Comparison of Whole Salivary Cortisol and Interleukin 1-Beta Levels in Light Cigarette-Smokers and Users of Electronic Nicotine Delivery Systems before and after Non-Surgical Periodontal Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Institutional Review Approval
2.2. Study Location
2.3. Criteria for Eligibility
2.4. Questionnaire
2.5. Blinding
2.6. Periodontal Parameters
2.7. Collection of Whole Saliva and Assessment of Cortisol and IL-1β Levels
2.8. Non-Surgical Periodontal Treatment
2.9. Power and Statistical Analyses
3. Results
3.1. Demographics
3.2. Periodontal Parameters
3.3. Whole Salivary Cortisol and IL-1β Levels
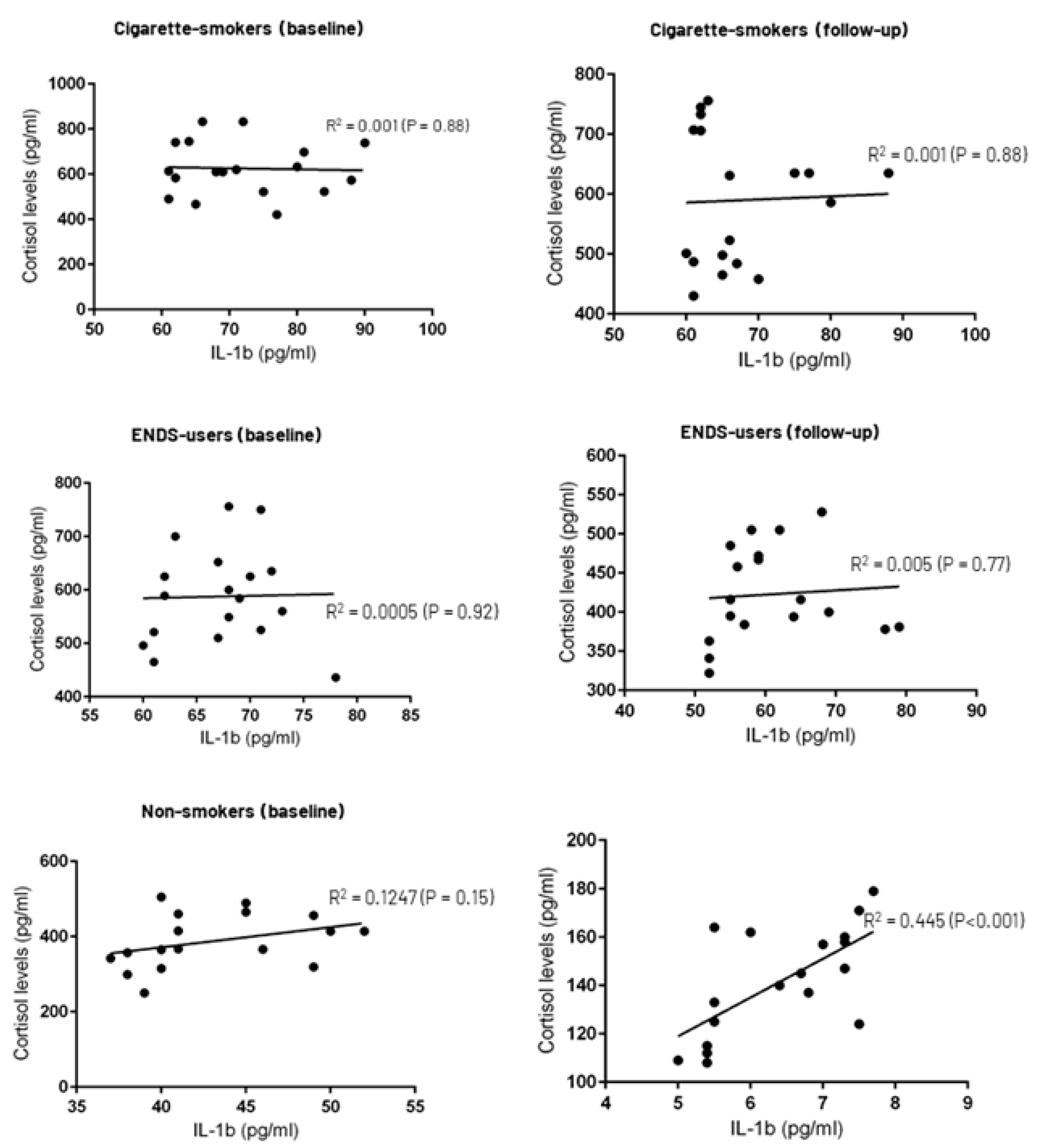
3.4. Correlation between Whole Salivary Cortisol and IL-1β Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rom, O.; Pecorelli, A.; Valacchi, G.; Reznick, A.Z. Are E-cigarettes a safe and good alternative to cigarette smoking? Ann. N. Y. Acad. Sci. 2015, 1340, 65–74. [Google Scholar] [CrossRef] [PubMed]
- ALHarthi, S.S.; BinShabaib, M.; Akram, Z.; Rahman, I.; Romanos, G.E.; Javed, F. Impact of cigarette smoking and vaping on the outcome of full-mouth ultrasonic scaling among patients with gingival inflammation: A prospective study. Clin. Oral Investig. 2019, 23, 2751–2758. [Google Scholar] [CrossRef] [PubMed]
- Madison, M.C.; Landers, C.T.; Gu, B.H.; Chang, C.Y.; Tung, H.Y.; You, R.; Hong, M.J.; Baghaei, N.; Song, L.Z.; Porter, P.; et al. Electronic cigarettes disrupt lung lipid homeostasis and innate immunity independent of nicotine. J. Clin. Investig. 2019, 129, 4290–4304. [Google Scholar] [CrossRef] [PubMed]
- Kavousi, M.; Pisinger, C.; Barthelemy, J.C.; Smedt, D.; Koskinas, K.; Marques-Vidal, P.; Panagiotakos, D.; Prescott, E.B.; Tiberi, M.; Vassiliou, V.S.; et al. Electronic cigarettes and health with special focus on cardiovascular effects: Position paper of the European Association of Preventive Cardiology (EAPC). Eur. J. Prev. Cardiol. 2020, 28, 1552–1566. [Google Scholar] [CrossRef] [PubMed]
- Münzel, T.; Hahad, O.; Kuntic, M.; Keaney, J.F.; Deanfield, J.E.; Daiber, A. Effects of tobacco cigarettes, e-cigarettes, and waterpipe smoking on endothelial function and clinical outcomes. Eur. Heart J. 2020, 41, 4057–4070. [Google Scholar] [CrossRef]
- Javed, F.; Kellesarian, S.V.; Sundar, I.K.; Romanos, G.E.; Rahman, I. Recent updates on electronic cigarette aerosol and inhaled nicotine effects on periodontal and pulmonary tissues. Oral Dis. 2017, 23, 1052–1057. [Google Scholar] [CrossRef]
- Alanazi, H.; Park, H.J.; Chakir, J.; Semlali, A.; Rouabhia, M. Comparative study of the effects of cigarette smoke and electronic cigarettes on human gingival fibroblast proliferation, migration and apoptosis. Food Chem. Toxicol. 2018, 118, 390–398. [Google Scholar] [CrossRef]
- Sundar, I.K.; Javed, F.; Romanos, G.E.; Rahman, I. E-cigarettes and flavorings induce inflammatory and pro-senescence responses in oral epithelial cells and periodontal fibroblasts. Oncotarget 2016, 7, 77196–77204. [Google Scholar] [CrossRef]
- Harris, A.C.; Muelken, P.; Smethells, J.R.; Yershova, K.; Stepanov, I.; Olson, T.T.; Kellar, K.J.; LeSage, M.G. Effects of nicotine-containing and “nicotine-free” e-cigarette refill liquids on intracranial self-stimulation in rats. Drug Alcohol Depend. 2018, 185, 1–9. [Google Scholar] [CrossRef]
- Chang, J.; Meng, H.W.; Lalla, E.; Lee, C.T. The impact of smoking on non-surgical periodontal therapy: A systematic review and meta-analysis. J. Clin. Periodontol. 2021, 48, 60–75. [Google Scholar] [CrossRef]
- Kotsakis, G.A.; Javed, F.; Hinrichs, J.E.; Karoussis, I.K.; Romanos, G.E. Impact of cigarette smoking on clinical outcomes of periodontal flap surgical procedures: A systematic review and meta-analysis. J. Periodontol. 2015, 86, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.L.; Wu, S.L.; Tsai, C.C.; Ko, S.Y.; Yang, J.W. Serum cortisol level and disc displacement disorders of the temporomandibular joint. J. Oral Rehabil. 2016, 43, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, O.; Tasdemir, Z.; Aral, C.A.; Dundar, S.; Koca, H.B. Gingival crevicular fluid and saliva stress hormone levels in patients with chronic and aggressive periodontitis. J. Clin. Periodontol. 2016, 43, 1024–1031. [Google Scholar] [CrossRef] [PubMed]
- Dubar, M.; Clerc-Urmès, I.; Baumann, C.; Clément, C.; Alauzet, C.; Bisson, C. Relations of Psychosocial Factors and Cortisol with Periodontal and Bacterial Parameters: A Prospective Clinical Study in 30 Patients with Periodontitis before and after Non-Surgical Treatment. Int. J. Environ. Res. Public Health 2020, 17, 7651. [Google Scholar] [CrossRef]
- Alresayes, S.; Al-Aali, K.; Javed, F.; Alghamdi, O.; Mokeem, S.A.; Vohra, F.; Abduljabbar, T. Assessment of self-rated pain perception and whole salivary cortisol levels among adolescents with and without temporomandibular disorders. Cranio 2021, 1–7. [Google Scholar] [CrossRef]
- Alresayes, S.; Al-Askar, M.; Mokeem, S.A.; Javed, F.; Vohra, F.; Abduljabbar, T. Cortisol levels in the peri-implant sulcular fluid among patients with and without peri-implantitis. J. Periodontal Res. 2021, 56, 746–752. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, B.; Pan, C.; Zhang, A. To evaluate the serum cortisol, salivary cortisol, and serum interleukin-1 B level in patients of chronic periodontitis with smoking and stress and without smoking and stress. Medicine 2021, 100, e26757. [Google Scholar] [CrossRef]
- Liukkonen, J.; Gürsoy, U.K.; Pussinen, P.J.; Suominen, A.L.; Könönen, E. Salivary Concentrations of Interleukin (IL)-1β, IL-17A, and IL-23 Vary in Relation to Periodontal Status. J. Periodontol. 2016, 87, 1484–1491. [Google Scholar] [CrossRef]
- Sánchez, G.A.; Miozza, V.A.; Delgado, A.; Busch, L. Salivary IL-1β and PGE2 as biomarkers of periodontal status, before and after periodontal treatment. J. Clin. Periodontol. 2013, 40, 1112–1117. [Google Scholar] [CrossRef]
- Mokeem, S.A.; Alasqah, M.N.; Michelogiannakis, D.; Al-Kheraif, A.A.; Romanos, G.E.; Javed, F. Clinical and radiographic periodontal status and whole salivary cotinine, IL-1β and IL-6 levels in cigarette- and waterpipe-smokers and E-cig users. Environ. Toxicol. Pharmacol. 2018, 61, 38–43. [Google Scholar] [CrossRef]
- Javed, F.; Al-Zawawi, A.S.; Allemailem, K.S.; Almatroudi, A.; Mehmood, A.; Divakar, D.D.; Al-Kheraif, A.A. Periodontal Conditions and Whole Salivary IL-17A and -23 Levels among Young Adult Cannabis sativa (Marijuana)-Smokers, Heavy Cigarette-Smokers and Non-Smokers. Int. J. Environ. Res. Public Health 2020, 17, 7435. [Google Scholar] [CrossRef] [PubMed]
- Javed, F.; Näsström, K.; Benchimol, D.; Altamash, M.; Klinge, B.; Engström, P.E. Comparison of periodontal and socioeconomic status between subjects with type 2 diabetes mellitus and non-diabetic controls. J. Periodontol. 2007, 78, 2112–2119. [Google Scholar] [CrossRef] [PubMed]
- Javed, F.; Abduljabbar, T.; Vohra, F.; Malmstrom, H.; Rahman, I.; Romanos, G.E. Comparison of Periodontal Parameters and Self-Perceived Oral Symptoms among Cigarette-Smokers, Individuals Vaping Electronic-Cigarettes and Never-Smokers: A Pilot Study. J. Periodontol. 2017, 88, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- Ainamo, J.; Bay, I. Problems and proposals for recording gingivitis and plaque. Int. Dent. J. 1975, 25, 229–235. [Google Scholar]
- Armitage, G.C. Diagnosis of periodontal diseases. J. Periodontol. 2003, 74, 1237–1247. [Google Scholar] [CrossRef]
- Updegrave, W.J. The paralleling extension-cone technique in intraoral dental radiography. Oral Surg. Oral Med. Oral Pathol. 1951, 4, 1250–1261. [Google Scholar] [CrossRef]
- Khocht, A.; Janal, M.; Harasty, L.; Chang, K.M. Comparison of direct digital and conventional intraoral radiographs in detecting alveolar bone loss. J. Am. Dent. Assoc. 2003, 134, 1468–1475. [Google Scholar] [CrossRef]
- Kobus, A.; Kierklo, A.; Zalewska, A.; Kuźmiuk, A.; Szajda, S.D.; Ławicki, S.; Bagińska, J. Unstimulated salivary flow, pH, proteins and oral health in patients with Juvenile Idiopathic Arthritis. BMC Oral Health 2017, 17, 94. [Google Scholar] [CrossRef]
- Chinthakanan, S.; Laosuwan, K.; Boonyawong, P.; Kumfu, S.; Chattipakorn, N.; Chattipakorn, S.C. Reduced heart rate variability and increased saliva cortisol in patients with TMD. Arch. Oral Biol. 2018, 90, 125–129. [Google Scholar] [CrossRef]
- Nociti, F.H., Jr.; Casati, M.Z.; Duarte, P.M. Current perspective of the impact of smoking on the progression and treatment of periodontitis. Periodontology 2000 2015, 67, 187–210. [Google Scholar] [CrossRef]
- Haber, J. Smoking is a major risk factor for periodontitis. Curr. Opin. Periodontol. 1994, 12–18. [Google Scholar]
- Lee, Y.H.; Shin, M.H.; Kweon, S.S.; Choi, J.S.; Rhee, J.A.; Ahn, H.R.; Yun, W.J.; Ryu, S.Y.; Kim, B.H.; Nam, H.S.; et al. Cumulative smoking exposure, duration of smoking cessation, and peripheral arterial disease in middle-aged and older Korean men. BMC Public Health 2011, 11, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, F.O.; Cota, L.O.M. Cumulative smoking exposure and cessation associated with the recurrence of periodontitis in periodontal maintenance therapy: A 6-year follow-up. J. Periodontol. 2019, 90, 856–865. [Google Scholar] [CrossRef] [PubMed]
- Taghavi, S.; Khashyarmanesh, Z.; Moalemzadeh-Haghighi, H.; Nassirli, H.; Eshraghi, P.; Jalali, N.; Hassanzadeh-Khayyat, M. Nicotine content of domestic cigarettes, imported cigarettes and pipe tobacco in iran. Addict. Health 2012, 4, 28–35. [Google Scholar]
- Attin, T.; Hornecker, E. Tooth brushing and oral health: How frequently and when should tooth brushing be performed? Oral Health Prev. Dent. 2005, 3, 135–140. [Google Scholar]
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89 (Suppl. 1), S173–S182. [Google Scholar] [CrossRef]
- Chapple, I.L.C.; Mealey, B.L.; Van Dyke, T.E.; Bartold, P.M.; Dommisch, H.; Eickholz, P.; Geisinger, M.L.; Genco, R.J.; Glogauer, M.; Goldstein, M.; et al. Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium: Consensus report of workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89 (Suppl. 1), S74–S84. [Google Scholar] [CrossRef]
- Antwi-Amoabeng, D.; Islam, R. Vaping Is Not Safe: A Case of Acute Eosinophilic Pneumonia following Cannabis Vapor Inhalation. Case Rep. Pulmonol. 2020, 2020, 9496564. [Google Scholar] [CrossRef]
- Wigginton, B.; Gartner, C.; Rowlands, I.J. Is It Safe to Vape? Analyzing Online Forums Discussing E-Cigarette Use during Pregnancy. Womens Health Issues 2017, 27, 93–99. [Google Scholar] [CrossRef]
- Zhang, Y.; Florath, I.; Saum, K.U.; Brenner, H. Self-reported smoking, serum cotinine, and blood DNA methylation. Environ. Res. 2016, 146, 395–403. [Google Scholar] [CrossRef]
- Kim, J.; Lee, S. Daily Cigarette Consumption and Urine Cotinine Level between Dual Users of Electronic and Conventional Cigarettes, and Cigarette-Only Users. J. Psychoact. Drugs 2020, 52, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Etter, J.F. A longitudinal study of cotinine in long-term daily users of e-cigarettes. Drug Alcohol Depend. 2016, 160, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Callahan-Lyon, P. Electronic cigarettes: Human health effects. Tob. Control 2014, 23 (Suppl. 2), ii36–ii40. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.W.; Ein, N.; Peck, K.; Huang, V.; Pruessner, J.C.; Vickers, K. Sex differences in salivary cortisol reactivity to the Trier Social Stress Test (TSST): A meta-analysis. Psychoneuroendocrinology 2017, 82, 26–37. [Google Scholar] [CrossRef] [Green Version]
| Parameters | Cigarette-Smokers | ENDS-Users | Non-Smokers |
|---|---|---|---|
| Patients (n) | 18 | 18 | 18 |
| Gender | 14 males | 12 males | 10 males |
| 4 females | 6 females | 8 females | |
| Mean age | 45.6 ± 2.8 years | 41.3 ± 1.8 years | 42.2 ± 3.5 years |
| Duration of smoking pack years | 12.7 ± 2.2 pack-years | NA | NA |
| Duration of vaping | NA | 6.8 ± 0.5 years | NA |
| Puffs per vaping session | NA | 4.3 ± 0.5 puffs/session | NA |
| Family history of smoking | 11 (61.1%) | 12 (66.7%) | 3 (16.7%) |
| Tooth brushing | |||
| Once daily | 7 (38.9%) | 8 (44.4%) | 5 (27.8%) |
| Twice daily | 11 (61.1%) | 10 (55.6%) | 13 (72.2%) |
| Flossing | |||
| Once daily | None | None | None |
| Baseline | 12-Weeks’ Follow-Up | |||||
|---|---|---|---|---|---|---|
| Parameters | Cigarette-Smokers (n = 18) | ENDS-Users (n = 18) | Non-Smokers (n = 18) | Cigarette-Smokers (n = 18) | ENDS-Users (n = 18) | Non-Smokers (n = 18) |
| Missing teeth (n) | 5.2 ± 0.4 teeth | 4.5 ± 0.1 teeth | 4.4 ± 0.5 teeth | 5.2 ± 0.4 teeth | 4.5 ± 0.1 teeth | 4.4 ± 0.5 teeth |
| Plaque index | 2.1 ± 0.2 | 1.8 ± 0.2 | 2.1 ± 0.3 | 1.5 ± 0.3 † | 1.2 ± 0.04 † | 0.5 ± 0.007 |
| Gingival index | 0.5 ± 0.07 * | 0.7 ± 0.05 * | 2.5 ± 0.2 | 0.5 ± 0.04 | 0.5 ± 0.06 | 0.4 ± 0.003 |
| Probing depth | 4.5 ± 0.3 mm | 4.4 ± 0.5 mm | 4.6 ± 0.5 mm | 2.5 ± 0.2 † mm | 2.6 ± 0.3 † mm | 0.6 ± 0.03 mm |
| Clinical attachment loss | 1.7 ± 0.07 mm | 1.4 ± 0.1 mm | 1.4 ± 0.2 mm | 1.7 ± 0.04 mm | 1.5 ± 0.08 mm | 1.4 ± 0.06 mm |
| Marginal bone loss (mesial surface) | 3.2 ± 0.4 mm | 2.9 ± 0.5 mm | 2.7 ± 0.4 mm | NA | NA | NA |
| Marginal bone loss (distal surface) | 3.3 ± 0.5 mm | 3.04 ± 0.3 mm | 2.7 ± 0.5 mm | NA | NA | NA |
| Baseline | 12-Weeks’ Follow-Up | |||||
|---|---|---|---|---|---|---|
| Parameters | Cigarette-Smokers (n = 18) | ENDS-Users (n = 18) | Non-Smokers (n = 18) | Cigarette-Smokers (n = 18) | ENDS-Users (n = 18) | Non-Smokers (n = 18) |
| Salivary flow rate (mL/min) | 0.15 ± 0.03 mL/min | 0.13 ± 0.01 mL/min | 0.11 ± 0.02 mL/min | 0.13 ± 0.02 mL/min | 0.12 ± 0.01 mL/min | 0.12 ± 0.01 mL/min |
| Interleukin 1β (pg/mL) | 72.2 ± 9.3 pg/mL | 67.3 ± 5.1 pg/mL | 42.8 ± 3.7 pg/mL * | 54.1 ± 7.2 pg/mL | 60.7 ± 5.5 pg/mL | 6.4 ± 1.3 pg/mL |
| Cortisol levels (pg/mL) | 625.4 ± 204.8 pg/mL | 589.7 ± 153.3 pg/mL | 386.4 ± 87.5 pg/mL | 516.8 ± 143.8 pg/mL | 422.8 ± 108.3 pg/mL | 141.8 ± 33.7 pg/mL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhumaidan, A.A.; Al-Aali, K.A.; Vohra, F.; Javed, F.; Abduljabbar, T. Comparison of Whole Salivary Cortisol and Interleukin 1-Beta Levels in Light Cigarette-Smokers and Users of Electronic Nicotine Delivery Systems before and after Non-Surgical Periodontal Therapy. Int. J. Environ. Res. Public Health 2022, 19, 11290. https://doi.org/10.3390/ijerph191811290
Alhumaidan AA, Al-Aali KA, Vohra F, Javed F, Abduljabbar T. Comparison of Whole Salivary Cortisol and Interleukin 1-Beta Levels in Light Cigarette-Smokers and Users of Electronic Nicotine Delivery Systems before and after Non-Surgical Periodontal Therapy. International Journal of Environmental Research and Public Health. 2022; 19(18):11290. https://doi.org/10.3390/ijerph191811290
Chicago/Turabian StyleAlhumaidan, Abdulkareem A., Khulud A. Al-Aali, Fahim Vohra, Fawad Javed, and Tariq Abduljabbar. 2022. "Comparison of Whole Salivary Cortisol and Interleukin 1-Beta Levels in Light Cigarette-Smokers and Users of Electronic Nicotine Delivery Systems before and after Non-Surgical Periodontal Therapy" International Journal of Environmental Research and Public Health 19, no. 18: 11290. https://doi.org/10.3390/ijerph191811290
APA StyleAlhumaidan, A. A., Al-Aali, K. A., Vohra, F., Javed, F., & Abduljabbar, T. (2022). Comparison of Whole Salivary Cortisol and Interleukin 1-Beta Levels in Light Cigarette-Smokers and Users of Electronic Nicotine Delivery Systems before and after Non-Surgical Periodontal Therapy. International Journal of Environmental Research and Public Health, 19(18), 11290. https://doi.org/10.3390/ijerph191811290







