Mental Fatigue Effects on the Produced Perception of Effort and Its Impact on Subsequent Physical Performances
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Exprimental Protocol
2.3. Obtaining Psychological and Dynamometric Parameters
2.3.1. Brunel Mood Scale
2.3.2. NASA Task Load Index (NASA-TLX)
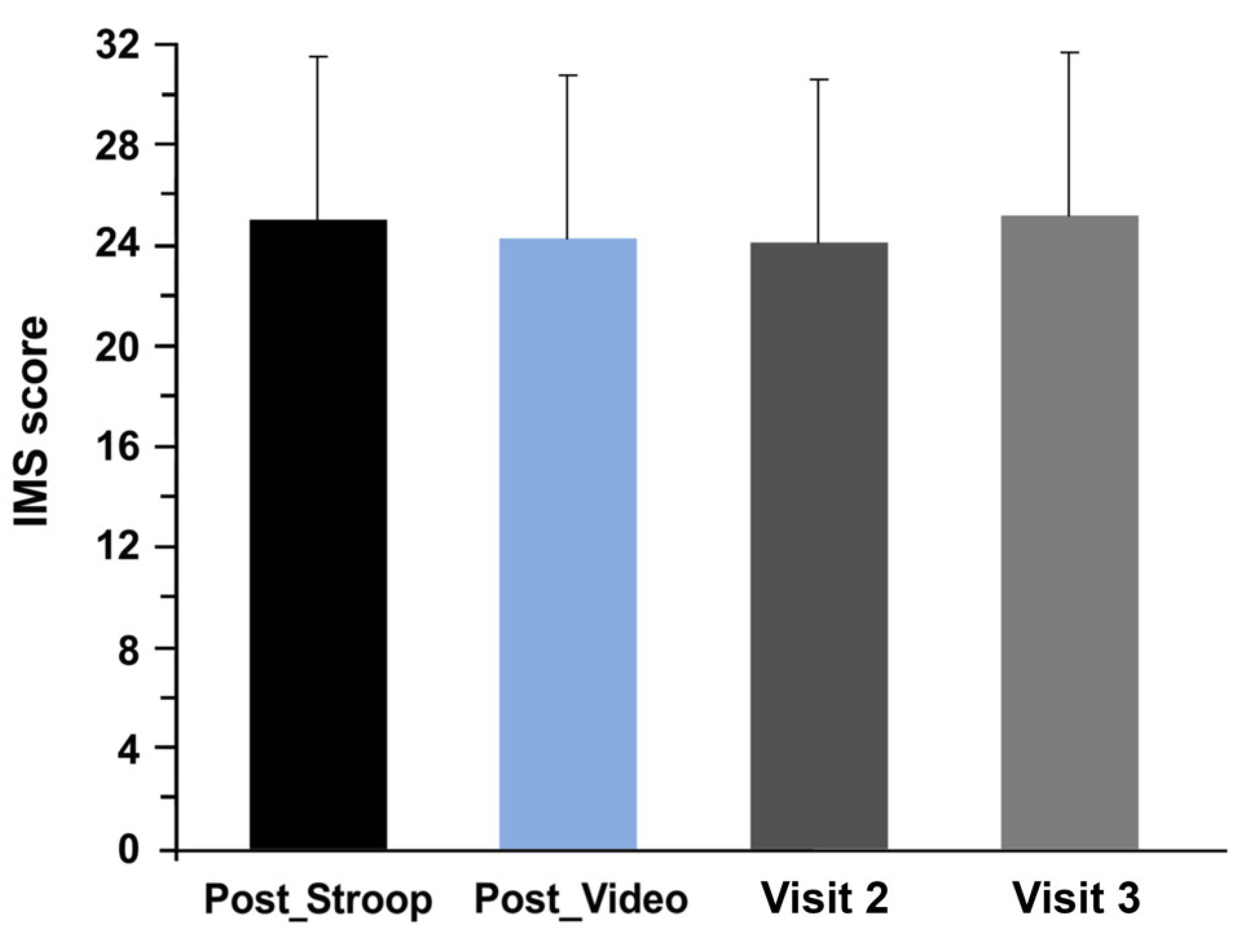
2.3.3. Intrinsic Motivation Scale (IMS)
2.3.4. The Mood Questionnaire (QM)
2.4. EMG Procedure
2.5. Statistical Analysis
3. Results
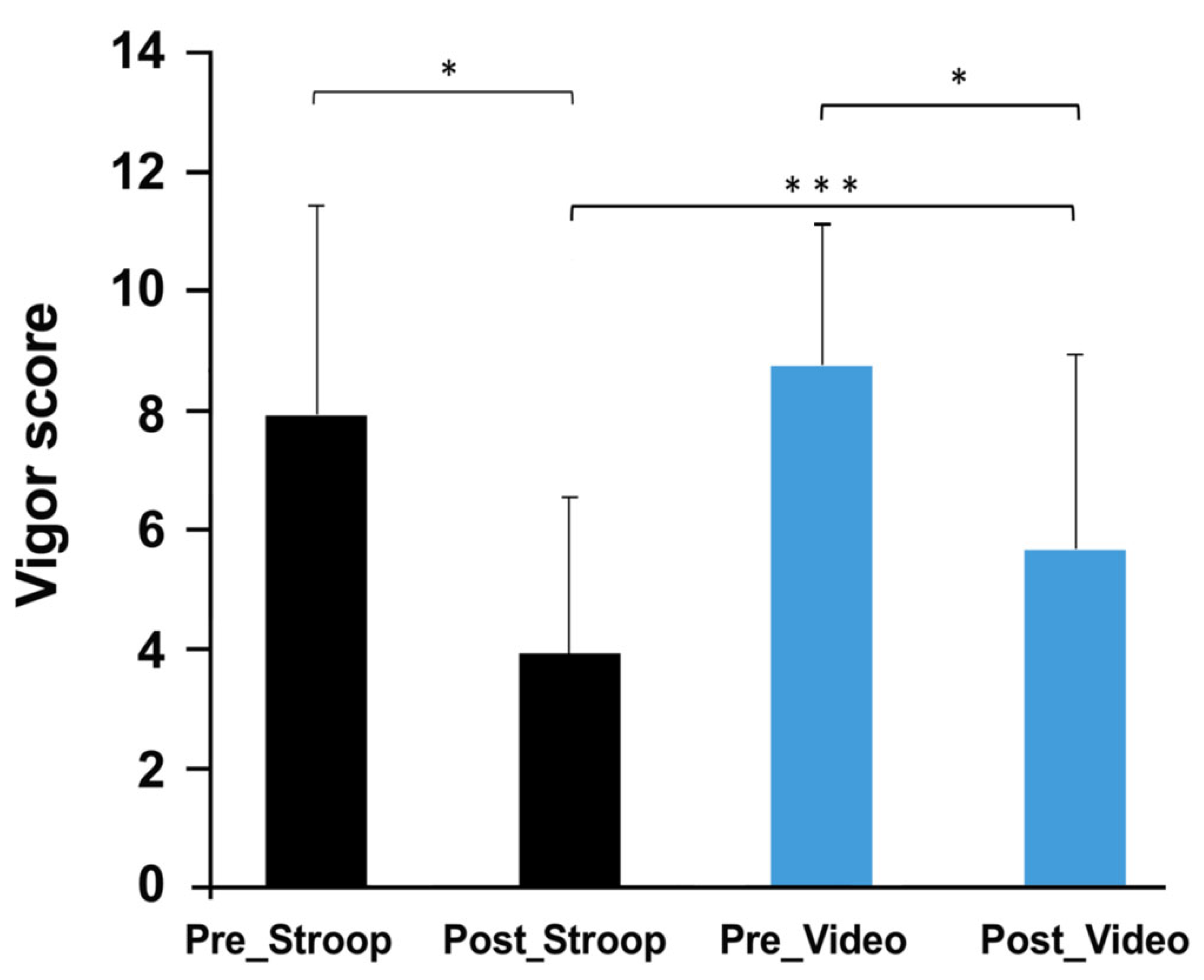
3.1. Evolution of Mood and Motivation
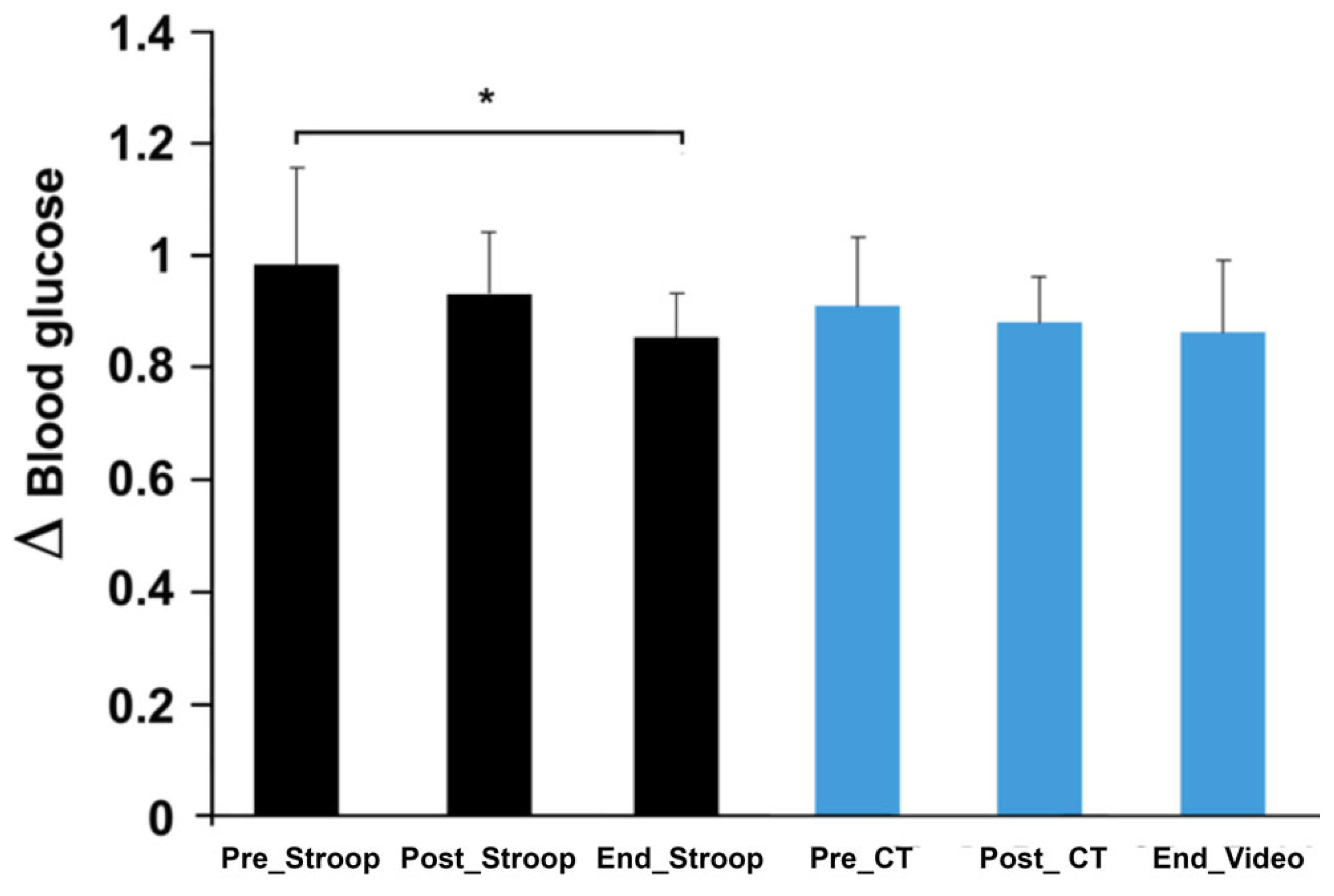
3.2. Effects of the Response Inhibition on Blood Sugar and the Subjective Workload
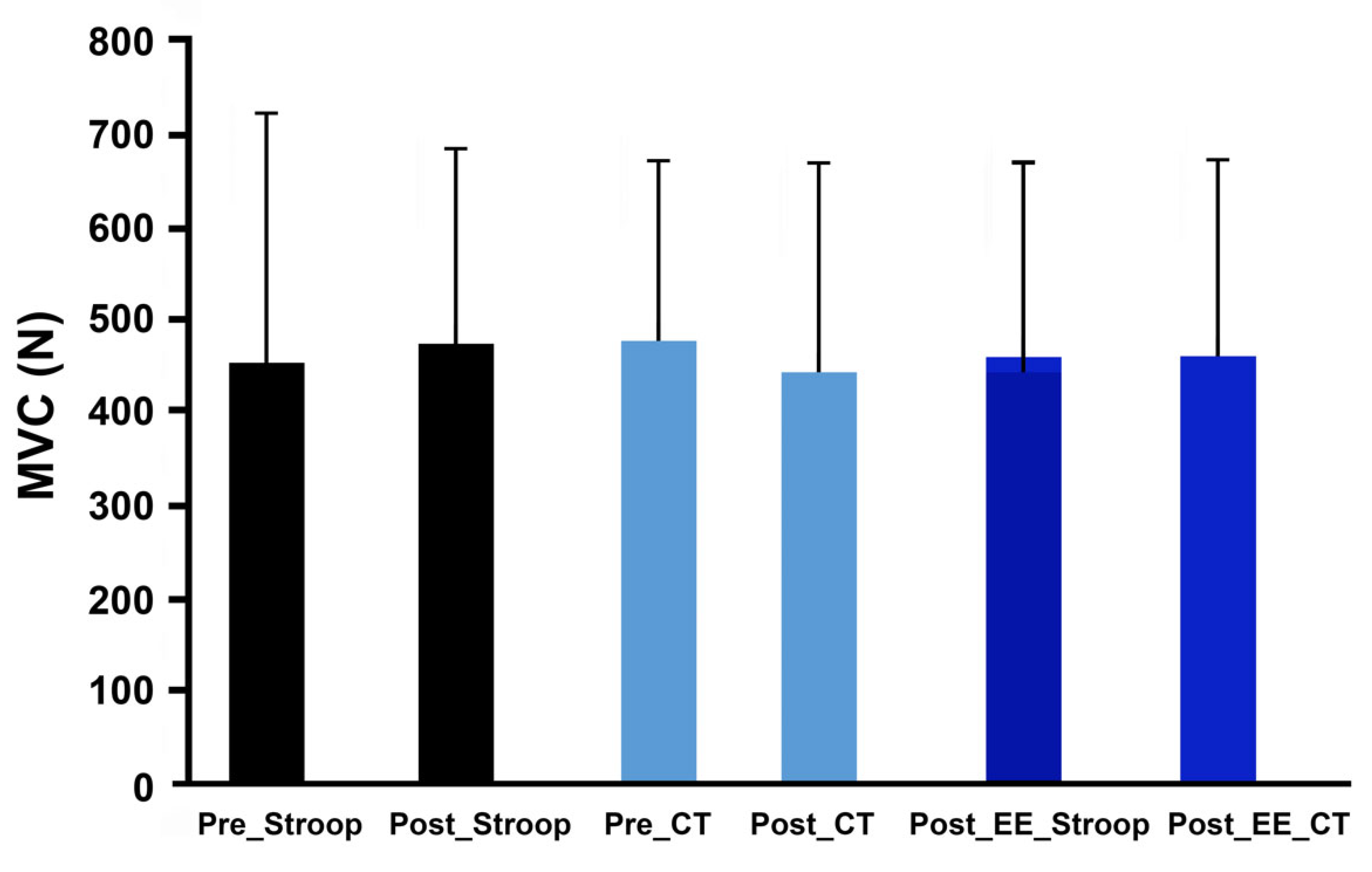
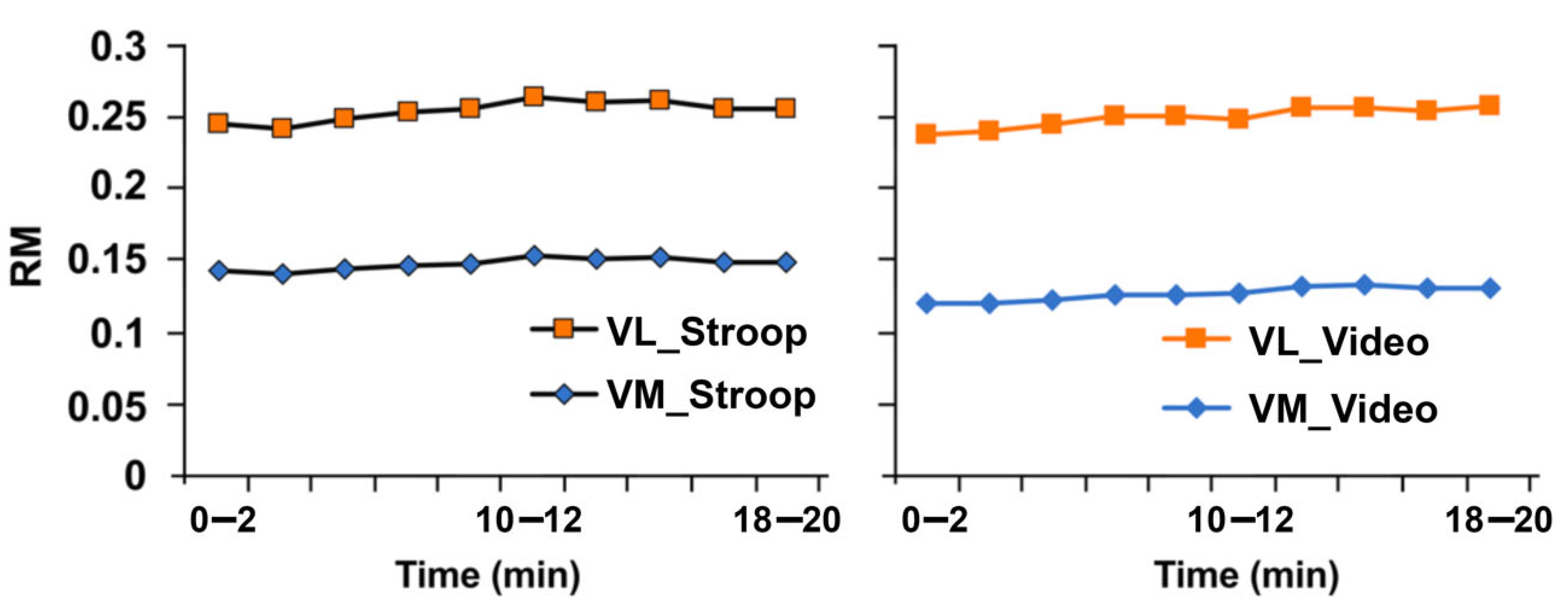
3.3. Effects of Response Inhibition on Neuromuscular Functions
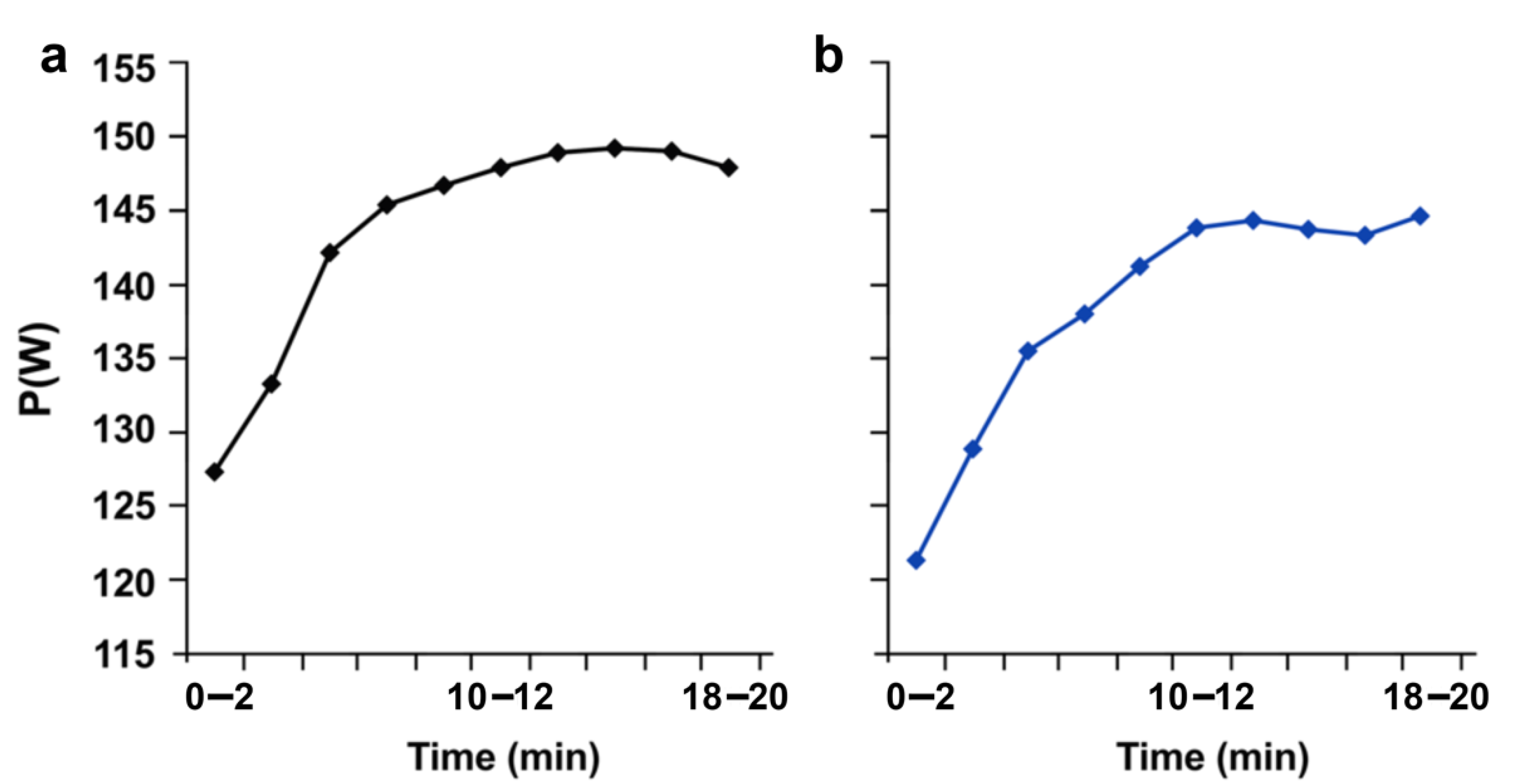
3.4. Effects of Response Inhibition on Endurance Exercise
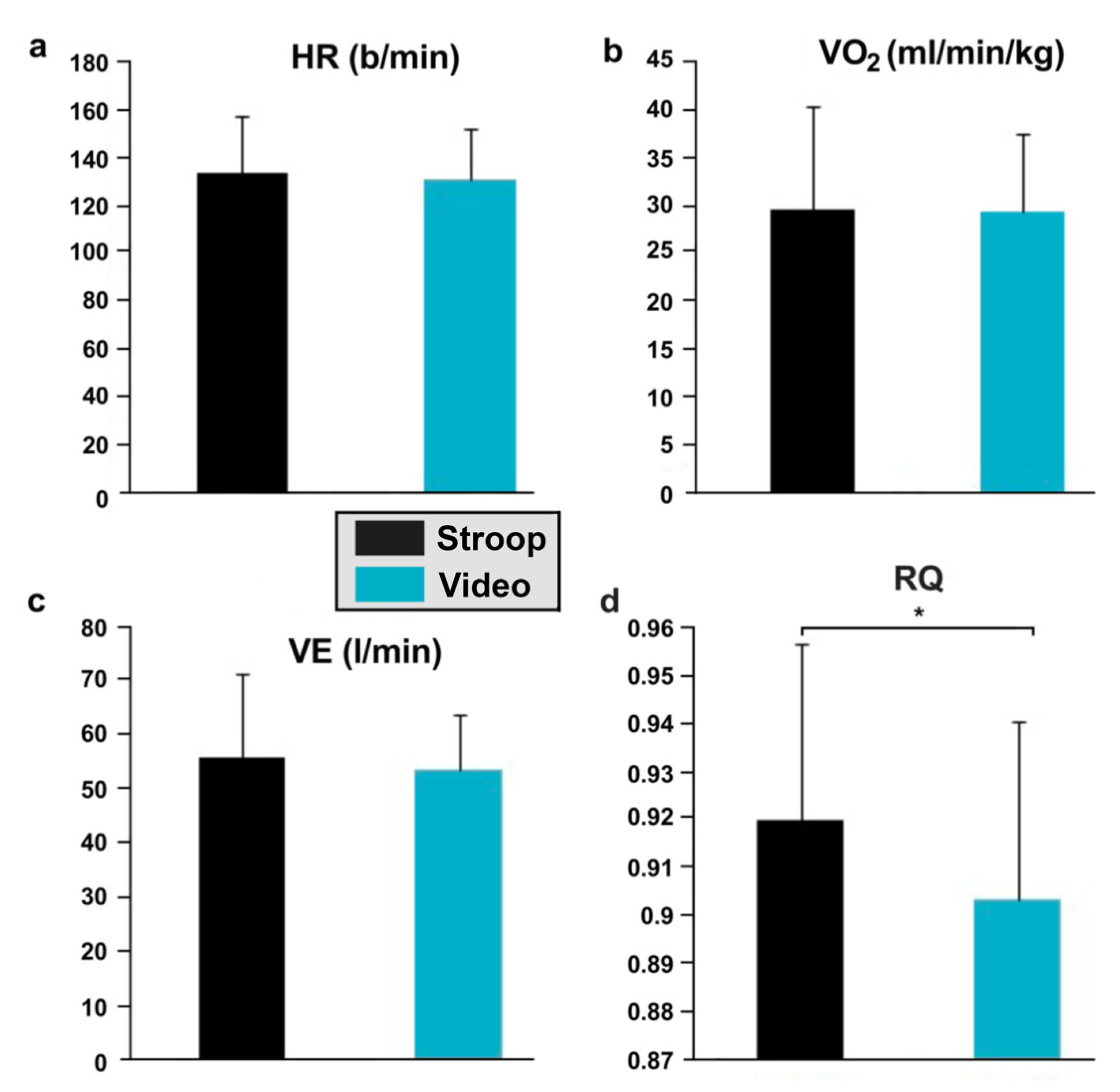
3.5. Effects of Each Condition on Cardiorespiratory Factors during Pedaling Exercise
4. Discussion
4.1. Response Inhibition and Mental Fatigue
4.2. Mental Fatigue and Neuromuscular Functions
4.3. Mental Effort and Subsequent Endurance Exercise
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Angelo, M.J.M.F.l.T. Lafatica. 1891. Available online: https://www.researchgate.net/figure/Front-page-of-La-Fatica-Fatigue-which-was-published-in-1891-by-Treves-in-Milano_fig2_7071491o (accessed on 29 August 2022).
- Hancock, P.A.; Desmond, P.A. Stress, Workload, and Fatigue; Lawrence Erlbaum: Mahwah, NJ, USA, 2001. [Google Scholar]
- Hernández-Mendo, A.; Reigal, R.E.; López-Walle, J.M.; Serpa, S.; Samdal, O.; Morales-Sánchez, V.; Juárez-Ruizde Mier, R.; Tristán-Rodríguez, J.L.; Rosado, A.F.; Falco, C. Physical Activity, Sports Practice, and Cognitive Functioning: The Current Research Status. Front. Psychol. 2019, 10, 2658. [Google Scholar] [CrossRef]
- Marcora, S.M.; Staiano, W.; Manning, V. Mental fatigue impairs physical performance in humans. J. Appl. Physiol. 2009, 106, 857–864. [Google Scholar] [CrossRef]
- Pageaux, B.; Lepers, R.; Dietz, K.C.; Marcora, S.M. Response inhibition impairs subsequent self-paced endurance performance. Eur. J. Appl. Physiol. 2014, 114, 1095–1105. [Google Scholar] [CrossRef]
- MacMahon, C.; Schücker, L.; Hagemann, N.; Strauss, B. Cognitive fatigue effects on physical performance during running. J. Sport Exerc. Psychol. 2014, 36, 375–381. [Google Scholar] [CrossRef]
- Smith, M.R.; Coutts, A.J.; Merlini, M.; Deprez, D.; Lenoir, M.; Marcora, S.M. Mental Fatigue Impairs Soccer-Specific Physical and Technical Performance. Med. Sci. Sports Exerc. 2016, 48, 267–276. [Google Scholar] [CrossRef]
- Martin, K.; Staiano, W.; Menaspà, P.; Hennessey, T.; Marcora, S.; Keegan, R.; Thompson, K.G.; Martin, D.; Halson, S.; Rattray, B. Superior Inhibitory Control and Resistance to Mental Fatigue in Professional Road Cyclists. PLoS ONE 2016, 11, e0159907. [Google Scholar] [CrossRef]
- Royal, K.A.; Farrow, D.; Mujika, I.; Halson, S.L.; Pyne, D.; Abernethy, B. The effects of fatigue on decision making and shooting skill performance in water polo players. J. Sports Sci. 2006, 24, 807–815. [Google Scholar] [CrossRef]
- Russell, S.; Jenkins, D.; Halson, S.; Kelly, V. Changes in subjective mental and physical fatigue during netball games in elite development athletes. J. Sci. Med. Sport 2020, 23, 615–620. [Google Scholar] [CrossRef]
- Bangsbo, J.; Iaia, F.M.; Krustrup, P. Metabolic response and fatigue in soccer. Int. J. Sports Physiol. Perform. 2007, 2, 111–127. [Google Scholar] [CrossRef]
- Sun, H.; Soh, K.G.; Roslan, S.; Wazir, M.R.W.N.; Soh, K.L. Does mental fatigue affect skilled performance in athletes? A systematic review. PLoS ONE 2021, 16, e0258307. [Google Scholar] [CrossRef]
- Sun, H.; Soh, K.G.; Mohammadi, A.; Wang, X.; Bin, Z.; Zhao, Z. Effects of mental fatigue on technical performance in soccer players: A systematic review with a meta-analysis. Front. Public Health 2022, 10, 922630. [Google Scholar] [CrossRef] [PubMed]
- Magariño, L.S.; Madhivanan, P. Cognitive fatigue effects on physical performance: A systematic review and meta-analysis—Correspondence. Physiol. Behav. 2019, 198, 159–160. [Google Scholar] [CrossRef] [PubMed]
- Le Mansec, Y.; Pageaux, B.; Nordeza, A.; Dorela, S.; Jubeaua, M. Mental fatigue alters the speed and the accuracy of the ball in table tennis. J. Sports Sci. 2017, 36, 2751–2759. [Google Scholar] [CrossRef]
- Moreira, A.; Aoki, M.S.; Franchini, E.; da Silva Machado, D.G.; Paludo, A.C.; Okano, A.H. Mental fatigue impairs technical performance and alters neuroendocrine and autonomic responses in elite young basketball players. Physiol. Behav. 2018, 196, 112–118. [Google Scholar] [CrossRef]
- Sansone, A.P.; Tessitore, A.; Lukonaitiene, I.; Paulauskas, H. Technical-tactical profile, perceived exertion, mental demands and enjoyment of different tactical tasks and training regimes in basketball small-sided games. Biol. Sport 2020, 37, 14–23. [Google Scholar] [CrossRef]
- Farahani, J.; Soltani, P.; Rezlescu, C. Assessing decision-making in elite academy footballers using real world video clips. Prog. Brain Res. 2020, 253, 59–70. [Google Scholar] [CrossRef]
- Cook, D.B.; O’Connor, P.J.; Lange, G.; Steffener, J. Functional neuroimaging correlates of mental fatigue induced by cognition among chronic fatigue syndrome patients and controls. Neuroimage 2007, 36, 108–122. [Google Scholar] [CrossRef]
- Williamson, J.W.; Fadel, P.J.; Mitchell, J.H. New insights into central cardiovascular control during exercise in humans: A central commandupdate. Exp. Physiol. 2006, 91, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Wright, R.A. Refining the prediction of effort: Brehm’s distinction be-tween potential motivation and motivation intensity. Soc. Pers. Psychol. Compass 2008, 2, 682–701. [Google Scholar] [CrossRef]
- Critchley, H.D.; Mathias, C.J.; Josephs, O.; O’Doherty, J.; Zanini, S.; Dewar, B.K.; Cipolotti, L.; Shallice, T.; Dolan, R.J. Human cingulate cortex and autonomic control: Converging neuroimaging and clinical evidence. Brain 2003, 126, 2139–2152. [Google Scholar] [CrossRef] [Green Version]
- Burnley, M.; Jones, A.M. Oxygen uptake kinetics as a determinant of sports performance. Eur. J. Sport Sci. 2007, 7, 63–79. [Google Scholar] [CrossRef]
- Ward, S.A. Muscle-energetic and cardio-pulmonary determinants of exercise tolerance in humans: Muscle-energetic and cardio-pulmonary determinants of exercise tolerance in humans. Exp. Physiol. 2007, 92, 321–322. [Google Scholar] [CrossRef] [PubMed]
- Lewczuk, K.; Wizła, M.; Oleksy, T.; Wyczesany, M. Emotion Regulation, Effort and Fatigue: Complex Issues Worth Investigating. Front. Psychol. 2022, 13, 742557. [Google Scholar] [CrossRef] [PubMed]
- Habay, J.; van Cutsem, J.; Verschueren, J.; de Bock, S.; Proost, M.; de Wachter, J.; Tassignon, B.; Meeusen, R.; Roelands, B. Mental Fatigue and Sport-Specific Psychomotor Performance: A Systematic Review. Sports Med. 2021, 51, 1527–1548. [Google Scholar] [CrossRef] [PubMed]
- Brownsberger, J.; Edwards, A.; Crowther, R.; Cottrell, D. Impact of mental fatigue on self-paced exercise. Int. J. Sports Med. 2013, 34, 1029–1036. [Google Scholar] [CrossRef]
- Borg, G. Perceived exertion as an indicator of somatic stress. Scand. J. Rehabil. Med. 1970, 2, 92–98. [Google Scholar]
- Faulkner, J.; Eston, R.G. Perceived exertion research in the 21st century: Developments, reflections and questions for the future. J. Exerc. Sci. Fit. 2008, 6, 1–14. [Google Scholar]
- McMorris, T. Cognitive Fatigue Effects on Physical Performance: The Role of Interoception. Sports Med. 2020, 50, 1703–1708. [Google Scholar] [CrossRef]
- Fairclough, S.H.; Houston, K. A metabolic measure of mental effort. Biol. Psychol. 2004, 66, 177–190. [Google Scholar] [CrossRef]
- Stroop, J.R. Studies of Interference in Serial Verbal Reactions. J. Exp. Psychol. 1935, 18, 643–662. [Google Scholar] [CrossRef]
- Brandt, R.; Herrero, D.; Massetti, T.; Crocetta, T.B.; Guarnieri, R.; Monteiro, C.B.D.M.; Viana, M.D.S.; Bevilacqua, G.G.; de Abreu, L.C.; Andrade, A. The Brunel Mood Scale rating in mental health for physically active and apparently healthy populations. Health 2016, 8, 125–132. [Google Scholar] [CrossRef]
- Terry, P.C.; Lane, A.M.; Fogarty, G.J. Construct validity of the Profile of Mood States-Adolescents for use with adults. Psychol. Sport Exerc. 2003, 4, 125–139. [Google Scholar] [CrossRef] [Green Version]
- Colligan, L.; Potts, H.W.; Finn, C.T.; Sinkin, R.A. Cognitive workload changes for nurses transitioning from a legacy system with paper documentation to a commercial electronic health record. Int. J. Med. Inform. 2015, 84, 469–476. [Google Scholar] [CrossRef]
- Hart, S.; Staveland, L. Development of NASA-TLX (Task Load Index): Results of Empirical and Theoretical Research. In Human Mental Workload; Hancock, P., Meshkati, N., Eds.; North Holland Press: Amsterdam, The Netherlands, 1988; pp. 139–183. [Google Scholar]
- Duman, I.; Horzum, M.B.; Randler, C. Adaptation of the intrinsic motivation inventory to Turkish. Int. J. Psychol. Educ. Stud. 2020, 7, 26–33. [Google Scholar] [CrossRef]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of recommendations for SEMG sensors and sensor placement procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef]
- Letafatkar, A.; Hatefi, M.; Babakhani, F.; Abbaszadeh Ghanati, H.; Wallace, B. The influence of hip rotations on muscle activity during unilateral weight-bearing exercises in individuals with and without genu varum: A cross-sectional study. Phys. Ther. Sport 2020, 43, 224–229. [Google Scholar] [CrossRef]
- Soderberg, G.L.; Knutson, L.M. A guide for use and interpretation of kinesiologic electromyographic data. Phys. Ther. 2000, 80, 485–498. [Google Scholar] [CrossRef]
- Gailliot, M.T. Unlocking the Energy Dynamics of Executive Functioning: Linking Executive Functioning to Brain Glycogen. Perspect. Psychol. Sci. 2008, 3, 245–263. [Google Scholar] [CrossRef]
- Rozand, V.; Pageaux, B.; Marcora, S.M.; Papaxanthis, C.; Lepers, R. Does mental exertion alter maximal muscle activation? Front. Hum. Neurosci. 2014, 8, 755. [Google Scholar] [CrossRef]
- Mizuno, K.; Tanaka, M.; Nozaki, S.; Yamaguti, K.; Mizuma, H.; Sasabe, T.; Sugino, T.; Shirai, T.; Kataoka, Y.; Kajimoto, Y.; et al. Mental fatigue-induced decrease in levels of several plasma amino acids. J. Neural Transm. 2007, 114, 555–561. [Google Scholar] [CrossRef]
- Nozaki, S.; Tanaka, M.; Mizuno, K.; Ataka, S.; Mizuma, H.; Tahara, T.; Sugino, T.; Shirai, T.; Eguchi, A.; Okuyama, K.; et al. Mental and physical fatigue-related biochemical alterations. Nutrition 2009, 25, 51–57. [Google Scholar] [CrossRef]
- Kuettel, A.; Larsen, C.H. Risk and protective factors for mental health in elite athletes: A scoping review. Int. Rev. Sport Exerc. Psychol. 2020, 13, 231–265. [Google Scholar] [CrossRef]

| Fatigue | Vigor | Motivation | |||||
|---|---|---|---|---|---|---|---|
| Pre- Cognitive | Post- Cognitive | Post- Pedaling | Pre- Cognitive | Post- Cognitive | Post- Pedaling | Pre- Pedaling | |
| ST | 2.67 ± 2.61 | 4.25 ± 2.86 | 3.42 ± 2.19 | 7.92 ± 3.53 | 3.92 ± 2.61 * | 6.92 ± 4.52 | 25 ± 6.48 |
| CT | 3 ± 2.17 | 5.08 ± 2.5 | 3.42 ± 2.78 | 8.75 ± 2.38 | 5.67 ± 3.26 | 9.08 ± 3.94 | 24.25 ± 5.44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hakim, H.; Khemiri, A.; Chortane, O.G.; Boukari, S.; Chortane, S.G.; Bianco, A.; Marsigliante, S.; Patti, A.; Muscella, A. Mental Fatigue Effects on the Produced Perception of Effort and Its Impact on Subsequent Physical Performances. Int. J. Environ. Res. Public Health 2022, 19, 10973. https://doi.org/10.3390/ijerph191710973
Hakim H, Khemiri A, Chortane OG, Boukari S, Chortane SG, Bianco A, Marsigliante S, Patti A, Muscella A. Mental Fatigue Effects on the Produced Perception of Effort and Its Impact on Subsequent Physical Performances. International Journal of Environmental Research and Public Health. 2022; 19(17):10973. https://doi.org/10.3390/ijerph191710973
Chicago/Turabian StyleHakim, Hassen, Aymen Khemiri, Oussema Gaied Chortane, Samia Boukari, Sabri Gaied Chortane, Antonino Bianco, Santo Marsigliante, Antonino Patti, and Antonella Muscella. 2022. "Mental Fatigue Effects on the Produced Perception of Effort and Its Impact on Subsequent Physical Performances" International Journal of Environmental Research and Public Health 19, no. 17: 10973. https://doi.org/10.3390/ijerph191710973
APA StyleHakim, H., Khemiri, A., Chortane, O. G., Boukari, S., Chortane, S. G., Bianco, A., Marsigliante, S., Patti, A., & Muscella, A. (2022). Mental Fatigue Effects on the Produced Perception of Effort and Its Impact on Subsequent Physical Performances. International Journal of Environmental Research and Public Health, 19(17), 10973. https://doi.org/10.3390/ijerph191710973









