Revisiting Standard and Novel Therapeutic Approaches in Halitosis: A Review
Abstract
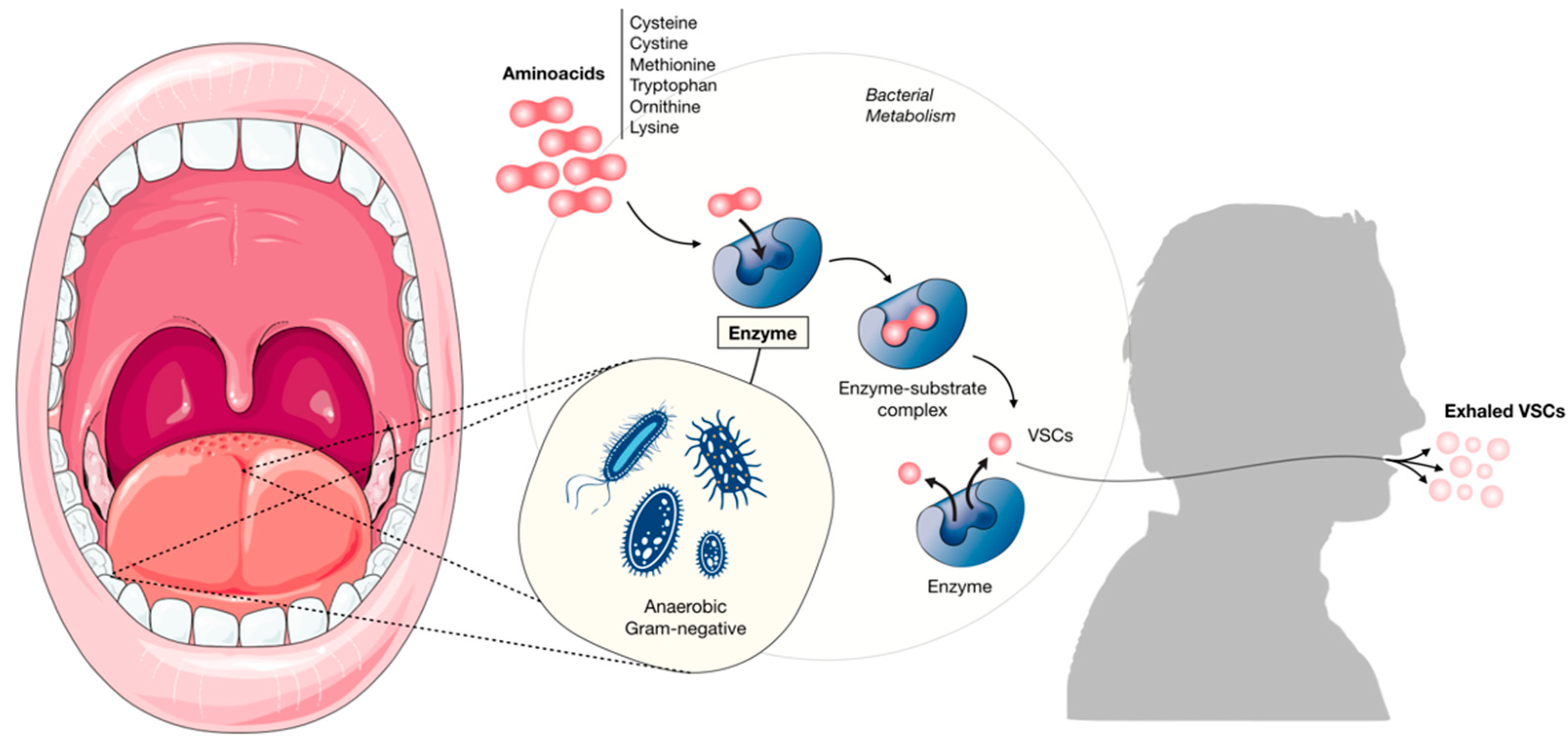
1. Introduction
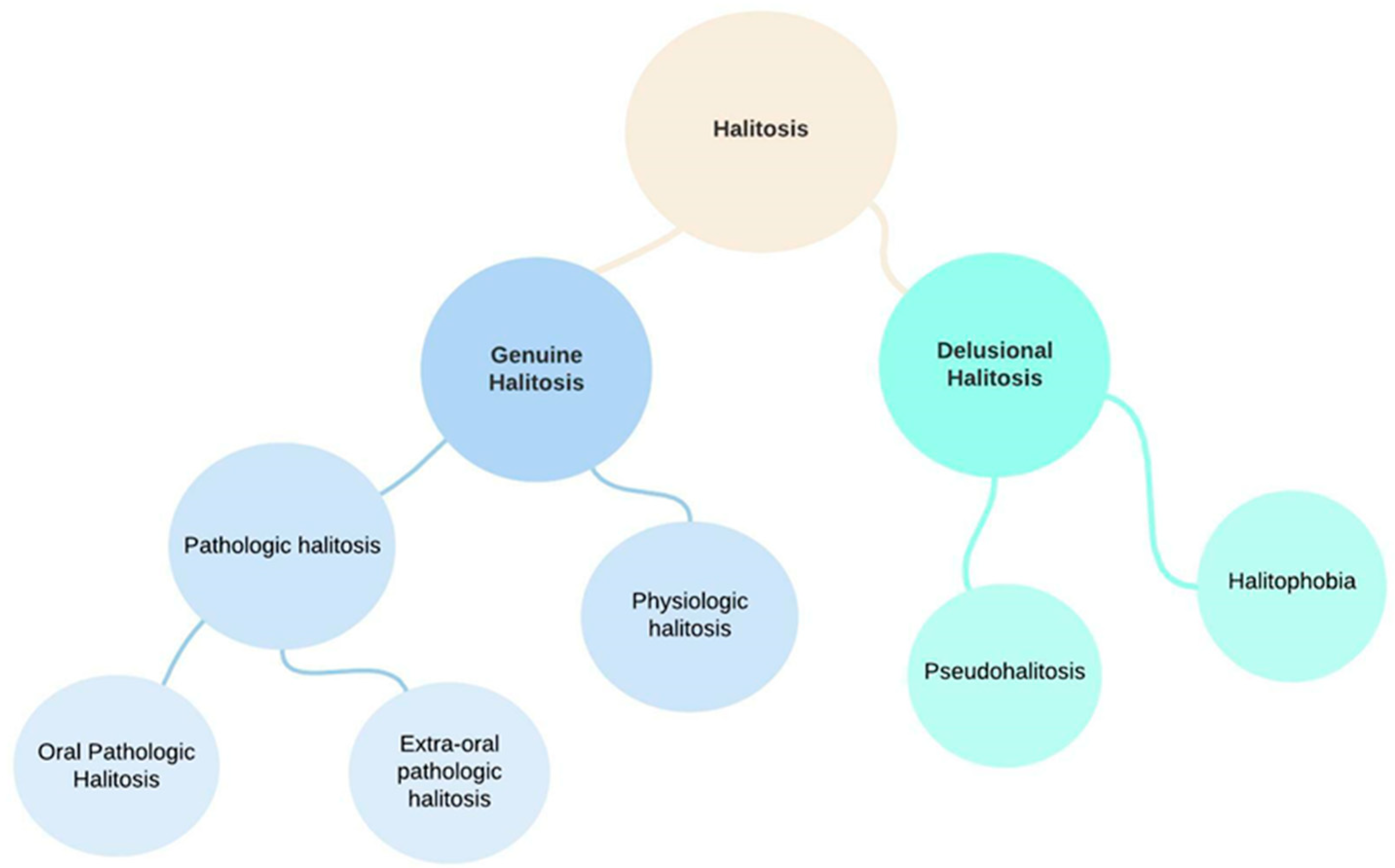
2. Classification of Halitosis
- Genuine halitosis is an oral malodor that is noticeable and exceeds the socially acceptable level.Genuine halitosis can be classified into physiological halitosis or pathological halitosis [14]. Pathological halitosis can originate from oral diseases—intra-oral halitosis, (e.g., tongue coating, periodontal infections, odontogenic infections, xerostomia, mucosal lesions) or systemic diseases—extra-oral pathologic halitosis (e.g., respiratory tract infections, gastrointestinal disease, metabolic disorders, endocrine system disorders, medication).
- 2.
- Delusional halitosis includes pseudohalitosis and halitophobia.
2.1. Risk Factors for Halitosis
2.1.1. Behavioral Factors
2.1.2. Dry Mouth/Xerostomia
2.2. Causes of Halitosis
2.2.1. Extra-Oral Causes
Respiratory System
Gastrointestinal
Metabolic Disorders
Hepatology and Endocrinology
Medication
2.2.2. Intra-Oral Causes
Odontogenic Halitosis
Tongue Coating
Periodontal Disease
- (1)
- Periodontal patients have a higher prevalence of intraoral bacteria (bacterial plaque and tongue coating), decreased pH, which is necessary for amino acid putrefaction and formation of VSCs [78].
- (2)
- (3)
- VSCs also aid in bacterial invasion of connective tissue by their toxic effects on epithelial cells, while methyl mercaptan prevents the growth and proliferation of epithelial cells [25].
Oral Candidiasis
Oral Cancer
Other Oral Sources
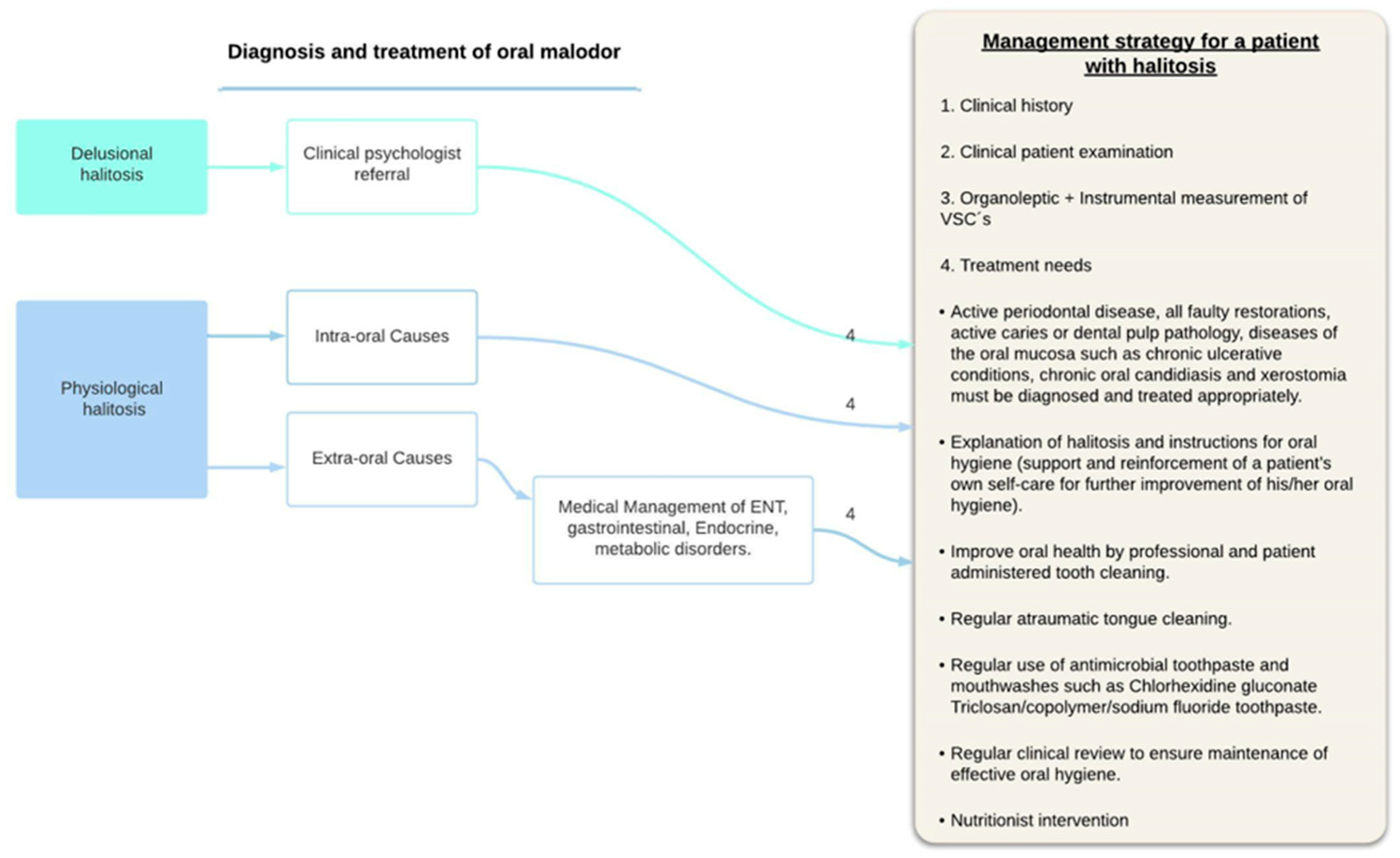
3. Treatment
3.1. Treatments for Intra-Oral Causes
3.1.1. Mechanical Reduction
3.1.2. Chemical Reduction
- Chlorhexidine (CHX): considered the gold standard mouth rinse for halitosis treatment [102]. Its use at a concentration of 0.2% causes a 43% reduction in VSCs and a 50% reduction in organoleptic scores throughout the day [99]. CHX in combination with CPC produce greater reductions in VSCs level, and both aerobic and anaerobic bacterial counts showed the lowest percentage of survival in a randomized, double-blind, cross-over study design [102]. Combined effects of zinc and CHX were studied in a study conducted in 10 participants, Zinc (0.3%) and CHX (0.025%) in low concentration led to 0.16% drop in H2S levels after 1 h, 0.4% drop after 2 h and 0.75% drop after 3 h showing a synergistic effect of the two [103]. However, patients may be reluctant to use CHX long-term as it has an unpleasant taste and can cause (reversible) staining of the teeth [104].
- Essential oils: these products give only a short-term and restricted effect (25% reduction) for 3 h. Furthermore, the reduction in odor-producing bacteria is limited [105]. Usage of Listerine containing essential oils resulted in significant reduction in halitosis-producing bacteria in healthy subjects [106].
- Chlordioxide: It is a strong oxidant that can reduce halitosis by oxidizing H2S, CH3SH, cysteine and methionine. A 29% reduction in odor was reported after 4 h [107].
- A formulation of triclosan/copolymer/sodium fluoride in 3 weeks randomized double blind trial by Hu et al. seemed to be particularly effective in reducing VSC, oral bacteria, and halitosis [109].
3.1.3. Probiotics
3.1.4. Transformation of Volatile Sulfur Components
3.1.5. Masking Effect
3.2. Treatments for Extra-Oral Causes
3.2.1. Halitophobia
3.2.2. Dry Mouth/Xerostomia
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rayman, S.; Almas, K. Halitosis among racially diverse populations: An update. Int. J. Dent. Hyg. 2008, 6, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Nadanovsky, P.; Carvalho, L.B.M.; de Leon, A.P. Oral malodour and its association with age and sex in a general population in Brazil. Oral Dis. 2007, 13, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Lopes, R.G.; De Godoy, C.H.L.; Deana, A.M.; De Santi, M.E.S.O.; Prates, R.A.; França, C.M.; Fernandes, K.P.S.; Mesquita-Ferrari, R.A.; Bussadori, S.K. Photodynamic therapy as a novel treatment for halitosis in adolescents: Study protocol for a randomized controlled trial. Trials 2014, 15, 443. [Google Scholar] [CrossRef] [PubMed]
- Loesche, W.J.; Kazor, C. Microbiology and treatment of halitosis. Periodontol. 2000 2002, 28, 256–279. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, A.C.; McKenzie, D.; Riggio, M.P.; Hodge, P.J.; Rolph, H.; Flanagan, A.; Bagg, J. Microbiological culture analysis of the tongue anaerobic microflora in subjects with and without halitosis. Oral Dis. 2005, 11 (Suppl. S1), 61–63. [Google Scholar] [CrossRef]
- Tonzetich, J. Production and origin of oral malodor: A review of mechanisms and methods of analysis. J. Periodontol. 1977, 48, 13–20. [Google Scholar] [CrossRef]
- Tonzetich, J. Oral malodour: An indicator of health status and oral cleanliness. Int. Dent. J. 1978, 28, 309–319. [Google Scholar]
- Ratcliff, P.A.; Johnson, P.W. The relationship between oral malodor, gingivitis, and periodontitis: A review. J. Periodontol. 1999, 70, 485–489. [Google Scholar] [CrossRef]
- Miyazaki, H.; Sakao, S.; Katoh, Y.; Takehara, T. Correlation between volatile sulphur compounds and certain oral health measurements in the general population. J. Periodontol. 1995, 66, 679–684. [Google Scholar] [CrossRef]
- McDowell, J.D.; Kassebaum, D.K. Diagnosing and Treating Halitosis. J. Am. Dent. Assoc. 1993, 124, 55–64. [Google Scholar] [CrossRef]
- Vandekerckhove, B.; Bollen, C. Epidemiology in the general population, specific populations and in a multidisciplinary halitosis consultation. In Ademgeur Houten; Prelum Uitgevers: Houten, The Netherlands, 2009; pp. 3–10. [Google Scholar]
- Kizhner, V.; Xu, D.; Krespi, Y.P. A New Tool Measuring Oral Malodor Quality of Life. Eur. Arch. Otorhinolaryngol. 2011, 268, 1227–1232. [Google Scholar] [CrossRef] [PubMed]
- Bornstein, M.M.; Kislig, K.; Hoti, B.B.; Seemann, R.; Lussi, A. Prevalence of halitosis in the population of the city of Bern, Switzerland: A study comparing self-reported and clinical data. Eur. J. Oral Sci. 2009, 117, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Bicak, D.A. A Current Approach to Halitosis and Oral Malodor—A Mini Review. Open Dent. J. 2018, 12, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Dossey, L. Listerine’s long shadow: Disease mongering and the selling of sickness. Explore 2006, 2, 379–385. [Google Scholar] [CrossRef]
- Wu, J.; Cannon, R.D.; Ji, P.; Farella, M.; Mei, L. Halitosis: Prevalence, risk factors, sources, measurement and treatment—A review of the literature. Aust. Dent. J. 2020, 65, 4–11. [Google Scholar] [CrossRef]
- Bornstein, M.M.; Stocker, B.L.; Seemann, R.; Bürgin, W.B.; Lussi, A. Prevalence of halitosis in young male adults: A study in swiss army recruits comparing self-reported and clinical data. J. Periodontol. 2009, 80, 24–31. [Google Scholar] [CrossRef]
- Setia, S.; Pannu, P.; Gambhir, R.S.; Galhotra, V.; Ahluwalia, P.; Sofat, A. Correlation of oral hygiene practices, smoking and oral health conditions with self perceived halitosis amongst undergraduate dental students. J. Nat. Sci. Biol. Med. 2014, 5, 67–72. [Google Scholar] [CrossRef]
- Khaira, N.; Palmer, R.M.; Wilson, R.F.; Scott, D.A.; Wade, W.G. Production of volatile sulphur compounds in diseased periodontal pockets is significantly increased in smokers. Oral Dis. 2008, 6, 371–375. [Google Scholar] [CrossRef]
- Hammad, M.M.; Darwazeh, A.M.; Al-Waeli, H.; Tarakji, B.; Alhadithy, T.T. Prevalence and awareness of halitosis in a sample of Jordanian population. J. Int. Soc. Prev. Community Dent. 2014, 4, S178–S186. [Google Scholar] [CrossRef]
- Hughes, F.J.; McNab, R. Oral malodour—A review. Arch. Oral Biol. 2008, 53 (Suppl. S1), S1–S7. [Google Scholar] [CrossRef]
- Spielman, A.I.; Bivona, P.; Rifkin, B.R. Halitosis. A common oral problem. N. Y. State Dent. J. 1996, 62, 36–42. [Google Scholar] [PubMed]
- Almståhl, A.; Wikstrom, M. Oral Microflora in Subjects with Reduced Salivary Secretion. J. Dent. Res. 1999, 78, 1410–1416. [Google Scholar] [CrossRef]
- Alamoudi, N.; Farsi, N.; Faris, J.; Masoud, I.; Merdad, K.; Meisha, D. Salivary characteristics of children and its relation to oral microorganism and lip mucosa dryness. J. Clin. Pediatr. Dent. 2004, 28, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, U.; Sharma, G.; Juneja, M.; Nagpal, A. Halitosis: Current concepts on etiology, diagnosis and management. Eur. J. Dent. 2016, 10, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Pajukoski, H.; Meurman, J.H.; Halonen, P.; Sulkava, R. Prevalence of subjective dry mouth and burning mouth in hospitalized elderly patients and outpatients in relation to saliva, medication, and systemic diseases. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2001, 92, 641–649. [Google Scholar] [CrossRef]
- Iwanicka-Grzegorek, K.; Lipkowska, E.; Kepa, J.; Michalik, J.; Wierzbicka, M. Comparison of ninhydrin method of detecting amine compounds with other methods of halitosis detection. Oral Dis. 2005, 11 (Suppl. S1), 37–39. [Google Scholar] [CrossRef]
- Traudt, M.; Kleinberg, I. Stoichiometry of oxygen consumption and sugar, organic acid and amino acid utilization in salivary sediment and pure cultures of oral bacteria. Arch. Oral Biol. 1996, 41, 965–978. [Google Scholar] [CrossRef]
- Aylıkcı, B.U.; Colak, H. Halitosis: From diagnosis to management. J. Nat. Sci. Biol. Med. 2013, 4, 14–23. [Google Scholar] [CrossRef]
- Tangerman, A.; Winkel, E.G. Intra- and extra-oral halitosis: Finding of a new form of extra-oral blood-borne halitosis caused by dimethyl sulphide. J. Clin. Periodontol. 2007, 34, 748–755. [Google Scholar] [CrossRef]
- Durham, T.M.; Malloy, T.; Hodges, E.D. Halitosis: Knowing when ‘bad breath’ signals systemic disease. Geriatrics 1993, 48, 55–59. [Google Scholar]
- Lanza, D.C. Diagnosis of Chronic Rhinosinusitis. Ann. Otol. Rhinol. Laryngol. 2004, 113, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Richter, J.L. Diagnosis and treatment of halitosis. Compend. Contin. Educ. Dent. 1996, 17, 370–372, 374–376 passim; quiz 388. [Google Scholar] [PubMed]
- Kleinberg, I.; Westbay, G. Oral Malodor. Crit. Rev. Oral Biol. Med. 1990, 1, 247–259. [Google Scholar] [CrossRef]
- Gokdogan, O.; Catli, T.; Ileri, F. Halitosis in Otorhinolaryngology Practice. Iran. J. Otorhinolaryngol. 2015, 27, 145–153. [Google Scholar] [CrossRef]
- Bogdasarian, R.S. Halitosis. Otolaryngol. Clin. N. Am. 1986, 19, 111–117. [Google Scholar]
- Pruet, C.W.; Duplan, D.A. Tonsil concretions and tonsilloliths. Otolaryngol. Clin. N. Am. 1987, 20, 305–309. [Google Scholar] [CrossRef]
- Fletcher, S.M.; Blair, P.A. Chronic halitosis from tonsilloliths: A common etiology. J. La. State Med. Soc. 1988, 140, 7–9. [Google Scholar]
- Mulwafu, W.; Fagan, J.J.; Isaacs, S. Adult tonsillectomy—Are long waiting lists putting patients at risk? S. Afr. J. Surg. 2006, 44, 66–68. [Google Scholar]
- Rosenberg, M. Bad breath, diagnosis and treatment. Univ. Tor. Dent. J. 1990, 3, 7–11. [Google Scholar]
- McNamara, T.F.; Alexander, J.F.; Lee, M. The role of microorganisms in the production of oral malodor. Oral Surg. Oral Med. Oral Pathol. 1972, 34, 41–48. [Google Scholar] [CrossRef]
- Lorber, B. "Bad breath": Presenting manifestation of anaerobic pulmonary infection. Am. Rev. Respir. Dis. 1975, 112, 875–877. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, A.R. Bad Breath. JAMA 1988, 260, 2665. [Google Scholar] [CrossRef]
- Guffin, T.N.W.J.; Lucente, F.E. Bad breath. In Essentials of Otolaryngology; Raven Press: New York, NY, USA, 1993; pp. 257–277. [Google Scholar]
- Greenberger, N.J. Gastrointestinal disorders: A pathophysiologic approach. In Gastrointestinal Disorders: A Pathophysiologic Approach; Year Book: Chicago, IL, USA, 1981; pp. 14–15. [Google Scholar]
- Lu, D.P. Halitosis: An etiologic classification, a treatment approach, and prevention. Oral Surg. Oral Med. Oral Pathol. 1982, 54, 521–526. [Google Scholar] [CrossRef]
- Keles, M.; Tozoglu, U.; Uyanik, A.; Eltas, A.; Bayindir, Y.Z.; Cetinkaya, R.; Bilge, O.M. Does peritoneal dialysis affect halitosis in patients with end-stage renal disease? Perit. Dial. Int. 2011, 31, 168–172. [Google Scholar] [CrossRef]
- Scully, C.; Greenman, J. Halitology (breath odour: Aetiopathogenesis and management). Oral Dis. 2012, 18, 333–345. [Google Scholar] [CrossRef]
- Van Steenberge, D. Endocrinological Aspects; Prelum Uitgevers: Houten, The Netherlands, 2009; pp. 107–115. [Google Scholar]
- Van den Velde, S.; Quirynen, M.; Van Hee, P.; van Steenberghe, D. Halitosis associated volatiles in breath of healthy subjects. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007, 853, 54–61. [Google Scholar] [CrossRef]
- Bollen, C.M.; Rompen, E.H.; Demanez, J.P. Halitosis: A multidisciplinary problem. Rev. Med. Liege 1999, 54, 32–36. [Google Scholar]
- Feller, L.; Blignaut, E. Halitosis: A review. S. Afr. Dent. J. 2005, 60, 17–19. [Google Scholar]
- Whittle, C.L.; Fakharzadeh, S.; Eades, J.; Preti, G. Human Breath Odors and Their Use in Diagnosis. Ann. N. Y. Acad. Sci. 2007, 1098, 252–266. [Google Scholar] [CrossRef]
- Mackay, R.J.; McEntyre, C.J.; Henderson, C.; Lever, M.; George, P.M. Trimethylaminuria: Causes and diagnosis of a socially dis-tressing condition. Clin. Biochem. Rev. 2011, 32, 33–43. [Google Scholar]
- Tangerman, A.; Meuwese-Arends, M.T.; Jansen, J.B. Foetor hepaticus. Lancet 1994, 343, 1569. [Google Scholar] [CrossRef]
- Van der Velde, S.; Nevens, F.; Van Hee, P.; Van Steenberghe, D.; Quirynen, M. GC–MS analysis of breath odor compounds in liver patients. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2008, 875, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, A.; Sugano, N.; Motohashi, M.; Matsumoto, S.; Ito, K. Relationship between oral malodor and the menstrual cycle. J. Periodontal Res. 2010, 45, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Calil, C.M.; Lima, P.O.; Bernardes, C.F.; Groppo, F.C.; Bado, F.; Marcondes, F.K. Influence of gender and menstrual cycle on volatile sulphur compounds production. Arch. Oral Biol. 2008, 53, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Marx, R.E. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: A growing epidemic. J. Oral Maxillofac. Surg. 2003, 61, 1115–1117. [Google Scholar] [CrossRef]
- Stockmann, P.; Vairaktaris, E.; Wehrhan, F.; Seiss, M.; Schwarz, S.; Spriewald, B.; Neukam, F.-W.; Nkenke, E. Osteotomy and primary wound closure in bisphosphonate-associated osteonecrosis of the jaw: A prospective clinical study with 12 months follow-up. Support. Care Cancer 2010, 18, 449–460. [Google Scholar] [CrossRef]
- Mortazavi, H.; Rahbani Nobar, B.; Shafiei, S. Drug-related Halitosis: A Systematic Review. Oral Health Prev. Dent. 2020, 18, 399–407. [Google Scholar] [CrossRef]
- Allaker, R.P.; Waite, R.D.; Hickling, J.; North, M.; McNab, R.; Bosma, M.P.; Hughes, F.J. Topographic distribution of bacteria associated with oral malodour on the tongue. Arch. Oral Biol. 2008, 53 (Suppl. S1), S8–S12. [Google Scholar] [CrossRef]
- Takeuchi, H.; Machigashira, M.; Yamashita, D.; Kozono, S.; Nakajima, Y.; Miyamoto, M.; Takeuchi, N.; Setoguchi, T.; Noguchi, K. The association of periodontal disease with oral malodour in a Japanese population. Oral Dis. 2010, 16, 702–706. [Google Scholar] [CrossRef]
- Tonzetich, J.; Kestenbaum, R.C. Odour production by human salivary fractions and plaque. Arch. Oral Biol. 1969, 14, 815–827. [Google Scholar] [CrossRef]
- Ng, W.; Tonzetich, J. Effect of hydrogen sulfide and methyl mercaptan on the permeability of oral mucosa. J. Dent. Res. 1984, 63, 994–997. [Google Scholar] [CrossRef]
- Sukontapatipark, W.; El-Agroudi, M.A.; Selliseth, N.J.; Thunold, K.; Selvig, K.A. Bacterial colonization associated with fixed orthodontic appliances. A scanning electron microscopy study. Eur. J. Orthod. 2001, 23, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Calil, C.; Liberato, F.L.; Pereira, A.C.; Meneghim, M.D.C.; Goodson, J.M.; Groppo, F.C. The relationship between volatile sulphur compounds, tongue coating and periodontal disease. Int. J. Dent. Hyg. 2009, 7, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Roldán, S.; Herrera, D.; Sanz, M. Biofilms and the tongue: Therapeutical approaches for the control of halitosis. Clin. Oral Investig. 2003, 7, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Doel, J.J.; Benjamin, N.; Hector, M.P.; Rogers, M.; Allaker, R.P. Evaluation of bacterial nitrate reduction in the human oral cavity. Eur. J. Oral Sci. 2005, 113, 14–19. [Google Scholar] [CrossRef]
- Haraszthy, V.I.; Zambon, J.J.; Sreenivasan, P.K.; Zambon, M.M.; Gerber, D.; Rego, R.; Parker, C. Identification of oral bacterial species associated with halitosis. J. Am. Dent. Assoc. 2007, 138, 1113–1120. [Google Scholar] [CrossRef]
- Aimetti, M.; Perotto, S.; Castiglione, A.; Ercoli, E.; Romano, F. Prevalence estimation of halitosis and its association with oral health-related parameters in an adult population of a city in North Italy. J. Clin. Periodontol. 2015, 42, 1105–1114. [Google Scholar] [CrossRef] [PubMed]
- Kojima, K. Clinical studies on the coated tongue. Jpn. J. Oral Maxillofac. Surg. 1985, 31, 1659–1678. [Google Scholar] [CrossRef][Green Version]
- Quirynen, M.; Avontroodt, P.; Soers, C.; Zhao, H.; Pauwels, M.; Van Steenberghe, D. Impact of tongue cleansers on microbial load and taste. J. Clin. Periodontol. 2004, 31, 506–510. [Google Scholar] [CrossRef]
- Grapp, G.L. Fetor oris (halitosis). A medical and dental responsibility. Northwest Med. 1933, 32, 375–380. [Google Scholar]
- Attia, E.L.; Marshall, K.G. Halitosis. Can. Med. Assoc. J. 1982, 126, 1281–1285. [Google Scholar] [PubMed]
- Yaegaki, K.; Sanada, K. Volatile sulfur compounds in mouth air from clinically healthy subjects and patients with periodontal disease. J. Periodontal Res. 1992, 27, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Bolepalli, A.C.; Munireddy, C.; Peruka, S.; Polepalle, T.; Alluri, L.S.C.; Mishaeel, S. Determining the association between oral malodor and periodontal disease: A case control study. J. Int. Soc. Prev. Community Dent. 2015, 5, 413–418. [Google Scholar] [CrossRef]
- Yaegaki, K.; Coil, J.M. Examination, classification, and treatment of halitosis; clinical perspectives. J. Can. Dent. Assoc. 2000, 66, 257–261. [Google Scholar]
- Morita, M.; Wang, H.-L. Relationship between sulcular sulfide level and oral malodor in subjects with periodontal disease. J. Periodontol. 2001, 72, 79–84. [Google Scholar] [CrossRef]
- Setoguchi, T.; Machigashira, M.; Yamamoto, M.; Yotsumoto, Y.; Yoshimori, M.; Izumi, Y.; Yaegaki, K. The effects of methyl mercaptan on epithelial cell growth and proliferation. Int. Dent. J. 2002, 52 (Suppl. S3), 241–246. [Google Scholar] [CrossRef]
- Bosy, A.; Kulkarni, G.V.; Rosenberg, M.; McCulloch, C.A. Relationship of oral malodor to periodontitis: Evidence of independence in discrete subpopulations. J. Periodontol. 1994, 65, 37–46. [Google Scholar] [CrossRef]
- Stamou, E.; Kozlovsky, A.; Rosenberg, M. Association between oral malodour and periodontal disease-related parameters in a population of 71 Israelis. Oral Dis. 2005, 11 (Suppl. S1), 72–74. [Google Scholar] [CrossRef]
- Johnson, B.E. Halitosis, or the meaning of bad breath. J. Gen. Intern. Med. 1992, 7, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Sulser, G.F.; Brening, R.H.; Fosdick, L.S. Some conditions that effect the odor concentration of breath. J. Dent. Res. 1939, 18, 355–359. [Google Scholar] [CrossRef]
- Pratibha, P.K.; Bhat, K.M.; Bhat, G.S. Oral malodor: A review of the literature. J. Dent. Hyg. 2006, 80, 8. [Google Scholar] [PubMed]
- Delanghe, G.; Ghyselen, J.; van Steenberghe, D.; Feenstra, L. Multidisciplinary breath-odour clinic. Lancet 1997, 350, 187. [Google Scholar] [CrossRef]
- Haghgoo, R.; Abbasi, F. Evaluation of the use of a peppermint mouth rinse for halitosis by girls studying in Tehran high schools. J. Int. Soc. Prev. Community Dent. 2013, 3, 29–31. [Google Scholar] [CrossRef] [PubMed]
- Lodhia, P.; Yaegaki, K.; Khakbaznejad, A.; Imai, T.; Sato, T.; Tanaka, T.; Murata, T.; Kamoda, T. Effect of green tea on volatile sulfur compounds in mouth air. J. Nutr. Sci. Vitaminol. 2008, 54, 89–94. [Google Scholar] [CrossRef]
- Zeng, Q.C.; Wu, A.Z.; Pika, J. The effect of green tea extract on the removal of sulfur-containing oral malodor volatiles in vitro and its potential application in chewing gum. J. Breath Res. 2010, 4, 036005. [Google Scholar] [CrossRef]
- Dal Rio, A.C.C.; Nicola, E.M.D.; Teixeira, A.R.F. Halitosis-an assessment protocol proposal. Braz. J. Otorhinolaryngol. 2007, 73, 835–842. [Google Scholar] [CrossRef]
- Bradshaw, D.J.; Perring, K.D.; Cawkill, P.M.; Provan, A.F.; McNulty, D.A.; Saint, E.J.; Richards, J.; Munroe, M.J.; Behan, J.M. Creation of oral care flavours to deliver breath-freshening benefits. Oral Dis. 2005, 11, 75–79. [Google Scholar] [CrossRef]
- Armstrong, B.L.; Sensat, M.L.; Stoltenberg, J.L. Halitosis: A review of current literature. J. Dent. Hyg. 2010, 84, 65–74. [Google Scholar]
- Menon, M.V.; Coykendall, A.L. Effect of Tongue Scraping. J. Dent. Res. 1994, 73, 1492. [Google Scholar] [CrossRef]
- Van der Sleen, M.I.; Slot, D.E.; Trijffel, E.V.; Winkel, E.G.; Weijden, G.A.V.D. Effectiveness of mechanical tongue cleaning on breath odour and tongue coating: A systematic review. Int. J. Dent. Hyg. 2010, 8, 258–268. [Google Scholar] [CrossRef]
- Pedrazzi, V.; Sato, S.; Mattos, M.D.G.C.D.; Lara, E.H.G.; Panzeri, H. Tongue-Cleaning methods: A comparative clinical trial employing a toothbrush and a tongue scraper. J. Periodontol. 2004, 75, 1009–1012. [Google Scholar] [CrossRef] [PubMed]
- Outhouse, T.L.; Al-Alawi, R.; Fedorowicz, Z.; Keenan, J.V. Tongue scraping for treating halitosis. Cochrane Database Syst. Rev. 2006, CD005519. [Google Scholar] [CrossRef]
- Bollen, C.M.; Vandekerckhove, B.N.; Papaioannou, W.; Van Eldere, J.; Quirynen, M. Full- versus partial-mouth disinfection in the treatment of periodontal infections. A pilot study: Long-term microbiological observations. J. Clin. Periodontol. 1996, 23, 960–970. [Google Scholar] [CrossRef] [PubMed]
- Quirynen, M.; Zhao, H.; Soers, C.; DeKeyser, C.; Pauwels, M.; Coucke, W.; Steenberghe, D.V. The impact of periodontal therapy and the adjunctive effect of antiseptics on breath odor-related outcome variables: A double-blind randomized study. J. Periodontol. 2005, 76, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, M.; Gelernter, I.; Barki, M.; Bar-Ness, R. Day-Long reduction of oral malodor by a two-phase oil:water mouthrinse as compared to chlorhexidine and placebo rinses. J. Periodontol. 1992, 63, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Delanghe, G.; Ghyselen, J.; Bollen, C.; Van Steenberghe, D.; Vandekerckhove, B.N.; Feenstra, L. An inventory of patients’ response to treatment at a multidisciplinary breath odor clinic. Quintessence Int. 1999, 30, 307–310. [Google Scholar]
- Fedorowicz, Z.; Aljufairi, H.; Nasser, M.; Outhouse, T.L.; Pedrazzi, V. Mouthrinses for the treatment of halitosis. Cochrane Database Syst. Rev. 2008, CD006701. [Google Scholar] [CrossRef]
- Roldan, S.; Herrera, D.; Santa-Cruz, I.; O’Connor, A.; Gonzalez, I.; Sanz, M. Comparative effects of different chlorhexidine mouth-rinse formulations on volatile sulphur compounds and salivary bacterial counts. J. Clin. Periodontol. 2004, 31, 1128–1134. [Google Scholar] [CrossRef]
- Thrane, P.S.; Young, A.; Jonski, G.; Rölla, G. A new mouthrinse combining zinc and chlorhexidine in low concentrations provides superior efficacy against halitosis compared to existing formulations: A double-blind clinical study. J. Clin. Dent. 2007, 18, 82–86. [Google Scholar]
- Porter, S.R.; Scully, C. Oral malodour (halitosis). BMJ 2006, 333, 632–635. [Google Scholar] [CrossRef]
- Pitts, G.; Brogdon, C.; Hu, L.; Masurat, T.; Pianotti, R.; Schumann, P. Mechanism of action of an antiseptic, anti-odor mouthwash. J. Dent. Res. 1983, 62, 738–742. [Google Scholar] [CrossRef] [PubMed]
- Thaweboon, S.; Thaweboon, B. Effect of an essential oil-containing mouth rinse on VSC-producing bacteria on the tongue. Southeast Asian J. Trop. Med. Public Health 2011, 42, 456–462. [Google Scholar] [PubMed]
- Frascella, J.; Gilbert, R.; Fernandez, P. Odor reduction potential of a chlorine dioxide mouthrinse. J. Clin. Dent. 1998, 9, 39–42. [Google Scholar] [PubMed]
- Davies, R.M.; Ellwood, R.P. The effectiveness of a toothpaste containing triclosan and polyvinyl-methyl ether maleic acid copolymer in improving plaque control and gingival health. A systematic review. J. Clin. Periodontol. 2004, 31, 1029–1033. [Google Scholar] [CrossRef]
- Hu, D.; Zhang, Y.; Petrone, M.; Volpe, A.; DeVizio, W.; Giniger, M. Clinical effectiveness of a triclosan/copolymer/sodium fluoride dentifrice in controlling oral malodor: A 3-week clinical trial. Oral Dis. 2005, 11 (Suppl. S1), 51–53. [Google Scholar] [CrossRef] [PubMed]
- Navada, R.; Kumari, H.; Le, S.; Zhang, J. Oral malodor reduction from a zinc-containing toothpaste. J. Clin. Dent. 2008, 19, 69–73. [Google Scholar]
- Feng, X.; Chen, X.; Cheng, R.; Sun, L.; Zhang, Y.; He, T. Breath malodor reduction with use of a stannous-containing sodium fluoride dentifrice: A meta-analysis of four randomized and controlled clinical trials. Am. J. Dent. 2010, 23, 27B–31B. [Google Scholar]
- Sharma, N.C.; Galustians, H.J.; Qaqish, J.; Galustians, A.; Rustogi, K.; Petrone, M.E.; Chaknis, P.; García, L.; Volpe, A.R.; Proskin, H.M. Clinical effectiveness of a dentifrice containing triclosan and a copolymer for controlling breath odor. Am. J. Dent. 2007, 20, 79–82. [Google Scholar]
- Saïz, P.; Taveira, N.; Alves, R. Probiotics in Oral Health and Disease: A Systematic Review. Appl. Sci. 2021, 11, 8070. [Google Scholar] [CrossRef]
- Iwamoto, T.; Suzuki, N.; Tanabe, K.; Takeshita, T.; Hirofuji, T. Effects of probiotic Lactobacillus salivarius WB21 on halitosis and oral health: An open-label pilot trial. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2010, 110, 201–208. [Google Scholar] [CrossRef]
- Kang, M.-S.; Kim, B.-G.; Chung, J.; Lee, H.-C.; Oh, J.-S. Inhibitory effect of Weissella cibaria isolates on the production of volatile sulphur compounds. J. Clin. Periodontol. 2006, 33, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Henker, J.; Schuster, F.; Nissler, K. Successful treatment of gut-caused halitosis with a suspension of living non-pathogenic Escherichia coli bacteria—A case report. Eur. J. Pediatr. 2001, 160, 592–594. [Google Scholar] [CrossRef] [PubMed]
- Silwood, C.J.; Grootveld, M.C.; Lynch, E. A multifactorial investigation of the ability of oral health care products (OHCPs) to alleviate oral malodour. J. Clin. Periodontol. 2001, 28, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Van Steenberghe, D.; Avontroodt, P.; Peeters, W.; Pauwels, M.; Coucke, W.; Lijnen, A.; Quirynen, M. Effect of different mouthrinses on morning breath. J. Periodontol. 2001, 72, 1183–1191. [Google Scholar] [CrossRef]
- Young, A.; Jonski, G.; Rölla, G.; Wåler, S.M. Effects of metal salts on the oral production of volatile sulfur-containing compounds (VSC). J. Clin. Periodontol. 2001, 28, 776–781. [Google Scholar] [CrossRef]
- Young, A.; Jonski, G.; Rölla, G. Inhibition of orally produced volatile sulfur compounds by zinc, chlorhexidine or cetylpyridinium chloride-effect of concentration. Eur. J. Oral Sci. 2003, 111, 400–404. [Google Scholar] [CrossRef]
- Kleinberg, I.; Wolff, M.S.; Codipilly, D.M. Role of Saliva in Oral Dryness, Oral Feel and Oral Malodour. Int. Dent. J. 2002, 52 (Suppl. S3), 236–240. [Google Scholar] [CrossRef]
- Akpata, O.; Omoregie, O.F.; Akhigbe, K.; Ehikhamenor, E.E. Evaluation of Oral and Extra-Oral Factors Predisposing to Delusional Halitosis. Ghana Med. J. 2009, 43, 61–64. [Google Scholar] [CrossRef]
- Malasi, T.H.; el-Hilu, S.M.; Mirza, I.A.; el-Islam, M.F.; el-Hilu, S.R. Olfactory delusional syndrome with various aetiologies. Br. J. Psychiatry 1990, 156, 256–260. [Google Scholar] [CrossRef]
- Bohn, P. Imagined halitosis: A social phobia symptom? J. Calif. Dent. Assoc. 1997, 25, 161–164. [Google Scholar]
- Yaegaki, K.; Coil, J.M. Clinical dilemmas posed by patients with psychosomatic halitosis. Quintessence Int. 1999, 30, 328–333. [Google Scholar] [PubMed]
- Astor, F.C.; Hanft, K.L.; Ciocon, J.O. Xerostomia: A prevalent condition in the elderly. Ear Nose Throat J. 1999, 78, 476–479. [Google Scholar] [CrossRef] [PubMed]
- McDowell, J.D.; Kassebaum, D.K. Treatment of oral and nonoral sources of halitosis in elderly patients. Drugs Aging 1995, 6, 397–408. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Izidoro, C.; Botelho, J.; Machado, V.; Reis, A.M.; Proença, L.; Alves, R.C.; Mendes, J.J. Revisiting Standard and Novel Therapeutic Approaches in Halitosis: A Review. Int. J. Environ. Res. Public Health 2022, 19, 11303. https://doi.org/10.3390/ijerph191811303
Izidoro C, Botelho J, Machado V, Reis AM, Proença L, Alves RC, Mendes JJ. Revisiting Standard and Novel Therapeutic Approaches in Halitosis: A Review. International Journal of Environmental Research and Public Health. 2022; 19(18):11303. https://doi.org/10.3390/ijerph191811303
Chicago/Turabian StyleIzidoro, Catarina, João Botelho, Vanessa Machado, Ana Mafalda Reis, Luís Proença, Ricardo Castro Alves, and José João Mendes. 2022. "Revisiting Standard and Novel Therapeutic Approaches in Halitosis: A Review" International Journal of Environmental Research and Public Health 19, no. 18: 11303. https://doi.org/10.3390/ijerph191811303
APA StyleIzidoro, C., Botelho, J., Machado, V., Reis, A. M., Proença, L., Alves, R. C., & Mendes, J. J. (2022). Revisiting Standard and Novel Therapeutic Approaches in Halitosis: A Review. International Journal of Environmental Research and Public Health, 19(18), 11303. https://doi.org/10.3390/ijerph191811303










