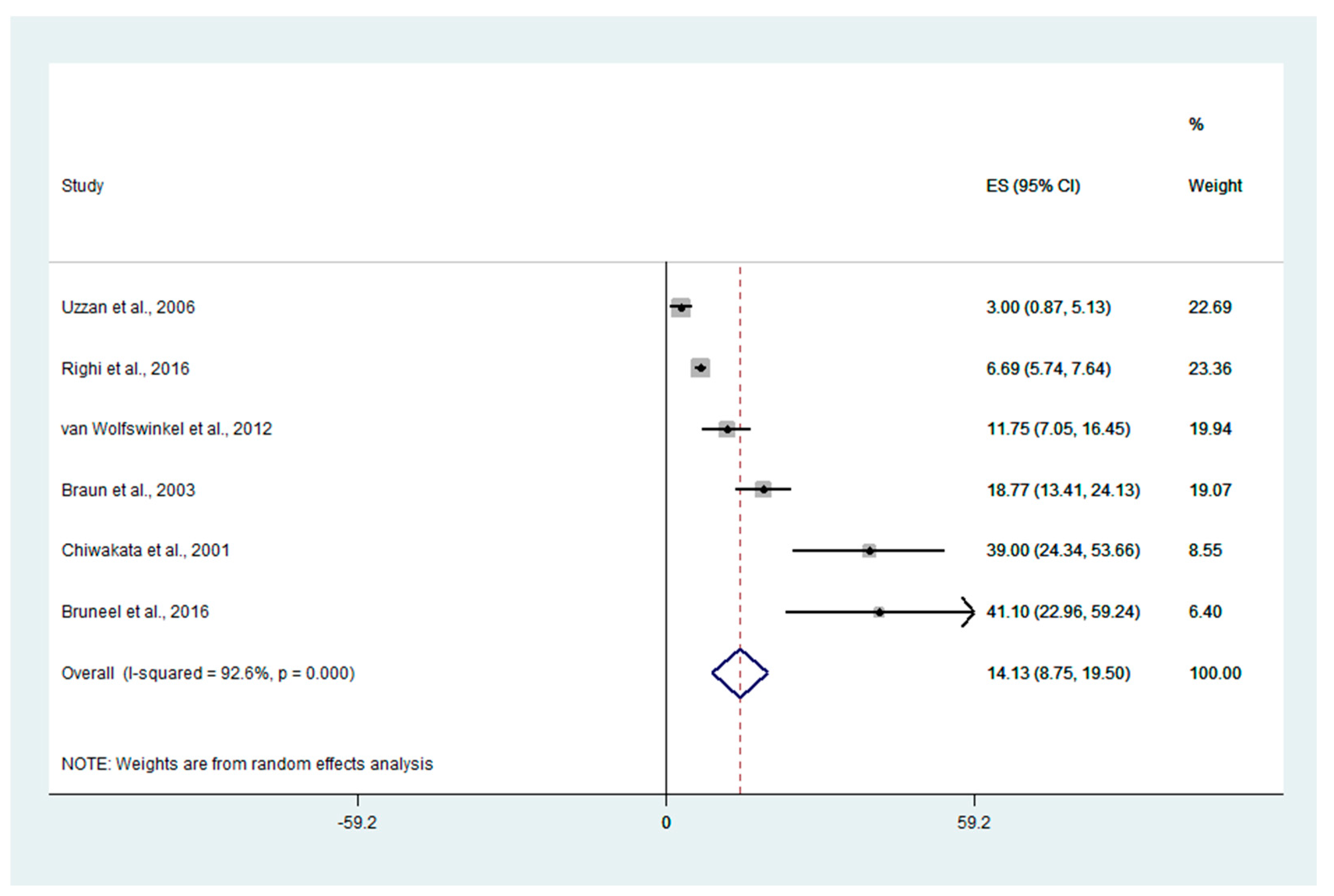
3.5. Differences in PCT Levels between Severe and Uncomplicated Malaria
3.6. Differences in Mean PCT Levels between Uncomplicated Malaria, Asymptomatic Malaria, and Healthy Controls
3.7. Other Information on PCT Levels in Patients with Malaria
3.8. Sensitivity Analysis
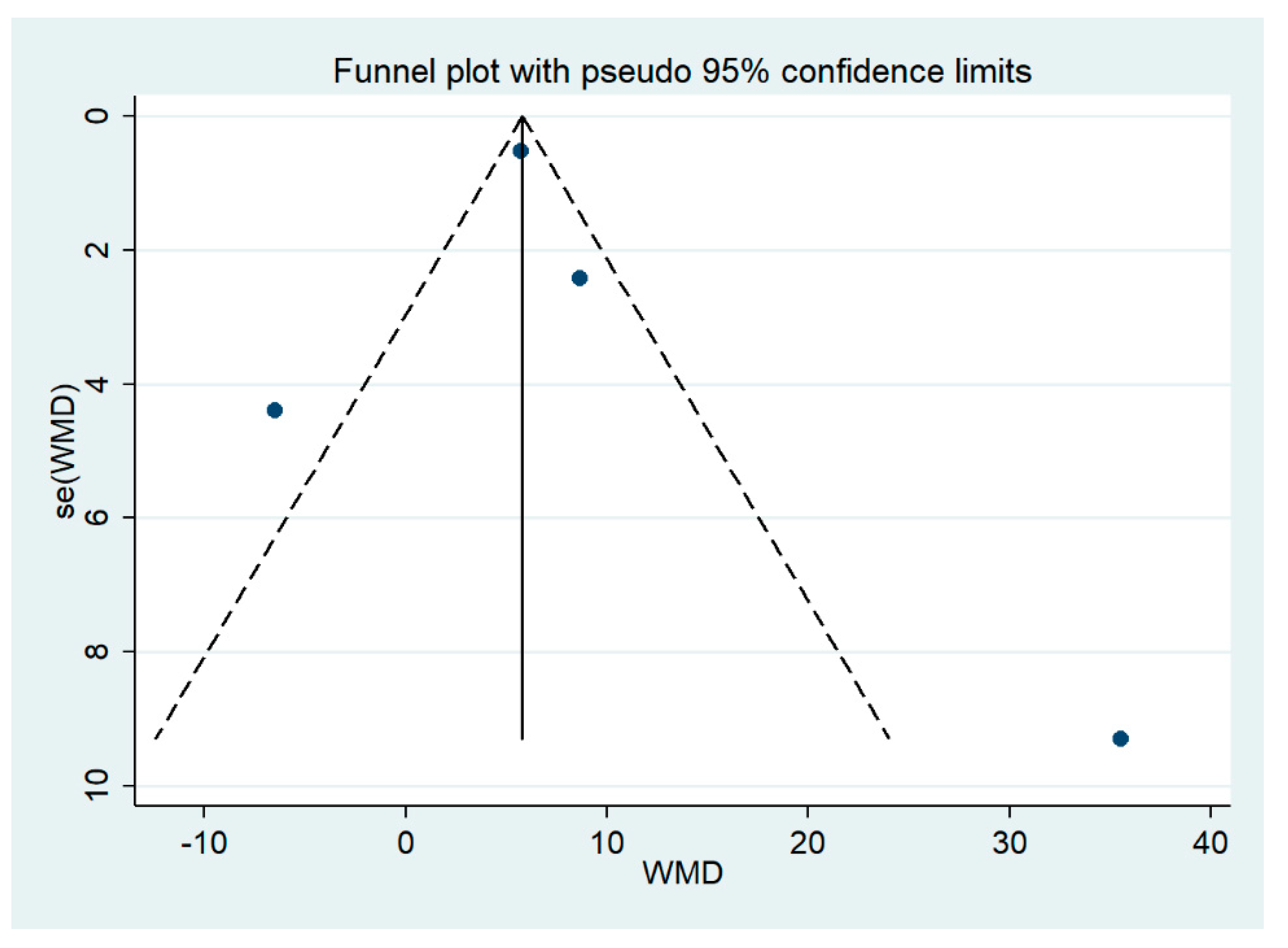
3.9. Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mahittikorn, A.; Masangkay, F.R.; Kotepui, K.U.; Milanez, G.J.; Kotepui, M. Comparison of Plasmodium ovale curtisi and Plasmodium ovale wallikeri infections by a meta-analysis approach. Sci. Rep. 2021, 11, 6409. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Malaria Report 2021; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Kotepui, M.; Kotepui, K.U.; Milanez, G.D.; Masangkay, F.R. Prevalence of severe Plasmodium knowlesi infection and risk factors related to severe complications compared with non-severe P. knowlesi and severe P. falciparum malaria: A systematic review and meta-analysis. Infect. Dis. Poverty 2020, 9, 106. [Google Scholar] [CrossRef]
- Kotepui, M.; Kotepui, K.U.; Milanez, G.J.; Masangkay, F.R. Prevalence and risk factors related to poor outcome of patients with severe Plasmodium vivax infection: A systematic review, meta-analysis, and analysis of case reports. BMC Infect. Dis. 2020, 20, 363. [Google Scholar] [CrossRef] [PubMed]
- Kotepui, M.; Kotepui, K.U.; Milanez, G.D.; Masangkay, F.R. Global prevalence and mortality of severe Plasmodium malariae infection: A systematic review and meta-analysis. Malar. J. 2020, 19, 274. [Google Scholar] [CrossRef] [PubMed]
- Kotepui, M.; Kotepui, K.U.; Milanez, G.D.; Masangkay, F.R. Severity and mortality of severe Plasmodium ovale infection: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0235014. [Google Scholar] [CrossRef] [PubMed]
- Mahittikorn, A.; Masangkay, F.R.; Kotepui, K.U.; Milanez, G.J.; Kotepui, M. Quantification of the misidentification of Plasmodium knowlesi as Plasmodium malariae by microscopy: An analysis of 1569 P. knowlesi cases. Malar. J. 2021, 20, 179. [Google Scholar] [CrossRef]
- Kotepui, M.; Masangkay, F.R.; Kotepui, K.U.; De Jesus Milanez, G. Misidentification of Plasmodium ovale as Plasmodium vivax malaria by a microscopic method: A meta-analysis of confirmed P. ovale cases. Sci. Rep. 2020, 10, 21807. [Google Scholar] [CrossRef]
- Wilairatana, P.; Mala, W.; Klangbud, W.K.; Kotepui, K.U.; Rattaprasert, P.; Kotepui, M. Prevalence, probability, and outcomes of typhoidal/non-typhoidal Salmonella and malaria co-infection among febrile patients: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 21889. [Google Scholar] [CrossRef]
- Wilairatana, P.; Chanmol, W.; Rattaprasert, P.; Masangkay, F.R.; Milanez, G.J.; Kotepui, K.U.; Kotepui, M. Prevalence and characteristics of malaria co-infection among individuals with visceral leishmaniasis in Africa and Asia: A systematic review and meta-analysis. Parasit. Vectors 2021, 14, 545. [Google Scholar] [CrossRef] [PubMed]
- Wilairatana, P.; Masangkay, F.R.; Kotepui, K.U.; Milanez, G.J.; Kotepui, M. Prevalence and characteristics of malaria among COVID-19 individuals: A systematic review, meta-analysis, and analysis of case reports. PLoS Negl. Trop. Dis. 2021, 15, e0009766. [Google Scholar] [CrossRef]
- Mahittikorn, A.; Kotepui, K.U.; De Jesus Milanez, G.; Masangkay, F.R.; Kotepui, M. A meta-analysis on the prevalence and characteristics of severe malaria in patients with Plasmodium spp. and HIV co-infection. Sci. Rep. 2021, 11, 16655. [Google Scholar] [CrossRef] [PubMed]
- Wilairatana, P.; Kuraeiad, S.; Rattaprasert, P.; Kotepui, M. Prevalence of malaria and scrub typhus co-infection in febrile patients: A systematic review and meta-analysis. Parasit. Vectors 2021, 14, 471. [Google Scholar] [CrossRef] [PubMed]
- Mala, W.; Wilairatana, P.; Kotepui, K.U.; Kotepui, M. Prevalence of malaria and chikungunya co-infection in febrile patients: A systematic review and meta-analysis. Trop. Med. Infect. Dis. 2021, 6, 119. [Google Scholar] [CrossRef]
- Wilairatana, P.; Mala, W.; Kotepui, M.; Kotepui, K.U. Alteration of blood lactate levels in severe falciparum malaria: A systematic review and meta-analysis. Biology 2021, 10, 1085. [Google Scholar] [CrossRef] [PubMed]
- van Wolfswinkel, M.E.; Hesselink, D.A.; Hoorn, E.J.; de Rijke, Y.B.; Koelewijn, R.; van Hellemond, J.J.; van Genderen, P.J. Copeptin does not accurately predict disease severity in imported malaria. Malar. J. 2012, 11, 6. [Google Scholar] [CrossRef]
- te Witt, R.; van Wolfswinkel, M.E.; Petit, P.L.; van Hellemond, J.J.; Koelewijn, R.; van Belkum, A.; van Genderen, P.J. Neopterin and procalcitonin are suitable biomarkers for exclusion of severe Plasmodium falciparum disease at the initial clinical assessment of travellers with imported malaria. Malar. J. 2010, 9, 255. [Google Scholar] [CrossRef] [PubMed]
- Hoffmeister, B.; Aguilar Valdez, A.D. Elevated admission C-reactive protein to albumin ratios are associated with disease severity and respiratory complications in adults with imported falciparum malaria. Trans. R. Soc. Trop. Med. Hyg. 2021, 116, 492–500. [Google Scholar] [CrossRef]
- Wilairatana, P.; Mahannop, P.; Tussato, T.; Hayeedoloh, I.M.; Boonhok, R.; Klangbud, W.K.; Mala, W.; Kotepui, K.U.; Kotepui, M. C-reactive protein as an early biomarker for malaria infection and monitoring of malaria severity: A meta-analysis. Sci. Rep. 2021, 11, 22033. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Ahmed, M.Z.; Sharma, S.; Nayak, A.; Anvikar, A.R.; Pande, V. C-reactive protein as a prognostic marker of Plasmodium falciparum malaria severity. J. Vector Borne Dis. 2019, 56, 122–126. [Google Scholar]
- Davies, J. Procalcitonin. J. Clin. Pathol. 2015, 68, 675–679. [Google Scholar] [CrossRef]
- Wiedermann, F.J.; Kaneider, N.; Egger, P.; Tiefenthaler, W.; Wiedermann, C.J.; Lindner, K.H.; Schobersberger, W. Migration of human monocytes in response to procalcitonin. Crit. Care Med. 2002, 30, 1112–1117. [Google Scholar] [CrossRef] [PubMed]
- Meisner, M. Update on procalcitonin measurements. Ann. Lab. Med. 2014, 34, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Moola, S.; Munn, Z.; Tufanaru, C.; Aromataris, E.; Sears, K.; Sfetcu, R.; Currie, M.; Qureshi, R.; Mattis, P.; Lisy, K.; et al. Chapter 7: Systematic Reviews of Etiology and Risk. JBI. 2020. Available online: https://synthesismanual.jbi.global (accessed on 5 January 2022).
- DerSimonian, R.; Kacker, R. Random-effects model for meta-analysis of clinical trials: An update. Contemp. Clin. Trials 2007, 28, 105–114. [Google Scholar] [CrossRef]
- Willis, B.H.; Riley, R.D. Measuring the statistical validity of summary meta-analysis and meta-regression results for use in clinical practice. Stat. Med. 2017, 36, 3283–3301. [Google Scholar] [CrossRef]
- Braun, N.; Marfo, Y.; Von Gärtner, C.; Burchard, G.D.; Zipfel, P.F.; Browne, N.E.; Fleischer, B.; Bröker, B.M. CTLA-4 positive T cells in contrast to procalcitonin plasma levels discriminate between severe and uncomplicated Plasmodium falciparum malaria in Ghanaian children. Trop. Med. Int. Health 2003, 8, 1018–1024. [Google Scholar] [CrossRef] [PubMed]
- Righi, E.; Merelli, M.; Arzese, A.; Siega, P.D.; Scarparo, C.; Bassetti, M. Determination of PCT on admission is a useful tool for the assessment of disease severity in travelers with imported Plasmodium falciparum malaria. Acta Parasitol. 2016, 61, 412–418. [Google Scholar] [CrossRef]
- Bruneel, F.; Tubach, F.; Mira, J.P.; Houze, S.; Gibot, S.; Huisse, M.G.; Megarbane, B.; Choquet, C.; Corne, P.; Peytel, E.; et al. Imported falciparum malaria in adults: Host- and parasite-related factors associated with severity. The French prospective multicenter PALUREA cohort study. Intensive Care Med. 2016, 42, 1588–1596. [Google Scholar] [CrossRef]
- Lin, J.; Huang, X.; Qin, G.; Zhang, S.; Sun, W.; Wang, Y.; Ren, K.; Xu, J.; Han, X. Manual exchange transfusion for severe imported falciparum malaria: A retrospective study. Malar. J. 2018, 17, 32. [Google Scholar] [CrossRef]
- Prodjosoewojo, S.; Riswari, S.F.; Djauhari, H.; Kosasih, H.; van Pelt, L.J.; Alisjahbana, B.; van der Ven, A.J.; de Mast, Q. A novel diagnostic algorithm equipped on an automated hematology analyzer to differentiate between common causes of febrile illness in Southeast Asia. PLoS Negl. Trop. Dis. 2019, 13, e0007183. [Google Scholar] [CrossRef]
- Chiwakata, C.B.; Manegold, C.; Bönicke, L.; Waase, I.; Jülch, C.; Dietrich, M. Procalcitonin as a parameter of disease severity and risk of mortality in patients with Plasmodium falciparum malaria. J. Infect. Dis. 2001, 183, 1161–1164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, B.; Yang, J.G.; He, Y.K.; Xia, S.L.; Shao, J.X.; Yin, Y.X. Prognostic value of procalcitonin recovery level for malaria recrudescence. Jundishapur J. Microbiol. 2019, 12, 5. [Google Scholar] [CrossRef]
- Uzzan, B.; Izri, A.; Durand, R.; Deniau, M.; Bouchaud, O.; Perret, G.Y. Serum procalcitonin in uncomplicated falciparum malaria: A preliminary study. Travel Med. Infect. Dis. 2006, 4, 77–80. [Google Scholar] [CrossRef]
- Hollenstein, U.; Looareesuwan, S.; Aichelburg, A.; Thalhammer, F.; Stoiser, B.; Amradee, S.; Chullawichit, S.; El Menyawi, I.; Burgmann, H. Serum procalcitonin levels in severe Plasmodium falciparum malaria. Am. J. Trop. Med. Hyg. 1998, 59, 860–863. [Google Scholar] [CrossRef]
- Erdman, L.K.; Dhabangi, A.; Musoke, C.; Conroy, A.L.; Hawkes, M.; Higgins, S.; Rajwans, N.; Wolofsky, K.T.; Streiner, D.L.; Liles, W.C.; et al. Combinations of host biomarkers predict mortality among Ugandan children with severe malaria: A retrospective case-control study. PLoS ONE 2011, 6, e17440. [Google Scholar] [CrossRef]
- Lubell, Y.; Blacksell, S.D.; Dunachie, S.; Tanganuchitcharnchai, A.; Althaus, T.; Watthanaworawit, W.; Paris, D.H.; Mayxay, M.; Peto, T.J.; Dondorp, A.M.; et al. Performance of C-reactive protein and procalcitonin to distinguish viral from bacterial and malarial causes of fever in Southeast Asia. BMC Infect. Dis. 2015, 15, 511. [Google Scholar] [CrossRef] [PubMed]
- Hesselink, D.A.; Burgerhart, J.S.; Bosmans-Timmerarends, H.; Petit, P.; van Genderen, P.J. Procalcitonin as a biomarker for severe Plasmodium falciparum disease: A critical appraisal of a semi-quantitative point-of-care test in a cohort of travellers with imported malaria. Malar. J. 2009, 8, 206. [Google Scholar] [CrossRef] [PubMed]
- Mbengue, B.; Diatta, B.; Niang, B.; Diagne, N.; Ndiaye, M.; Marrama, L.; Perraut, R.; Dieye, A. Differential kinetics of plasma procalcitonin levels in cerebral malaria in urban Senegalese patients according to disease outcome. Microbiol. Res. 2011, 2, 80–84. [Google Scholar] [CrossRef]
- Mohapatra, M.K.; Thomas, A.G.; Bariha, P.K.; Patel, D.K. Serum procalcitonin: As a triage tool for severe Plasmodium falciparum malaria. J. Trop. Dis. 2013, 1, 4. [Google Scholar] [CrossRef]
- Becker, K.L.; Snider, R.; Nylen, E.S. Procalcitonin in sepsis and systemic inflammation: A harmful biomarker and a therapeutic target. Br. J. Pharmacol. 2010, 159, 253–264. [Google Scholar] [CrossRef]
- Snider, R.H., Jr.; Nylen, E.S.; Becker, K.L. Procalcitonin and its component peptides in systemic inflammation: Immunochemical characterization. J. Investig. Med. 1997, 45, 552–560. [Google Scholar] [PubMed]
- Samsudin, I.; Vasikaran, S.D. Clinical utility and measurement of procalcitonin. Clin. Biochem. Rev. 2017, 38, 59–68. [Google Scholar] [PubMed]
- Katte, J.C.; Penanje, K.; Agoons, B.B.; Djahmeni, E.N.; Mbacham-Ngwafor, S.; Moor, V.J.A.; Koki, P.; Mbacham, W. Procalcitonin levels in children affected by severe malaria compared to those with uncomplicated malaria in the absence of bacterial infection: A cross-sectional study. Trop. Dis. Travel Med. Vaccines 2022, 8, 5. [Google Scholar] [CrossRef] [PubMed]
- Al-Nawas, B.; Shah, P. Procalcitonin in acute malaria. Eur. J. Med. Res. 1997, 2, 206–208. [Google Scholar] [PubMed]
- Schuetz, P.; Briel, M.; Christ-Crain, M.; Stolz, D.; Bouadma, L.; Wolff, M.; Luyt, C.E.; Chastre, J.; Tubach, F.; Kristoffersen, K.B.; et al. Procalcitonin to guide initiation and duration of antibiotic treatment in acute respiratory infections: An individual patient data meta-analysis. Clin. Infect. Dis. 2012, 55, 651–662. [Google Scholar] [CrossRef]
- Li, H.; Luo, Y.F.; Blackwell, T.S.; Xie, C.M. Meta-analysis and systematic review of procalcitonin-guided therapy in respiratory tract infections. Antimicrob. Agents Chemother. 2011, 55, 5900–5906. [Google Scholar] [CrossRef] [PubMed]
- Post, A.; Kaboré, B.; Bognini, J.; Diallo, S.; Lompo, P.; Kam, B.; Herssens, N.; van Opzeeland, F.; van der Gaast-de Jongh, C.E.; Langereis, J.D.; et al. Infection Manager System (IMS) as a new hemocytometry-based bacteremia detection tool: A diagnostic accuracy study in a malaria-endemic area of Burkina Faso. PLoS Negl. Trop. Dis. 2021, 15, e0009187. [Google Scholar] [CrossRef]
- Akech, S.O.; Kinuthia, D.W.; Macharia, W. Serum procalcitonin levels in children with clinical syndromes for targeting antibiotic use at an Emergency Department of a Kenyan Hospital. J. Trop. Pediatr. 2019, 66, 29–37. [Google Scholar] [CrossRef]
- Page, A.L.; de Rekeneire, N.; Sayadi, S.; Aberrane, S.; Janssens, A.C.; Dehoux, M.; Baron, E. Diagnostic and prognostic value of procalcitonin and C-reactive protein in malnourished children. Pediatrics 2014, 133, e363–e370. [Google Scholar] [CrossRef]
- Ciccone, E.J.; Kabugho, L.; Baguma, E.; Muhindo, R.; Juliano, J.J.; Mulogo, E.; Boyce, R.M. Rapid Diagnostic tests to guide case management of and improve antibiotic stewardship for pediatric acute respiratory illnesses in resource-constrained settings: A prospective cohort study in Southwestern Uganda. Microbiol. Spectr. 2021, 9, e0169421. [Google Scholar] [CrossRef]
- Díez-Padrisa, N.; Bassat, Q.; Machevo, S.; Quintó, L.; Morais, L.; Nhampossa, T.; O’Callaghan-Gordo, C.; Torres, A.; Alonso, P.L.; Roca, A. Procalcitonin and C-reactive protein for invasive bacterial pneumonia diagnosis among children in Mozambique, a malaria-endemic area. PLoS ONE 2010, 5, e13226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fievet, N.; Ezinmegnon, S.; Agbota, G.; Sossou, D.; Ladekpo, R.; Gbedande, K.; Briand, V.; Cottrell, G.; Vachot, L.; Marcos, J.Y.; et al. SEPSIS project: A protocol for studying biomarkers of neonatal sepsis and immune responses of infants in a malaria-endemic region. BMJ Open 2020, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Sinha, M.; Desai, S.; Mantri, S.; Kulkarni, A. Procalcitonin as an adjunctive biomarker in sepsis. Indian J. Anaesth. 2011, 55, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Wangrangsimakul, T.; Althaus, T.; Mukaka, M.; Kantipong, P.; Wuthiekanun, V.; Chierakul, W.; Blacksell, S.D.; Day, N.P.; Laongnualpanich, A.; Paris, D.H. Causes of acute undifferentiated fever and the utility of biomarkers in Chiangrai, northern Thailand. PLoS Negl. Trop. Dis. 2018, 12, e0006477. [Google Scholar] [CrossRef] [PubMed]
- Zammarchi, L.; Antonelli, A.; Bartolini, L.; Pecile, P.; Trotta, M.; Rogasi, P.G.; Santini, M.G.; Dilaghi, B.; Grifoni, S.; Rossolini, G.M.; et al. Louse-borne relapsing fever with meningeal involvement in an immigrant from Somalia to Italy, October 2015. Vector Borne Zoonotic Dis. 2016, 16, 352–355. [Google Scholar] [CrossRef]
- Kafkas, N.; Venetsanou, K.; Patsilinakos, S.; Voudris, V.; Antonatos, D.; Kelesidis, K.; Baltopoulos, G.; Maniatis, P.; Cokkinos, D.V. Procalcitonin in acute myocardial infarction. Acute Card. Care 2008, 10, 30–36. [Google Scholar] [CrossRef]
- Parli, S.E.; Trivedi, G.; Woodworth, A.; Chang, P.K. Procalcitonin: Usefulness in acute care surgery and trauma. Surg. Infect. 2018, 19, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Picariello, C.; Lazzeri, C.; Chiostri, M.; Gensini, G.F.; Valente, S. Kinetic of procalcitonin in patients with cardiogenic shock following acute myocardial infarction: Preliminary data. HSR Proc. Intensive Care Cardiovasc. Anesth. 2010, 2, 201–207. [Google Scholar]
| No. | Reference Study | Study Design | Study Location | Year | Characteristics of Participants | Number of Participants | Plasmodium Spp. | Mean Age (Years or Months) | Age Groups | Male Percentage | Procalcitonin (Mean ± SD or Median, Range) (ng/mL) | Parasite Density (Per Microliter) | Method for Malaria Detections | Method for Procalcitonin |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Braun et al., 2003 [28] | Case-control study | Ghana | NS | Severe malaria (18), Uncomplicated malaria (18), Asymptomatic malaria (21), Healthy controls (13) | 70 | P. falciparum | Severe malaria (18): mean 20.9 months (95%CI 6.6–35.2), Uncomplicated malaria (18): 44.2 (32.2–56.3), Asymptomatic malaria (21): 67.3 (55.5–79.1), Healthy controls (13): 77.5 (58.2–96.9) | Children | NS | Severe malaria (18): 18.77 ± 11.60, Uncomplicated malaria (18): 25.27 ± 14.55, Asymptomatic malaria (21): 0.42 ± 0.27, Healthy controls (13): 0.64 ± 0.32 | Severe malaria (18): mean 17,150 (5394–28,910), Uncomplicated malaria (18): 70,320 (36,380–104,300), Asymptomatic malaria (21): 1709 (1136–2282) | Microscopy | LUMI-Test (Brahms Hennigsdorf, Germany) |
| 2 | Bruneel et al., 2016 [30] | Cohort study | France | 2007–2010 | Severe malaria (155), Uncomplicated malaria (140) | 295 | P. falciparum | Severe malaria (155): 44.4 ± 14.0, Uncomplicated malaria (140): 39.3 ± 12.5 | Adults | Severe malaria (155): 67.1, Uncomplicated malaria (140): 71.4 | Severe malaria (155): 41.1 ± 115.2, Uncomplicated malaria (140): 5.6 ± 11.2 | Severe malaria (155): 8.0% (3.3–15.0), Uncomplicated malaria (140): 0.5% (0.1–2.3) | Microscopy | PCT sensitive Kryptor Analyzer (Brahms, Hennigsdorf, Germany) |
| 3 | Chiwakata et al., 2001 [33] | Prospective observational study | Germany | NS | Severe malaria (25), Uncomplicated malaria (36) | 61 | P. falciparum | NS | NS | NS | Severe malaria (25): 10.67 (2.61–132.1), Uncomplicated malaria (36): 1.52 (0.5–5.6) | NS | Microscopy | Immunoluminometric assay (LUMItest PCT, (BRAHMS Diagnostica)) |
| 4 | Erdman et al., 2011 [37] | Case-control study | Uganda | 2007–2009 | Uncomplicated malaria (53), Cerebral malaria (44), Severe malarial anemia (59) | 156 | P. falciparum | Uncomplicated malaria (53): 4.4 (2.1–8.1), Cerebral malaria (44): 3.0 (1.5–4.3), Severe malarial anemia (59): 1.3 (0.9–2.0) | 6 months and 12 years | Uncomplicated malaria (53): 54.7, Cerebral malaria (44): 47.7, Severe malarial anemia (59): 50.8 | PCT was elevated in children with severe malaria compared to uncomplicated malaria | Uncomplicated malaria (53): 38,600 (16,600–126,000), Cerebral malaria (44): 98,600 (15,600–276,000), Severe malarial anemia (59): 266,00 (7460–126,000) | Microscopy | ELISAs (Ray BioTech) |
| 5 | Hesselink et al., 2009 [39] | Prospective observational study | Netherlands | NS | Severe falciparum malaria (6), Uncomplicated falciparum malaria (65), Uncomplicated non-falciparum malaria (29) | 100 | P. falciparum and non-P. falciparum (P. vivax, P. ovale, P. malariae) | Severe falciparum malaria (6): 48 (29–55), Uncomplicated falciparum malaria (65): 36 (9–67), Uncomplicated non-falciparum malaria (29): 36 (15–62) | Adults | Severe falciparum malaria (6): 66.7, Uncomplicated falciparum malaria (65): 72.3, Uncomplicated non-falciparum malaria (29): 75.9 | Severe falciparum malaria (6): increased 6/6, Uncomplicated falciparum malaria (65): 42/65, Uncomplicated non-falciparum malaria (29): 25/29 | Severe falciparum malaria (6): 174,900 (80,500–567,000), Uncomplicated falciparum malaria (65): 3422 (23–385,000), Uncomplicated non-falciparum malaria (29): NS | Microscopy, RDT, QBC (Quantitative Buffy Coat) | BRAHMS PCT-Q® test (Brahms Diagnostics, Germany) |
| 6 | Hollenstein et al., 1998 [36] | Case-control study | Thailand | NS | Severe malaria (27) | 27 | P. falciparum | 24.8 ± 8.3 | 16–45 | 85.2 | 40 ng/mL (range 0.04–662), elevated 26/27 | 290,680 (533–1,147,040) | Microscopy | Immunoluminometric assay (LUMItest, PCT; Brahms Diagnostica GmbH, Berlin, Germany) |
| 7 | Huang et al., 2019 [34] | Retrospective study | Congo, Angola, Pakistan, African countries | 2014–2018 | Severe malaria (1), Uncomplicated malaria (21) | 22 | P. falciparum (19), P. vivax (2), P. ovale (1) | 27.2 ± 4.8 | 20–48 | 100 | 3.28 (0.95, 13.11), elevated 21/22 | NS | Microscopy and RDT | A Roche Cobas E601 automatic electrochemical luminescence analyzer and an auxiliary reagent (ElecsysBRAHMSprocalcitonin, RocheDiagnostics GmbH) |
| 8 | Lin et al., 2018 [31] | Retrospective study | China | 2007–2016 | Severe malaria (27): exchange transfusion (15), controls (12) | 27 | P. falciparum | Exchange transfusion (15): 47.8 ± 4.4, controls (12): 46.0 ± 5.8 | NS | NS | Exchange transfusion (15): 53.83 ± 29.41 | Exchange transfusion (15): 742,000 ± 518,000, controls (12): 587,000 ± 264,000 | NS | NS |
| 9 | Lubell et al., 2015 [38] | Retrospective study | Cambodia, Laos and, Thailand | NS | Uncomplicated malaria (125) | 125 | NS | NS | NS | NS | Procalcitonin levels were significantly higher in malaria infections as compared with viral infections | NS | NS | Enzyme-Linked Fluorescent Assay technique via the Mini-VIDAS platform (BioMérieux, 69,280 Marcy-l’Etoile, France) |
| 10 | Mbengue et al., 2011 [40] | Prospective observational study | Senegal | 2000–2003 | Cerebral malaria (98) | 98 | P. falciparum | NS | NS | NS | Mean PCT levels were elevated in patients with active infection, with a large range of values (0.1 to 280 nanog per mL) significantly higher on day 0 in fatal cases than in survival cases (53.6 vs. 27.3; p = 0.01). | NS | Microscopy and RDT | Double site sandwich immunoluminometric assay according to the manufacturer’s instructions (LUMI-test® PCT; Brahms Diagnostica Gmbh, Berlin, Germany) |
| 11 | Mohapatra et al., 2013 [41] | Prospective observational study | India | 2011–2012 | Severe malaria (41), Uncomplicated malaria (19) | 60 | P. falciparum | Severe malaria (41): 37.10 ± 13.238, Uncomplicated malaria (19): 37.84 ± 15.5 | Adults | Severe malaria (41): 56.1, Uncomplicated malaria (19): 63.2 | Severe malaria (41): increased 41/41, Uncomplicated malaria (19): 12/19 | Severe malaria (41): 8578.82 ± 214.56, Uncomplicated malaria (19): 4569.58 ± 178.9 | Microscopy | B·R·A·H·M·S PCT-Q (B·R·A·H·M·S, Aktiengesellschaft Neuendorfstrasse 25 D-16761 Hennigsdorf, Germany) |
| 12 | Prodjosoewojo et al., 2019 [32] | Cohort study | Indonesia | 2004–2016 | Patients with malaria (4) | 4 | NS | 25 (19.8–35) | NS | 75 | 34.2 (20.7–43) | NS | Microscopy | BRAHMS PCT, Elecsys and Cobas e 411 analyzers (Roche Diagnostics GmbH, US) |
| 13 | Righi et al., 2016 [29] | Prospective observational study | Italy | 2011–2013 | Severe malaria (9), Uncomplicated malaria (21) | 30 | P. falciparum | Severe malaria (9): 42 ± 13.2, Uncomplicated malaria (21): 41 ± 9.9 | Adults | Severe malaria (9): 89, Uncomplicated malaria (21): 81 | Severe malaria (9): 6.88 (4–9), Uncomplicated malaria (21): 0.38 (0.30–2.72) | Severe malaria (9): 6.25% (3.25–13), Uncomplicated malaria (21): 1% (0.3–1.5) | Microscopy | Automated immunofluorescent assays (B·R·A·H·M·S PCT sensitive KRYPTOR, Brahms Diagnostics, Germany) |
| 14 | Uzzan et al., 2006 [35] | Prospective observational study | France | NS | Severe malaria (3), Uncomplicated malaria (25) | 28 | P. falciparum | 37 ± 13 | Adults | 64.3 | Malaria (18): 3.0 ± 4.6, increased in 14/18 | NS | Microscopy | Immunoluminometric assay on antibody-coated tubes (Lumitestw PCT, BRAHMS Diagnostica GmbH, Berlin, Germany) |
| 15 | van Wolfswinkel et al., 2012 [16] | Cohort study | Netherlands | 1999–2010 | Severe falciparum malaria (25), Uncomplicated falciparum malaria (116), Uncomplicated non-falciparum malaria (63) | 204 | P. falciparum and non-P. falciparum (P. vivax, P. ovale, P. malariae) | Severe falciparum malaria (25): 44 (23–70), Uncomplicated falciparum malaria (116): 41 (11–69), Uncomplicated non-falciparum malaria (63): 38 (8–62) | Adults | Severe falciparum malaria (25): 60, Uncomplicated falciparum malaria (116): 79, Uncomplicated non-falciparum malaria (63): 70 | Severe falciparum malaria (25): 1.9 (0.9–42.3), Uncomplicated falciparum malaria (116): 0.6 (0.0–11.2), Uncomplicated non-falciparum malaria (63): 1.6 (0.0–42.6) | Severe falciparum malaria (25): 284,005 (39,600–1,380,600), Uncomplicated falciparum malaria (116): 22,657 (2–156,600), Uncomplicated non-falciparum malaria (63): NS | Microscopy, RDT, QBC (Quantitative Buffy Coat) | EIA test (VIDAS BRAHMS Procalcitonin, bioMérieux, Lyon, France) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahittikorn, A.; Kotepui, K.U.; Mala, W.; Wilairatana, P.; Kotepui, M. Procalcitonin as a Candidate Biomarker for Malarial Infection and Severe Malaria: A Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 11389. https://doi.org/10.3390/ijerph191811389
Mahittikorn A, Kotepui KU, Mala W, Wilairatana P, Kotepui M. Procalcitonin as a Candidate Biomarker for Malarial Infection and Severe Malaria: A Meta-Analysis. International Journal of Environmental Research and Public Health. 2022; 19(18):11389. https://doi.org/10.3390/ijerph191811389
Chicago/Turabian StyleMahittikorn, Aongart, Kwuntida Uthaisar Kotepui, Wanida Mala, Polrat Wilairatana, and Manas Kotepui. 2022. "Procalcitonin as a Candidate Biomarker for Malarial Infection and Severe Malaria: A Meta-Analysis" International Journal of Environmental Research and Public Health 19, no. 18: 11389. https://doi.org/10.3390/ijerph191811389
APA StyleMahittikorn, A., Kotepui, K. U., Mala, W., Wilairatana, P., & Kotepui, M. (2022). Procalcitonin as a Candidate Biomarker for Malarial Infection and Severe Malaria: A Meta-Analysis. International Journal of Environmental Research and Public Health, 19(18), 11389. https://doi.org/10.3390/ijerph191811389







