Potential Toxicity Risk Assessment and Priority Control Strategy for PAHs Metabolism and Transformation Behaviors in the Environment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Environmental and Human Health Toxicity Characterization of PAHs
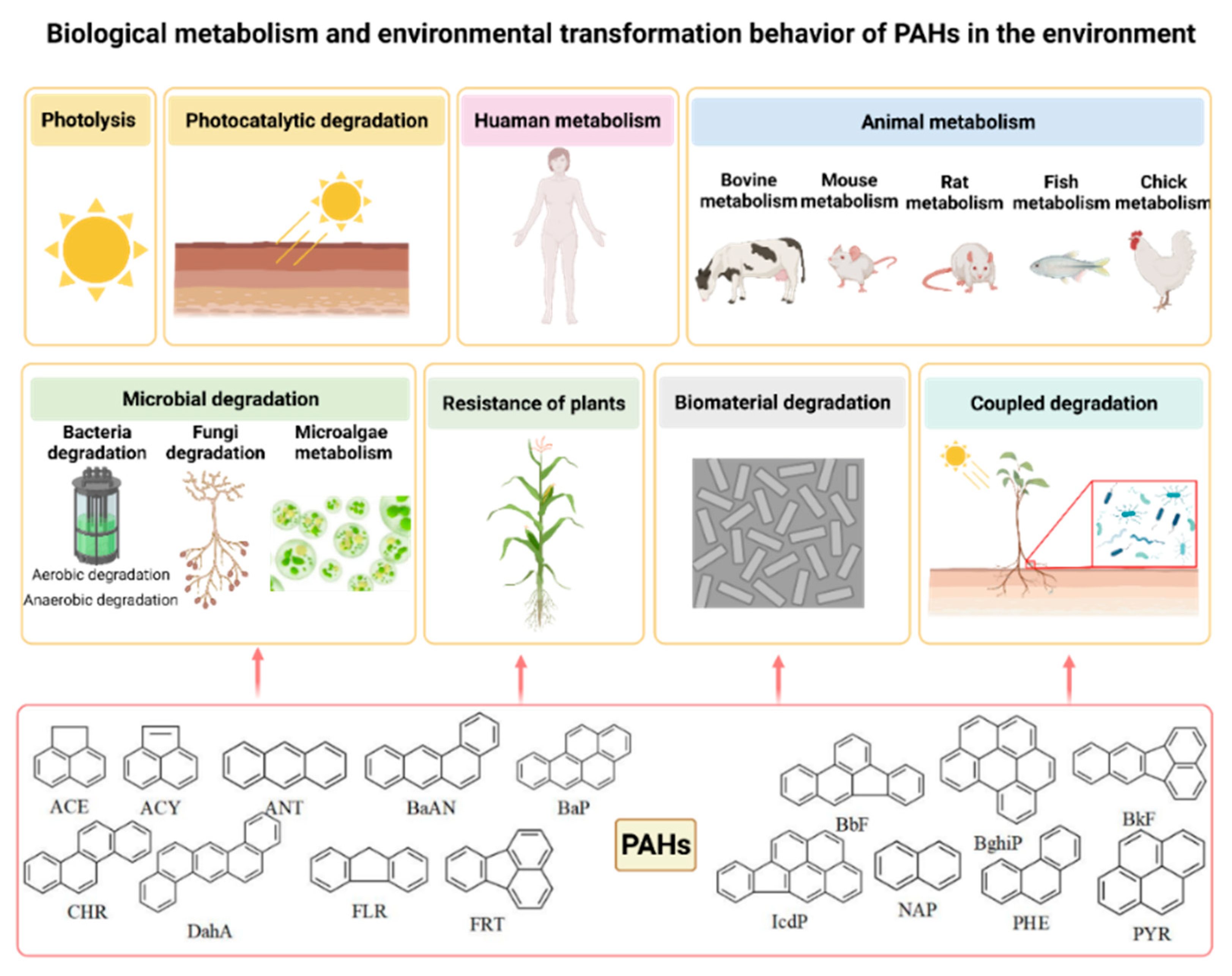
2.2. Identification and Screening of Biological Metabolism and Environmental Transformation Products of PAHs
2.3. Neurotoxicity, Immunotoxicity and Phytotoxicity Prediction of PAHs and Their Derivatives Using a 3D-QSAR Model Method
2.3.1. Molecular Structure Drawing of PAHs and Their Derivatives and Screening of Neurotoxic, Immunotoxic and Phytotoxic Receptor Proteins
2.3.2. Characterization of PAHs’ Neurotoxicity, Immunotoxicity and Phytotoxicity Effect: Molecular Docking and Molecular Dynamics Method
2.3.3. Construction of 3D-QSAR Models for PAHs’ Neurotoxicity, Immunotoxicity and Phytotoxicity
2.4. Prediction of PAHs and Their Derivatives’ Genotoxicity and Developmental Toxicity Using TOPKAT Method
2.5. Prediction of PAHs and Their Derivatives’ Carcinogenicity and Endocrine Disruption Toxicity: Using VEGA Platform
2.6. Construction of a Priority Control Evaluation System for PAHs and Its Derivatives’ Toxicity Risk: Using Risk Entropy Method
3. Results and Discussion
3.1. Prediction for Neurotoxicity, Immunotoxicity and Phytotoxicity of PAH Derivatives Based on 3D-QSAR Models
3.1.1. Construction and Evaluation of 3D-QSAR Models for PAHs’ Neurotoxicity, Immunotoxicity and Phytotoxicity
3.1.2. Prediction of PAH Derivatives’ Neurotoxicity, Immunotoxicity, and Phytotoxicity
3.2. Prediction of PAH Derivatives’ Developmental Toxicity and Genotoxicity Based on TOPKAT Method
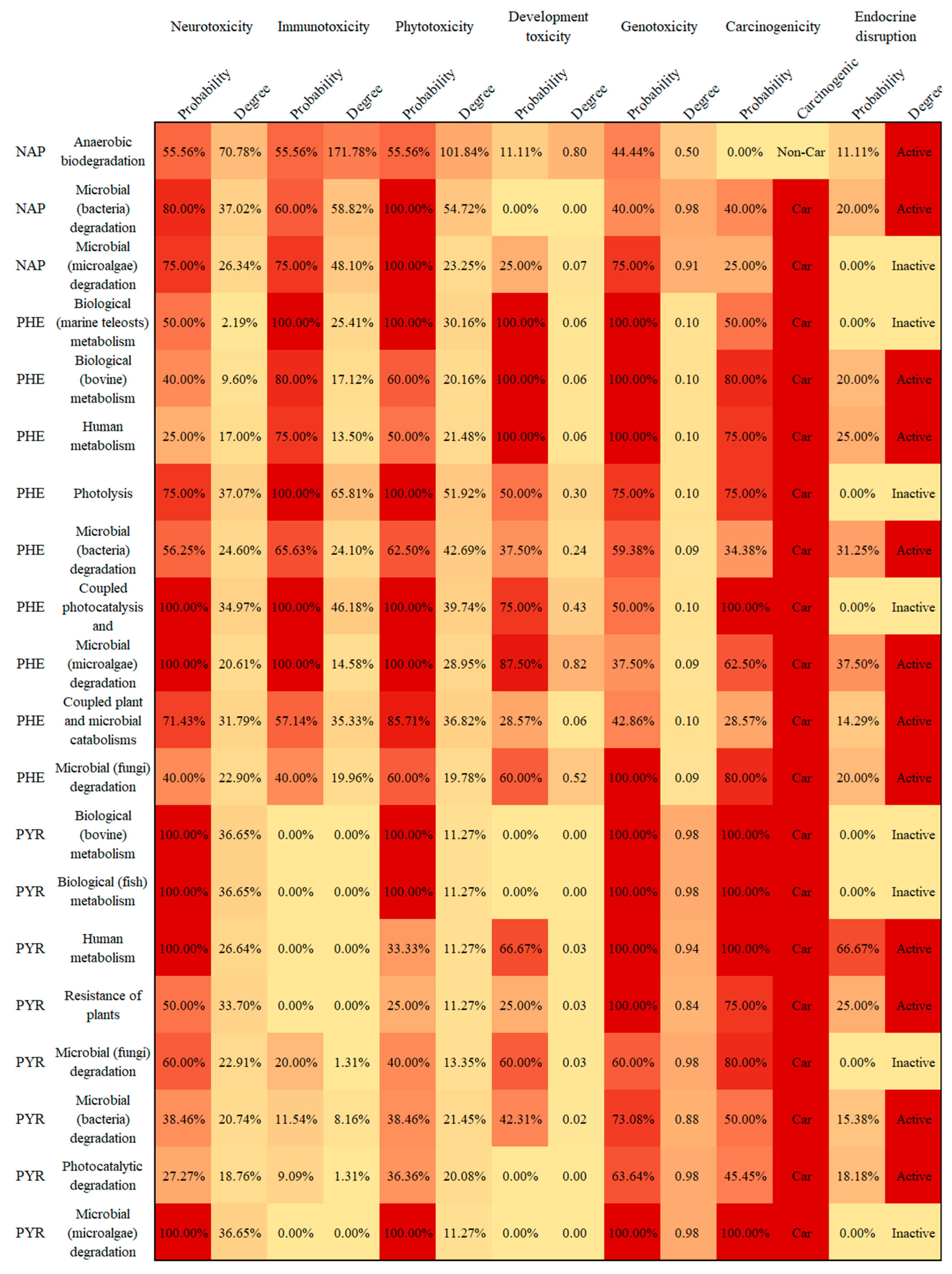
3.3. Prediction of PAH Derivatives’ Carcinogenicity and Endocrine-Disrupting Effect Based on VEGA Platform
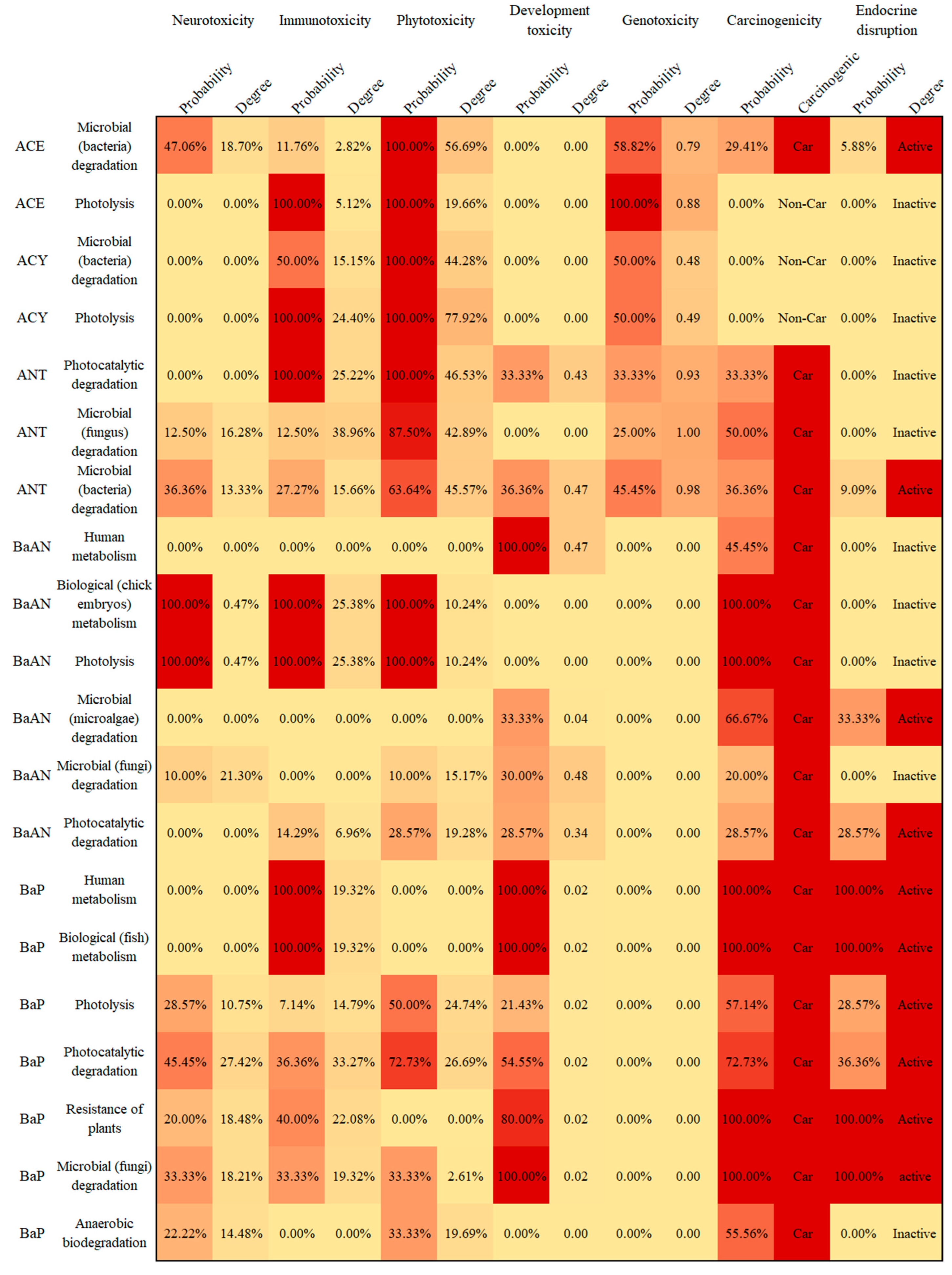
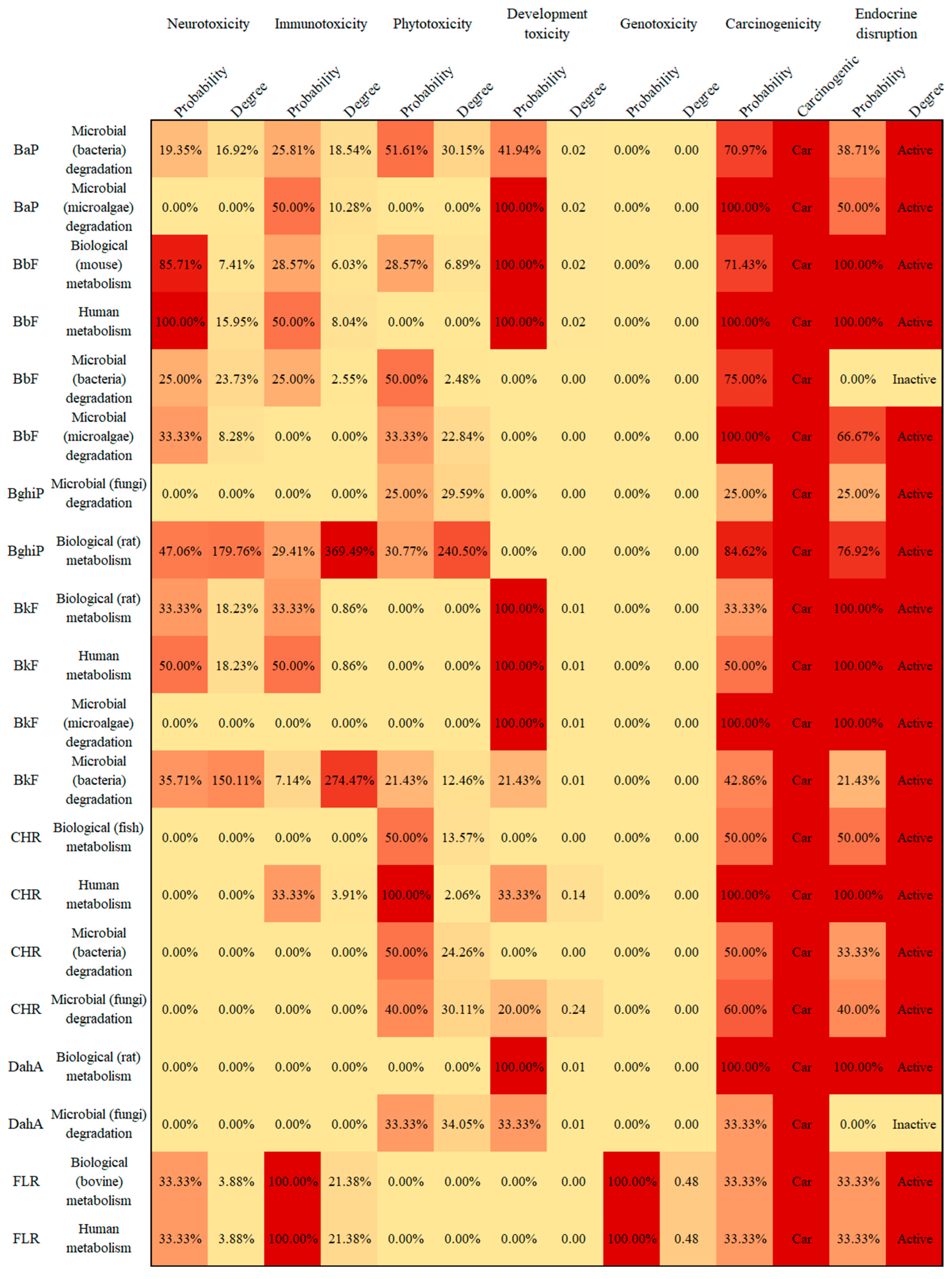
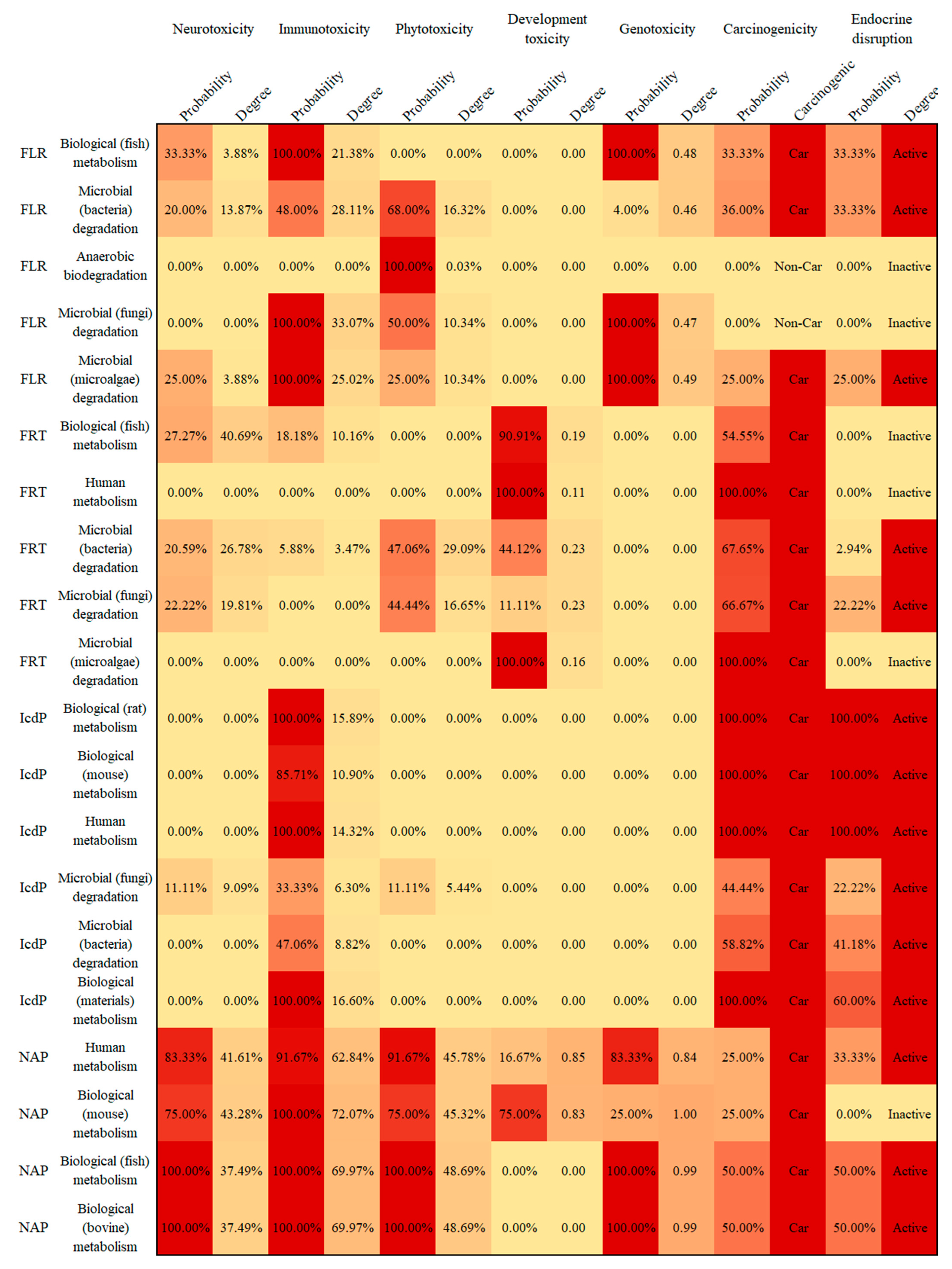
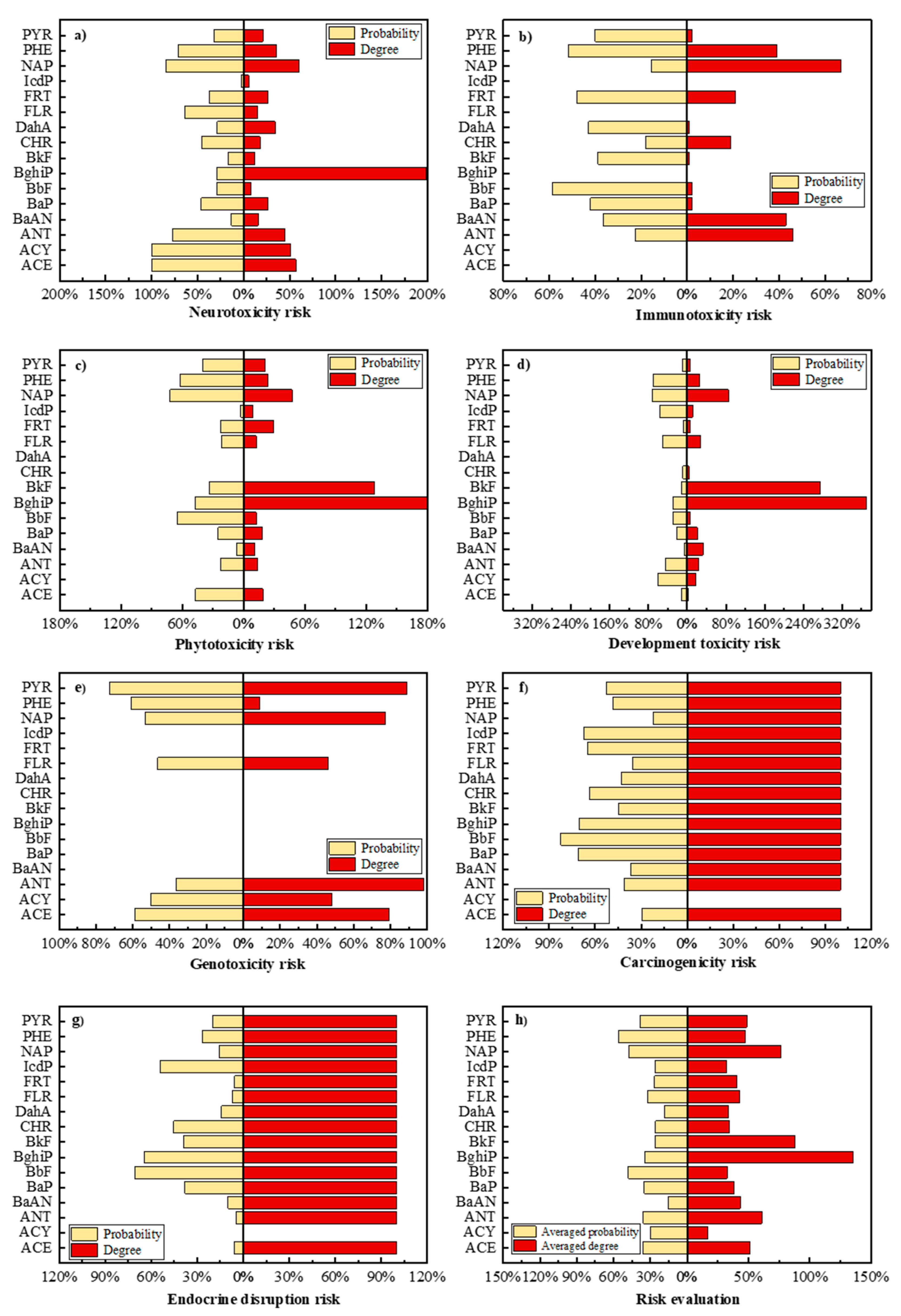
3.4. Potential Human and Environmental Health Risk Evaluation of PAH Derivatives
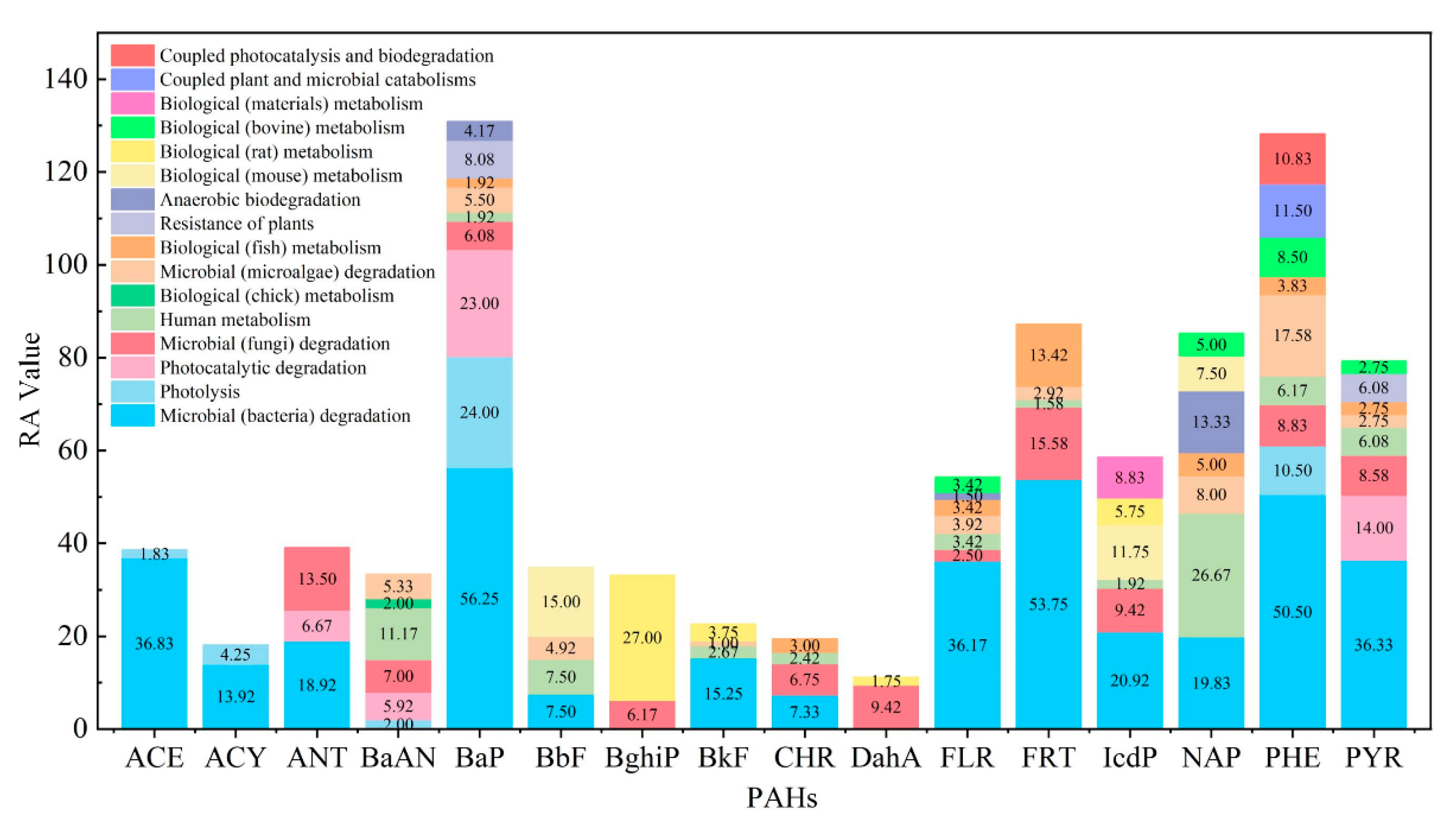
3.5. Construction of a Priority Control Evaluation System of Total Exposed Risk of PAHs’ Metabolism and Transformation Pathway Based on the Risk Entropy Method
4. Conclusions
- (1)
- The neurotoxicity, immunotoxicity, and phytotoxicity 3D-QSAR models were constructed separately. The evaluation parameters indicated the good evaluation stability and prediction ability of the three models.
- (2)
- Biological metabolism and environmental transformation behavior of 16 PAHs in the environment were summarized, and 473 PAH derivatives generated by different pathways were summarized.
- (3)
- The 3D-QSAR model, TOKPAT, and VEGA platform were used to predict the phytotoxicity, neurotoxicity, immunotoxicity, developmental toxicity, genotoxicity, carcinogenicity, and endocrine-disrupting effect of PAHs and their derivatives. The environmental toxicity (phytotoxicity) and human health toxicity (neurotoxicity, immunotoxicity, developmental toxicity, genotoxicity, carcinogenicity, and endocrine-disrupting effect) of PAH derivatives generated in different metabolic and transformation pathways were comprehensively and systematically predicted.
- (4)
- The potential risks (environmental and human toxicity risk) of the metabolic and transformation behaviors of the 16 PAHs were evaluated. The derivatives of BghiP produced in rat metabolisms presented a high degree of neurotoxicity risk (risk degree 179.76%), immunotoxicity risk (degree 369.49%), phytotoxicity risk (degree 240.50%), and potential carcinogenic and endocrine-disrupting risk. According to the risk assessment results, partial PAH derivatives should also be included in the critical monitoring objects to realize the risk control of the whole life cycle of PAHs in the environment.
- (5)
- The risk priority control evaluation system for PAHs based on the risk entropy method was constructed, which was of great significance for screening the priority control pathway of PAHs in the environment.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reizer, E.; Viskolcz, B.; Fiser, B. Formation and growth mechanisms of polycyclic aromatic hydrocarbons: A mini-review. Chemosphere 2022, 291, 132793. [Google Scholar] [CrossRef] [PubMed]
- Kariyawasam, T.; Doran, G.S.; Howitt, J.A.; Prenzler, P.D. Chemosphere Polycyclic aromatic hydrocarbon contamination in soils and sediments: Sustainable approaches for extraction and remediation. Chemosphere 2022, 291, 132981. [Google Scholar] [CrossRef]
- Krzyszczak, A.; Czech, B. Occurrence and toxicity of polycyclic aromatic hydrocarbons derivatives in environmental matrices. Sci. Total Environ. 2021, 788, 147738. [Google Scholar] [CrossRef]
- Méndez García, M.; García de Llasera, M.P. A review on the enzymes and metabolites identified by mass spectrometry from bacteria and microalgae involved in the degradation of high molecular weight PAHs. Sci. Total Environ. 2021, 797, 149035. [Google Scholar] [CrossRef]
- Recabarren-Villalón, T.; Ronda, A.C.; Oliva, A.L.; Cazorla, A.L.; Marcovecchio, J.E.; Arias, A.H. Seasonal distribution pattern and bioaccumulation of Polycyclic aromatic hydrocarbons (PAHs) in four bioindicator coastal fishes of Argentina. Environ. Pollut. 2021, 291, 118125. [Google Scholar] [CrossRef] [PubMed]
- Pii, Y.; Marastoni, L.; Gemassmer, E.; Valentinuzzi, F.; Mazzetto, F.; Mimmo, T.; Cesco, S. Phytotoxicity alleviation by bacterial species isolated from polycyclic aromatic hydrocarbons (PAHs) contaminated sites. Environ. Technol. Innov. 2019, 13, 104–112. [Google Scholar] [CrossRef]
- Meudec, A.; Poupart, N.; Dussauze, J.; Deslandes, E. Relationship between heavy fuel oil phytotoxicity and polycyclic aromatic hydrocarbon contamination in Salicornia fragilis. Sci. Total Environ. 2007, 381, 146–156. [Google Scholar] [CrossRef]
- Turja, R.; Sanni, S.; Stankevičiūtė, M.; Butrimavičienė, L.; Devier, M.H.; Budzinski, H.; Lehtonen, K.K. Biomarker responses and accumulation of polycyclic aromatic hydrocarbons in Mytilus trossulus and Gammarus oceanicus during exposure to crude oil. Environ. Sci. Pollut. Res. 2020, 27, 15498–15514. [Google Scholar] [CrossRef]
- Šimečková, P.; Pěnčíková, K.; Kováč, O.; Slavík, J.; Pařenicová, M.; Vondráček, J.; Machala, M. In vitro profiling of toxic effects of environmental polycyclic aromatic hydrocarbons on nuclear receptor signaling, disruption of endogenous metabolism and induction of cellular stress. Sci. Total Environ. 2022, 815, 151967. [Google Scholar] [CrossRef]
- Chauhan, A.; Fazlurrahman; Oakeshott, J.G.; Jain, R.K. Bacterial metabolism of polycyclic aromatic hydrocarbons: Strategies for bioremediation. Indian J. Microbiol. 2008, 48, 95–113. [Google Scholar] [CrossRef] [Green Version]
- Botta, C.; Di Giorgio, C.; Sabatier, A.S.; De Méo, M. Effects of UVA and visible light on the photogenotoxicity of benzo[a]pyrene and pyrene. Environ. Toxicol. 2009, 24, 492–505. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, R.A.; Taniguchi, S.; da Silva, J.; Gallotta, F.D.C.; Bícego, M.C. Polycyclic aromatic hydrocarbons in marine mammals: A review and synthesis. Mar. Pollut. Bull. 2021, 171, 112699. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, J.; Feng, S.; Xiao, Z.; Chen, C. Effects of ecohydrological interfaces on migrations and transformations of pollutants: A critical review. Sci. Total Environ. 2022, 804, 150140. [Google Scholar] [CrossRef]
- Xie, J.; Tao, L.; Wu, Q.; Lei, S.; Lin, T. Environmental profile, distributions and potential sources of halogenated polycyclic aromatic hydrocarbons. J. Hazard. Mater. 2021, 419, 126164. [Google Scholar] [CrossRef]
- Imam, A.; Kumar Suman, S.; Kanaujia, P.K.; Ray, A. Biological machinery for polycyclic aromatic hydrocarbons degradation: A review. Bioresour. Technol. 2022, 343, 126121. [Google Scholar] [CrossRef]
- Kouri, R.E.; Wood, A.W.; Levin, W.; Rude, T.H.; Yagi, H.; Mah, H.D.; Jerina, D.M.; Conney, A.H. Carcinogenicity of Benzo[a]pyrene and Thirteen of Its Derivatives in C3H/fCum Mice. J. Natl. Cancer Inst. 1980, 64, 617–623. [Google Scholar] [CrossRef]
- Masuda, M.; Wang, Q.; Tokumura, M.; Miyake, Y.; Amagai, T. Risk assessment of polycyclic aromatic hydrocarbons and their chlorinated derivatives produced during cooking and released in exhaust gas. Ecotoxicol. Environ. Saf. 2020, 197, 110592. [Google Scholar] [CrossRef]
- Geier, M.C.; Chlebowski, A.C.; Truong, L.; Simonich, S.L.M.; Anderson, K.A.; Tanguay, R.L. Comparative developmental toxicity of a comprehensive suite of polycyclic aromatic hydrocarbons. Arch. Toxicol. 2018, 92, 571–586. [Google Scholar] [CrossRef]
- Liu, R.; Ma, S.; Yu, Y.; Li, G.; Yu, Y.; An, T. Field study of PAHs with their derivatives emitted from e-waste dismantling processes and their comprehensive human exposure implications. Environ. Int. 2020, 144, 106059. [Google Scholar] [CrossRef]
- Tang, C.; Jin, J.; Peng, X. Research Advances in Computational Toxicological Assessment and Environmental Behavior Modeling of Pollutants. Environ. Monit. Assess. 2016, 8, 1–15. [Google Scholar]
- Wang, J.; Wang, S. Toxicity changes of wastewater during various advanced oxidation processes treatment: An overview. J. Clean. Prod. 2021, 315, 128202. [Google Scholar] [CrossRef]
- Zhou, L.; Fan, D.; Yin, W.; Gu, W.; Wang, Z.; Liu, J.; Xu, Y.; Shi, L.; Liu, M.; Ji, G. Comparison of seven in silico tools for evaluating of daphnia and fish acute toxicity: Case study on Chinese Priority Controlled Chemicals and new chemicals. BMC Bioinform. 2021, 22, 151. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Zhang, Y.; Pei, L.; Lou, Y.; Mu, Y.; Yun, J.; Li, F.; Wang, Y.; Han, Z.; Xi, S.; et al. Chemometric QSAR modeling of acute oral toxicity of Polycyclic Aromatic Hydrocarbons (PAHs) to rat using simple 2D descriptors and interspecies toxicity modeling with mouse. Ecotoxicol. Environ. Safe. 2021, 222, 112525. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Hou, Y.; Zhang, Z.; Guo, Z.; Yang, W.; Dou, G.; Lv, X.; Wang, X.; Ge, J.; Wu, B.; et al. Identification of novel natural inhibitors targeting AKT Serine/Threonine Kinase 1 (AKT1) by computational study. Bioengineered 2022, 13, 12003–12020. [Google Scholar] [CrossRef] [PubMed]
- Rioux, B.; Mention, M.M.; Alarcan, J.; Abiola, T.T.; Peyrot, C.; Brunissen, F.; Braeuning, A.; Stavros, V.G.; Allais, F. Sustainable synthesis, in silico evaluation of potential toxicity and environmental fate, antioxidant and UV-filtering/photostability activity of phenolic-based thiobarbituric derivatives. Green Chem. Lett. Rev. 2022, 15, 116–127. [Google Scholar] [CrossRef]
- Li, X.; Hou, Y.; Li, Q.; Gu, W.; Li, Y. Molecular design of high-efficacy and high drug safety Fluoroquinolones suitable for a variety of aerobic biodegradation bacteria. J. Environ. Manage. 2021, 299, 113628. [Google Scholar] [CrossRef]
- Benfenati, E.; Manganaro, A.; Gini, G. VEGA-QSAR: AI inside a platform for predictive toxicology. In Proceedings of the 2nd Workshop on Popularize Artificial Intelligence, Turin, Italy, 5 December 2013; Volume 1107, pp. 21–28. [Google Scholar]
- WHO. IARC Agents Classified by the IARC Monographs; WHO: Geneva, Switzerland, 2021; Volume 1–130, pp. 1–39. [Google Scholar]
- Ghosal, D.; Ghosh, S.; Dutta, T.K.; Ahn, Y. Current State of Knowledge in Microbial Degradation of Polycyclic Aromatic Hydrocarbons (PAHs): A Review. Front. Microbiol. 2016, 7, 1369. [Google Scholar] [CrossRef]
- Crowell, S.R.; Hanson-Drury, S.; Williams, D.E.; Corley, R.A. In vitro metabolism of benzo[a]pyrene and dibenzo[def,p]chrysene in rodent and human hepatic microsomes. Toxicol. Lett. 2014, 228, 48–55. [Google Scholar] [CrossRef]
- Álvarez-Barragán, J.; Cravo-Laureau, C.; Wick, L.Y.; Duran, R. Fungi in PAH-contaminated marine sediments: Cultivable diversity and tolerance capacity towards PAH. Mar. Pollut. Bull. 2021, 164, 112082. [Google Scholar] [CrossRef]
- Toyooka, T.; Ibuki, Y. DNA damage induced by coexposure to PAHs and light. Environ. Toxicol. Pharmacol. 2007, 23, 256–263. [Google Scholar] [CrossRef]
- Xu, C.; Dong, D.; Meng, X.; Su, X.; Zheng, X.; Li, Y. Photolysis of polycyclic aromatic hydrocarbons on soil surfaces under UV irradiation. J. Environ. Sci. 2013, 25, 569–575. [Google Scholar] [CrossRef]
- Zhao, S.; Jia, H.; Nulaji, G.; Gao, H.; Wang, F.; Wang, C. Photolysis of polycyclic aromatic hydrocarbons (PAHs) on Fe3+-montmorillonite surface under visible light: Degradation kinetics, mechanism, and toxicity assessments. Chemosphere 2017, 184, 1346–1354. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Xu, P.F.; Du, H.; Tang, Y.; Meng, Y.; Yuan, L.; Sheng, L.P. Degradation of polycyclic aromatic hydrocarbons: A review. Appl. Ecol. Environ. Res. 2018, 16, 6419–6440. [Google Scholar] [CrossRef]
- Muralidharan, M.; Kavitha, R.; Kumar, P.S.; Pooja, M.; Rajagopal, R.; Gayathri, K.V. Performance evaluation and mechanism analysis of halotolerant bacterial strains and cerium oxide nanoparticle to degrade Benzo[a]pyrene. Environ. Technol. Innov. 2021, 24, 101980. [Google Scholar] [CrossRef]
- Abo-State, M.A.M.; Osman, M.E.; Khattab, O.H.; El-Kelani, T.A.; Abdel-Rahman, Z.M. Degradative pathways of polycyclic aromatic hydrocarbons (PAHs) by Phanerochaete chrysosporium under optimum conditions. J. Radiat. Res. Appl. Sci. 2021, 14, 507–520. [Google Scholar] [CrossRef]
- Seo, J.S.; Keum, Y.S.; Li, Q.X. Bacterial degradation of aromatic compounds. Int. J. Environ. Res. Public Health 2009, 6, 278–309. [Google Scholar]
- Hernández Blanco, F.J.; García de Llasera, M.P. Monitoring dihydrodiol polyaromatic hydrocarbon metabolites produced by the freshwater microalgae Selenastrum capricornutum. Chemosphere 2016, 158, 80–90. [Google Scholar] [CrossRef]
- Olmos-Espejel, J.J.; García de Llasera, M.P.; Velasco-Cruz, M. Extraction and analysis of polycyclic aromatic hydrocarbons and benzo[a]pyrene metabolites in microalgae cultures by off-line/on-line methodology based on matrix solid-phase dispersion, solid-phase extraction and high-performance liquid chromatography. J. Chromatogr. A 2012, 1262, 138–147. [Google Scholar] [CrossRef]
- Gao, Y.; Li, H.; Gong, S. Ascorbic acid enhances the accumulation of polycyclic aromatic hydrocarbons (PAHs) in roots of tall fescue (Festuca arundinacea Schreb.). PLoS ONE 2012, 7, e50467. [Google Scholar] [CrossRef]
- Franco, M.E.; Ramirez, A.J.; Johanning, K.M.; Matson, C.W.; Lavado, R. In vitro-in vivo biotransformation and phase I metabolite profiling of benzo[a]pyrene in Gulf killifish (Fundulus grandis) populations with different exposure histories. Aquat. Toxicol. 2021, 243, 106057. [Google Scholar] [CrossRef]
- Tombolini, F.; Pigini, D.; Tranfo, G.; Paci, E.; Carosi, I.; Marini, F.; Bauleo, L.; Ancona, C.; Forastiere, F. Levels of urinary metabolites of four PAHs and cotinine determined in 1016 volunteers living in Central Italy. Environ. Sci. Pollut. Res. 2018, 25, 28772–28779. [Google Scholar] [CrossRef] [PubMed]
- Thareja, S.; Aggarwal, S.; Bhardwaj, T.R.; Kumar, M. Self-organizing molecular field analysis of 2,4-thiazolidinediones: A 3D-QSAR model for the development of human PTP1B inhibitors. Eur. J. Med. Chem. 2010, 45, 2537–2546. [Google Scholar] [CrossRef] [PubMed]
- Ismail, S.Y.; Uzairu, A. In silico QSAR and molecular docking studies of sulfur containing shikonin oxime derivatives as anti-cancer agent for colon cancer. Radiol. Infect. Dis. 2019, 6, 108–121. [Google Scholar] [CrossRef]
- Dubrovskaya, E.; Pozdnyakova, N.; Golubev, S.; Muratova, A.; Grinev, V.; Bondarenkova, A.; Turkovskaya, O. Peroxidases from root exudates of Medicago sativa and Sorghum bicolor: Catalytic properties and involvement in PAH degradation. Chemosphere 2017, 169, 224–232. [Google Scholar] [CrossRef]
- Chepelev, N.L.; Long, A.S.; Williams, A.; Kuo, B.; Gagné, R.; Kennedy, D.A.; Phillips, D.H.; Arlt, V.M.; White, P.A.; Yauk, C.L. Transcriptional profiling of dibenzo[def,p]chrysene-induced spleen atrophy provides mechanistic insights into its immunotoxicity in mutamouse. Toxicol. Sci. 2016, 149, 251–268. [Google Scholar] [CrossRef]
- Singh, A.K.; Kashyap, M.P.; Kumar, V.; Tripathi, V.K.; Yadav, D.K.; Khan, F.; Jahan, S.; Khanna, V.K.; Yadav, S.; Pant, A.B. 3-methylcholanthrene induces neurotoxicity in developing neurons derived from human CD34+thy1+ stem cells by activation of aryl hydrocarbon receptor. Neuromol. Med. 2013, 15, 570–592. [Google Scholar] [CrossRef]
- Sparfel, L.; Pinel-Marie, M.L.; Boize, M.; Koscielny, S.; Desmots, S.; Pery, A.; Fardel, O. Transcriptional signature of human macrophages exposed to the environmental contaminant benzo(a)pyrene. Toxicol. Sci. 2010, 114, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Hossain, R.; Yasmin, T.; Hosen, M.I.; Nabi, A.H.M.N. In silico identification of potential epitopes present in human adenovirus proteins for vaccine design and of putative drugs for treatment against viral infection. J. Immunol. Methods 2018, 455, 55–70. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zaidi, S.A.; Zhang, Y. Binding mode analyses of NAP derivatives as mu opioid receptor selective ligands through docking studies and molecular dynamics simulation. Bioorg. Med. Chem. 2017, 25, 2463–2471. [Google Scholar] [CrossRef]
- Yang, J.; Li, X.; Zhao, Y.; Yang, H.; Li, Y. The exposure of OPFRs in fish from aquaculture area: Backward tracing of the ecological risk regulation. Environ. Pollut. 2021, 293, 118550. [Google Scholar] [CrossRef]
- Gu, W.; Li, Q.; Li, Y. Environment-friendly PCN derivatives design and environmental behavior simulation based on a multi-activity 3D-QSAR model and molecular dynamics. J. Hazard. Mater. 2020, 393, 122339. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, W.; Zhao, Y.; Chen, B.; Zhu, Z.; Kang, Q.; Zhang, B. Dermal exposure to synthetic musks: Human health risk assessment, mechanism, and control strategy. Ecotoxicol. Environ. Saf. 2022, 236, 113463. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Yang, J.; Li, Y. A model for phthalic acid esters’ biodegradability and biotoxicity multi-effect pharmacophore and its application in molecular modification. J. Environ. Sci. Health Part A 2021, 56, 361–378. [Google Scholar] [CrossRef] [PubMed]
- Gbeddy, G.; Egodawatta, P.; Goonetilleke, A.; Ayoko, G.; Chen, L. Dataset for the quantitative structure-activity relationship (QSAR) modeling of the toxicity equivalency factors (TEFs) of PAHs and transformed PAH products. Data Br. 2020, 28, 104821. [Google Scholar] [CrossRef]
- Zhang, W.; Gu, W.; Sun, R.; Zhou, M.; Han, Z.; Li, Y. An adjusted 3D-QSAR model for the combined activity of fluoroquinolones photodegradation and microbial degradation assisted by dynamic simulation and its application in molecular modification. Ecotoxicol. Environ. Saf. 2021, 212, 111973. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Y.; Chen, B.; Zhu, Z.; Kang, Q.; Husain, T.; Zhang, B. Inhalation and ingestion of Synthetic musks in pregnant women: In silico spontaneous abortion risk evaluation and control. Environ. Int. 2022, 158, 106911. [Google Scholar] [CrossRef]
- Hou, Y.; Zhao, Y.; Li, Q.; Li, Y. Highly biodegradable fluoroquinolone derivatives designed using the 3D-QSAR model and biodegradation pathways analysis. Ecotoxicol. Environ. Saf. 2020, 191, 110186. [Google Scholar] [CrossRef]
- Li, X.; Gu, W.; Chen, B.; Zhu, Z.; Zhang, B. Functional modification of HHCB: Strategy for obtaining environmentally friendly derivatives. J. Hazard. Mater. 2021, 416, 126116. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, J.; Zhan, J.; Gao, H. Screening out anti-inflammatory or anti-viral targets in Xuanfei Baidu Tang through a new technique of reverse finding target. Bioorg. Chem. 2021, 116, 105274. [Google Scholar] [CrossRef]
- Kranthi Kumar, K.; Yugandhar, P.; Uma Devi, B.; Siva Kumar, T.; Savithramma, N.; Neeraja, P. Applications of in silico methods to analyze the toxicity and estrogen receptor-mediated properties of plant-derived phytochemicals. Food Chem. Toxicol. 2019, 125, 361–369. [Google Scholar] [CrossRef]
- Lavado, G.J.; Gadaleta, D.; Toma, C.; Golbamaki, A.; Toropov, A.A.; Toropova, A.P.; Marzo, M.; Baderna, D.; Arning, J.; Benfenati, E. Zebrafish AC50 modelling: (Q)SAR models to predict developmental toxicity in zebrafish embryo. Ecotoxicol. Environ. Saf. 2020, 202, 110936. [Google Scholar] [CrossRef] [PubMed]
- Saravanakumar, K.; Sivasantosh, S.; Sathiyaseelan, A.; Sankaranarayanan, A.; Naveen, K.V.; Zhang, X.; Jamla, M.; Vijayasarathy, S.; Vishnu Priya, V.; MubarakAli, D.; et al. Impact of benzo[a]pyrene with other pollutants induce the molecular alternation in the biological system: Existence, detection, and remediation methods. Environ. Pollut. 2022, 304, 119207. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ren, Z.; Yang, H.; Li, Y. A novel multi-criteria framework for optimizing ecotoxicological effects and human health risks of neonicotinoid insecticides: Characterization, assessment and regulation strategies. J. Hazard. Mater. 2022, 432, 128712. [Google Scholar] [CrossRef]
- Bermúdez-Saldaña, J.M.; Escuder-Gilabert, L.; Medina-Hernández, M.J.; Villanueva-Camañas, R.M.; Sagrado, S. Modelling bioconcentration of pesticides in fish using biopartitioning micellar chromatography. J. Chromatogr. A 2005, 1063, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Takarina, N.D.; Purwiyanto, A.I.S.; Suteja, Y. Cadmium (Cd), Copper (Cu), and Zinc (Zn) levels in commercial and non-commercial fishes in the Blanakan River Estuary, Indonesia: A preliminary study. Mar. Pollut. Bull. 2021, 170, 112607. [Google Scholar] [CrossRef]
- Chedadi, O.; El Aissouq, A.; El Ouardi, Y.; Bouachrine, M.; Ouammou, A. 3D-QSAR and molecular docking studies of 4-methyl quinazoline derivatives as PI3Kα inhibitors. J. Indian Chem. Soc. 2021, 98, 100183. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Y. Design of environmentally friendly neonicotinoid insecticides with bioconcentration tuning and Bi-directional selective toxic effects. J. Clean. Prod. 2019, 221, 113–121. [Google Scholar] [CrossRef]
- Seifinejad, A.; Vassalli, A.; Tafti, M. Neurobiology of cataplexy. Sleep Med. Rev. 2021, 60, 101546. [Google Scholar] [CrossRef]
- Tomasetig, F.; Tebby, C.; Graillot, V.; Zeman, F.; Pery, A.; Cravedi, J.P.; Audebert, M. Comparative genotoxic potential of 27 polycyclic aromatic hydrocarbons in three human cell lines. Toxicol. Lett. 2020, 326, 99–105. [Google Scholar] [CrossRef]
- Das, D.N.; Bhutia, S.K. Inevitable dietary exposure of Benzo[a]pyrene: Carcinogenic risk assessment an emerging issues and concerns. Curr. Opin. Food Sci. 2018, 24, 16–25. [Google Scholar] [CrossRef]
- Sun, K.; Song, Y.; He, F.; Jing, M.; Tang, J.; Liu, R. A review of human and animals exposure to polycyclic aromatic hydrocarbons: Health risk and adverse effects, photo-induced toxicity and regulating effect of microplastics. Sci. Total Environ. 2021, 773, 145403. [Google Scholar] [CrossRef] [PubMed]
- Jesus, F.; Pereira, J.L.; Campos, I.; Santos, M.; Ré, A.; Keizer, J.; Nogueira, A.; Gonçalves, F.J.M.; Abrantes, N.; Serpa, D. A review on polycyclic aromatic hydrocarbons distribution in freshwater ecosystems and their toxicity to benthic fauna. Sci. Total Environ. 2022, 820, 153282. [Google Scholar] [CrossRef]
- Ren, K.; Wei, Y.; Li, J.; Han, C.; Deng, Y.; Su, G. Polycyclic aromatic hydrocarbons (PAHs) and their derivatives (oxygenated PAHs, azaarenes, and sulfur/oxygen-containing heterocyclic PAHs) in surface soils from a typical city, south China. Chemosphere 2021, 283, 131190. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Lai, Z.; Zeng, Y.; Gao, Y.; Yang, W.; Mai, Y.; Wang, C. Occurrence, source identification, and ecological risk assessment of polycyclic aromatic hydrocarbons in sediments of the Pearl River Delta, China. Mar. Pollut. Bull. 2021, 170, 112666. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.; Turner, R. Leadership competency profiles of successful project managers. Int. J. Proj. Manag. 2010, 28, 437–448. [Google Scholar] [CrossRef]
- Lu, S.; Lin, C.; Lei, K.; Xin, M.; Wang, B.; Ouyang, W.; Liu, X.; He, M. Endocrine-disrupting chemicals in a typical urbanized bay of Yellow Sea, China: Distribution, risk assessment, and identification of priority pollutants. Environ. Pollut. 2021, 287, 117588. [Google Scholar] [CrossRef]
- Li, J.; Chen, Y.; Yao, X.; Chen, A. Risk Management Priority Assessment of heritage sites in China Based on Entropy Weight and TOPSIS. J. Cult. Herit. 2021, 49, 10–18. [Google Scholar] [CrossRef]
- MAllick, S. Biodegradation of acenaphthene by Sphingobacterium sp. strain RTSB involving trans-3-carboxy-2-hydroxybenzylidenepyruvic acid as a metabolite. Chemosphere 2019, 219, 748–755. [Google Scholar] [CrossRef]
- Marquès, M.; Mari, M.; Audí-Miró, C.; Sierra, J.; Soler, A.; Nadal, M.; Domingo, J.L. Climate change impact on the PAH photodegradation in soils: Characterization and metabolites identification. Environ. Int. 2016, 89–90, 155–165. [Google Scholar] [CrossRef]
- Poonthrigpun, S.; Pattaragulwanit, K.; Paengthai, S.; Kriangkripipat, T.; Juntongjin, K.; Thaniyavarn, S.; Petsom, A.; Pinphanichakarn, P. Novel intermediates of acenaphthylene degradation by Rhizobium sp. strain CU-A1: Evidence for naphthalene-1,8-dicarboxylic acid metabolism. Appl. Environ. Microbiol. 2006, 72, 6034–6039. [Google Scholar] [CrossRef]
- Kou, J.; Li, Z.; Yuan, Y.; Zhang, H.; Wang, Y.; Zou, Z. Visible-light-induced photocatalytic oxidation of polycyclic aromatic hydrocarbons over tantalum oxynitride photocatalysts. Environ. Sci. Technol. 2009, 43, 2919–2924. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, M.; Esposito, E.; Moody, J.D.; Canhos, V.P.; Cerniglia, C.E. Metabolism of aromatic hydrocarbons by the filamentous fungus Cyclothyrium sp. Chemosphere 2004, 57, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Hadibarata, T.; Khudhair, A.B.; Salim, M.R. Breakdown products in the metabolic pathway of anthracene degradation by a ligninolytic fungus Polyporus sp. S133. Water. Air. Soil Pollut. 2012, 223, 2201–2208. [Google Scholar] [CrossRef]
- Tarafdar, A.; Sinha, A.; Masto, R.E. Biodegradation of anthracene by a newly isolated bacterial strain, Bacillus thuringiensis AT.ISM.1, isolated from a fly ash deposition site. Lett. Appl. Microbiol. 2017, 65, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Sahali, Y.; Kidd, L.C.R.; Skipper, P.L.; Tannenbaum, S.R. Metabolism of benz[a]anthracene by human liver microsomes. Cancer Lett. 1994, 83, 299–303. [Google Scholar] [CrossRef]
- Westman, O.; Larsson, M.; Venizelos, N.; Hollert, H.; EngwAll, M. An oxygenated metabolite of benzo[a]pyrene increases hepatic β-oxidation of fatty acids in chick embryos. Environ. Sci. Pollut. Res. 2014, 21, 6243–6251. [Google Scholar] [CrossRef]
- Luo, J.; Deng, J.; Cui, L.; Chang, P.; Dai, X.; Yang, C.; Li, N.; Ren, Z.; Zhang, X. The potential assessment of green alga Chlamydomonas reinhardtii CC-503 in the biodegradation of benz(a)anthracene and the related mechanism analysis. Chemosphere 2020, 249, 126097. [Google Scholar] [CrossRef]
- Cajthaml, T.; Erbanová, P.; Šašek, V.; Moeder, M. Breakdown products on metabolic pathway of degradation of benz[a]anthracene by a ligninolytic fungus. Chemosphere 2006, 64, 560–564. [Google Scholar] [CrossRef]
- Rachna; Rani, M.; Shanker, U. Sunlight mediated improved Photocatalytic degradation of carcinogenic benz[a]anthracene and benzo[a]pyrene by zinc oxide encapsulated hexacyanoferrate nanocomposite. J. Photochem. Photobiol. A Chem. 2019, 381, 111861. [Google Scholar] [CrossRef]
- Yang, L.; Zhou, Y.; Sun, H.; Lai, H.; Liu, C.; Yan, K.; Yuan, J.; Wu, T.; Chen, W.; Zhang, X. Dose-response relationship between polycyclic aromatic hydrocarbon metabolites and risk of diabetes in the general Chinese population. Environ. Pollut. 2014, 195, 24–30. [Google Scholar] [CrossRef]
- Lourenço, D.; Silva, L.J.G.; Lino, C.M.; Morais, S.; Pena, A. SPE-LC-FD determination of polycyclic aromatic hydrocarbon monohydroxy derivatives in cephalopods. J. Agric. Food Chem. 2014, 62, 2685–2691. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Hong, H.S.; Mu, J.L.; Lin, J.Q.; Wang, S.H. Polycyclic aromatic hydrocarbon (PAH) metabolites in marine fishes as a specific biomarker to indicate PAH pollution in the marine coastal environment. J. Environ. Sci. Heal.-Part A Toxic/Hazardous Subst. Environ. Eng. 2008, 43, 219–226. [Google Scholar] [CrossRef]
- Gupta, H.; Gupta, B. Photocatalytic degradation of polycyclic aromatic hydrocarbon benzo[a]pyrene by iron oxides and identification of degradation products. Chemosphere 2015, 138, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Ill, P. Research Articles (Series): Impact Studies on PAHs. ESPR-Environ. Sci. Pollut. Res.-Int. 2004, 1, 22–32. [Google Scholar]
- Shu-yan, Z.; Pei-jun, L.I.; Xiao-cai, Y.U.; Kun, S.H.I.; Hui, Z.; Jing, C. Degradation of metabolites of benzo[a]pyrene by coupling Penicillium chrysogenum with KMn04. J. Environ. Sci. 2007, 19, 238–243. [Google Scholar]
- Qin, W.; Fan, F.; Zhu, Y.; Huang, X.; Ding, A.; Liu, X.; Dou, J. Anaerobic biodegradation of benzo(a)pyrene by a novel Cellulosimicrobium cellulans CWS2 isolated from polycyclic aromatic hydrocarbon-contaminated soil. Brazilian J. Microbiol. 2018, 49, 258–268. [Google Scholar] [CrossRef]
- Chen, S.; Yin, H.; Ye, J.; Peng, H.; Zhang, N.; He, B. Effect of copper(II) on biodegradation of benzo[a]pyrene by Stenotrophomonas maltophilia. Chemosphere 2013, 90, 1811–1820. [Google Scholar] [CrossRef] [PubMed]
- Geddie, J.E.; Amin, S.; Huie, K.; Hecht, S.S. Formation and tumorigenicity of benzo[b]fluoranthene metabolites in mouse epidermis. Carcinogenesis 1987, 8, 1579–1584. [Google Scholar] [CrossRef]
- Palazzi, P.; Mezzache, S.; Bourokba, N.; Hardy, E.M.; Schritz, A.; Bastien, P.; Emond, C.; Li, J.; Soeur, J.; Appenzeller, B.M.R. Exposure to polycyclic aromatic hydrocarbons in women living in the Chinese cities of BaoDing and Dalian revealed by hair analysis. Environ. Int. 2018, 121, 1341–1354. [Google Scholar] [CrossRef]
- Story, S.P.; Kline, E.L.; Hughes, T.A.; Riley, M.B.; Hayasaka, S.S. Degradation of aromatic hydrocarbons by Sphingomonas paucimobilis strain EPA505. Arch. Environ. Contam. Toxicol. 2004, 47, 168–176. [Google Scholar] [CrossRef]
- Mandal, S.K.; Das, N. Biodegradation of perylene and benzo [ghi] perylene (5-6 rings) using yeast consortium: Kinetic study, enzyme analysis and degradation pathway. J. Environ. Biol. 2018, 39, 5–15. [Google Scholar] [CrossRef]
- Platt, K.L.; Grupe, S. Microsomal biotransformation of benzo[ghi]perylene, a mutagenic polycyclic aromatic hydrocarbon without a “classic” bay region. Chem. Res. Toxicol. 2005, 18, 700–710. [Google Scholar] [CrossRef] [PubMed]
- Grova, N.; Faÿs, F.; Hardy, E.M.; Appenzeller, B.M.R. New insights into urine-based assessment of polycyclic aromatic hydrocarbon-exposure from a rat model: Identification of relevant metabolites and influence of elimination kinetics. Environ. Pollut. 2017, 228, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Maeda, A.H.; Nishi, S.; Hatada, Y.; Ozeki, Y.; Kanaly, R.A. Biotransformation of the high-molecular weight polycyclic aromatic hydrocarbon (PAH) benzo[k]fluoranthene by Sphingobium sp. strain KK22 and identification of new products of non-alternant PAH biodegradation by liquid chromatography electrospray ionizatio. Microb. Biotechnol. 2014, 7, 114–129. [Google Scholar] [CrossRef] [PubMed]
- Pampanin, D.M.; Le Goff, J.; Skogland, K.; Marcucci, C.R.; Øysæd, K.B.; Lorentzen, M.; Jørgensen, K.B.; Sydnes, M.O. Biological effects of polycyclic aromatic hydrocarbons (PAH) and their first metabolic products in in vivo exposed Atlantic cod (Gadus morhua). J. Toxicol. Environ. Heal.-Part A Curr. Issues 2016, 79, 633–646. [Google Scholar] [CrossRef]
- Al Farraj, D.A.; Hadibarata, T.; Yuniarto, A.; Syafiuddin, A.; Surtikanti, H.K.; Elshikh, M.S.; Al Khulaifi, M.M.; Al-Kufaidy, R. Characterization of pyrene and chrysene degradation by halophilic Hortaea sp. B15. Bioprocess Biosyst. Eng. 2019, 42, 963–969. [Google Scholar] [CrossRef]
- Nayak, A.S.; Sanjeev Kumar, S.; Santosh Kumar, M.; Anjaneya, O.; Karegoudar, T.B. A catabolic pathway for the degradation of chrysene by Pseudoxanthomonas sp. PNK-04. FEMS Microbiol. Lett. 2011, 320, 128–134. [Google Scholar] [CrossRef]
- Hidayat, A.; Tachibana, S.; Itoh, K. Determination of chrysene degradation under saline conditions by Fusarium sp. F092, a fungus screened from nature. Fungal Biol. 2012, 116, 706–714. [Google Scholar] [CrossRef]
- Guntup Alli, S.; Thunuguntla, V.B.S.C.; Chalasani, L.M.; Rao, C.V.; Bondili, J.S. Degradation and Metabolite Profiling of Benz (a) Anthracene, Dibenz (a, h) Anthracene and Indeno [1, 2, 3-cd] Pyrene by Aspergillus terricola. Polycycl. Aromat. Compd. 2019, 39, 84–92. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, L.; Liu, C.; Stryker, Z.; Loganathan, B.G.; Kannan, K. Phthalate Metabolites, Hydroxy-Polycyclic Aromatic Hydrocarbons, and Bisphenol Analogues in Bovine Urine Collected from China, India, and the United States. Environ. Sci. Technol. 2019, 53, 11524–11531. [Google Scholar] [CrossRef]
- Johnson-Restrepo, B.; Olivero-Verbel, J.; Lu, S.; Guette-Fernández, J.; Baldiris-Avila, R.; O’Byrne-Hoyos, I.; Aldous, K.M.; Addink, R.; Kannan, K. Polycyclic aromatic hydrocarbons and their hydroxylated metabolites in fish bile and sediments from coastal waters of Colombia. Environ. Pollut. 2008, 151, 452–459. [Google Scholar] [CrossRef]
- Tsai, J.C.; Kumar, M.; Lin, J.G. Anaerobic biotransformation of fluorene and phenanthrene by sulfate-reducing bacteria and identification of biotransformation pathway. J. Hazard. Mater. 2009, 164, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Garon, D.; Krivobok, S.; Seigle-Murandi, F. Fungal degradation of fluorene. Chemosphere 2000, 40, 91–97. [Google Scholar] [CrossRef]
- Ke, L.; Luo, L.; Wang, P.; Luan, T.; Tam, N.F.Y. Effects of metals on biosorption and biodegradation of mixed polycyclic aromatic hydrocarbons by a freshwater green alga Selenastrum capricornutum. Bioresour. Technol. 2010, 101, 6950–6961. [Google Scholar] [CrossRef] [PubMed]
- Hillenweck, A.; Canlet, C.; Mauffret, A.; Debrauwer, L.; Claireaux, G.; Cravedi, J.P. Characterization of biliary metabolites of fluoranthene in the common sole (Solea Solea). Environ. Toxicol. Chem. 2008, 27, 2575–2581. [Google Scholar] [CrossRef]
- Lee, S.E.; Seo, J.S.; Keum, Y.S.; Lee, K.J.; Li, Q.X. Fluoranthene metabolism and associated proteins in Mycobacterium sp. JS14. Proteomics 2007, 7, 2059–2069. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.C.F.S.; Birolli, W.G.; Araújo, B.R.; Marques, C.G.; Barbosa-Junior, A.M.; Diniz, L.E.C.; Porto, A.L.M.; Romão, L.P.C. Biodegradation of the polycyclic aromatic hydrocarbon fluoranthene by fungi strains from a brazilian tropical peat. J. Braz. Chem. Soc. 2021, 32, 1874–1883. [Google Scholar] [CrossRef]
- Luan, T.; Fang, S.; Zhong, Y.; Lin, L.; Chan, S.M.N.; Lan, C.; Tam, N.F.Y. Determination of hydroxy metabolites of polycyclic aromatic hydrocarbons by fully automated solid-phase microextraction derivatization and gas chromatography-mass spectrometry. J. Chromatogr. A 2007, 1173, 37–43. [Google Scholar] [CrossRef]
- Rice, J.E.; Hosted, T.J.; Defloria, M.C.; Lavoie, E.J.; Fischer, D.L.; Wiley, J.C. Tumor-initiating activity of major in vivo metabolites of indeno[1, 2, 3-CD]pyrene on mouse skin. Carcinogenesis 1986, 7, 1761–1764. [Google Scholar] [CrossRef]
- Miao, L.L.; Qu, J.; Liu, Z.P. Hydroxylation at Multiple Positions Initiated the Biodegradation of Indeno[1,2,3-cd]Pyrene in Rhodococcus aetherivorans IcdP1. Front. Microbiol. 2020, 11, 1–13. [Google Scholar] [CrossRef]
- Jacob, J.; Grimmer, G. Capillary gas chromatographical analysis and mass spectrometric identification of polycyclic aromatic hydrocarbon metabolites from biological materials. Rev. Anal. Chem. 1987, 9, 49–89. [Google Scholar] [CrossRef]
- Nilsson, R.; Antić, R.; Berni, A.; DAllner, G.; Dettbarn, G.; Gromadzinska, J.; Joksić, G.; Lundin, C.; Palitti, F.; Prochazka, G.; et al. Exposure to polycyclic aromatic hydrocarbons in women from Poland, Serbia and Italy-relation between PAH metabolite excretion, DNA damage, diet and genotype (the EU DIEPHY project). Biomarkers 2013, 18, 165–173. [Google Scholar] [CrossRef]
- Tang, C.; Tan, J.; Fan, R.; Zhao, B.; Tang, C.; Ou, W.; Jin, J.; Peng, X. Quasi-targeted analysis of hydroxylation-related metabolites of polycyclic aromatic hydrocarbons in human urine by liquid chromatography–mass spectrometry. J. Chromatogr. A 2016, 1461, 59–69. [Google Scholar] [CrossRef]
- Cho, T.M.; Rose, R.L.; Hodgson, E. In vitro metabolism of naphthalene by human liver microsomal cytochrome P450 enzymes. Drug Metab. Dispos. 2006, 34, 176–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayala, D.C.; Morin, D.; Buckpitt, A.R. Simultaneous quantification of multiple urinary naphthalene metabolites by liquid chromatography tandem mass spectrometry. PLoS ONE 2015, 10, e0121937. [Google Scholar] [CrossRef] [PubMed]
- Kleemann, R.; Meckenstock, R.U. Anaerobic naphthalene degradation by Gram-positive, iron-reducing bacteria. FEMS Microbiol. Ecol. 2011, 78, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Weyrauch, P.; Heker, I.; Zaytsev, A.V.; von Hagen, C.A.; Arnold, M.E.; Golding, B.T.; Meckenstock, R.U. The 5,6,7,8-Tetrahydro-2-naphthoyl-coenzyme a reductase reaction in the anaerobic degradation of naphthalene and identification of downstream metabolites. Appl. Environ. Microbiol. 2020, 86, e00996-20. [Google Scholar] [CrossRef]
- Cerniglia, C.E.; Van Baalen, C.; Gibson, D.T. Metabolism of naphthalene by the cyanobacterium Oscillatoria sp., strain JCM. J. Gen. Microbiol. 1980, 116, 485–494. [Google Scholar] [CrossRef]
- Pulster, E.L.; Main, K.; Wetzel, D.; Murawski, S. Species-specific metabolism of naphthalene and phenanthrene in 3 species of marine teleosts exposed to Deepwater Horizon crude oil. Environ. Toxicol. Chem. 2017, 36, 3168–3176. [Google Scholar] [CrossRef]
- Cai, H.; Sun, L.; Wang, Y.; Song, T.; Bao, M.; Yang, X. Unprecedented efficient degradation of phenanthrene in water by intimately coupling novel ternary composite Mn3O4/MnO2-Ag3PO4 and functional bacteria under visible light irradiation. Chem. Eng. J. 2019, 369, 1078–1092. [Google Scholar] [CrossRef]
- Safonova, E.; Kvitko, K.; Kuschk, P.; Möder, M.; Reisser, W. Biodegradation of phenanthrene by the green alga Scenedesmus obliquus ES-55. Eng. Life Sci. 2005, 5, 234–239. [Google Scholar] [CrossRef]
- Muratova, A.; Dubrovskaya, E.; Golubev, S.; Grinev, V.; Chernyshova, M.; Turkovskaya, O. The coupling of the plant and microbial catabolisms of phenanthrene in the rhizosphere of Medicago sativa. J. Plant Physiol. 2015, 188, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ruddock, P.J.; Bird, D.J.; McEvoy, J.; Peters, L.D. Bile metabolites of polycyclic aromatic hydrocarbons (PAHs) in European eels Anguilla anguilla from United Kingdom estuaries. Sci. Total Environ. 2003, 301, 105–117. [Google Scholar] [CrossRef]
- Gupta, B.; Gupta, H. Iron Oxide Mediated Degradation of Mutagen Pyrene and Determination of Degradation Products. Int. J. Environ. Sci. Dev. 2015, 6, 908–912. [Google Scholar] [CrossRef] [Green Version]
| PAHs | Acronym | 2D Structure | IACR Classifications Group [28] |
|---|---|---|---|
| Acenaphthene | ACE |  | 3 |
| Acenaphthylene | ACY | 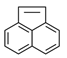 | * |
| Anthracene | ANT | 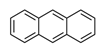 | 3 |
| Benz[a]anthracene | BaAN | 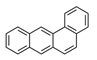 | 2B |
| Benzo[a]pyrene | BaP | 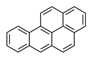 | 1 a |
| Benzo[b]fluoranthene | BbF | 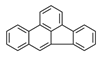 | 2B |
| Benzo[ghi]perylene | BghiP | 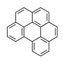 | 3 |
| Benzo[k]fluoranthene | BkF |  | 2B |
| Chrysene | CHR |  | 2B |
| Dibenz[a,h]anthracene | DahA |  | 2A b |
| Fluorene | FLR | 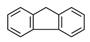 | 3 |
| Fluoranthene | FRT | 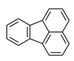 | 3 |
| Indeno[1,2,3-cd]pyrene | IcdP | 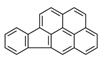 | 2B c |
| Naphthalene | NAP | 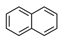 | 2B |
| Phenanthrene | PHE | 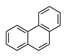 | 3 d |
| Pyrene | PYR | 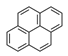 | 3 |
| 3D-QSAR Models | Q2 a | N b | R2 c | SEE d | F e | R2pred f |
|---|---|---|---|---|---|---|
| PAHs’ neurotoxicity model | 0.749 | 10 | 1.000 | 0.387 | 2370.819 | 0.789 |
| PAHs’ immunotoxicity model | 0.753 | 10 | 1.000 | 0.685 | 1991.539 | 0.639 |
| PAHs’ phytotoxicity model | 0.935 | 10 | 1.000 | 0.367 | 5032.620 | 0.650 |
| PAHs’ Neurotoxicity Model | PAHs’ Immunotoxicity Model | PAHs’ Phytotoxicity Model | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PAHs | Exp. (Binding Free Energy, kJ/mol) | Pred. (Binding Free Energy, kJ/mol) | Relative Error (%) | PAHs | Exp. (Binding Free Energy, kJ/mol) | Pred. (Binding Free Energy, kJ/mol) | Relative Error (%) | PAHs | Exp. (Binding Free Energy, kJ/mol) | Pred. (Binding Free Energy, kJ/mol) | Relative Error (%) |
| 1MNAP b | −43.422 | −47.198 | −8.70 | 1MNAP b | −61.808 | −41.487 | 32.88 | 1MNAP a | −37.273 | −37.162 | 0.30 |
| 2MNAP b | −46.392 | −47.670 | −2.75 | 2MNAP a | −47.673 | −47.812 | −0.29 | 2MNAP a | −42.014 | −42.319 | −0.73 |
| ACE a | −32.347 | −32.025 | 1.00 | ACE a | −68.893 | −68.577 | 0.46 | ACE a | −54.961 | −54.710 | 0.46 |
| ANY a | −43.915 | −44.111 | −0.45 | ACY a | −53.244 | −53.560 | −0.59 | ACY a | −53.263 | −53.552 | −0.54 |
| BaAN a | −55.501 | −55.371 | 0.23 | ANT a | −69.617 | −69.599 | 0.03 | ANT a | −64.852 | −64.844 | 0.01 |
| BANN a | −60.793 | −60.801 | −0.01 | BaAN a | −91.938 | −91.803 | 0.15 | BANN b | −97.418 | −109.937 | −12.85 |
| BaP a | −70.481 | −70.748 | −0.38 | BaP a | −97.864 | −97.145 | 0.73 | BaP a | −103.536 | −103.504 | 0.03 |
| BbF a | −44.365 | −44.287 | 0.18 | BbF a | −103.742 | −103.692 | 0.05 | BbF a | −100.331 | −100.260 | 0.07 |
| BeP a | −67.026 | −67.011 | 0.02 | BghiP a | −121.288 | −121.378 | −0.07 | BeP a | −74.155 | −73.930 | 0.30 |
| BghiP a | −64.460 | −64.604 | −0.22 | BkF a | −112.373 | −112.617 | −0.22 | BghiP a | −98.467 | −98.653 | −0.19 |
| BkF a | −50.842 | −50.926 | −0.17 | CHR a | −83.232 | −83.945 | −0.86 | BkF b | −81.132 | −60.361 | 25.60 |
| CHR a | −66.290 | −65.886 | 0.61 | CPPHN b | −78.146 | −66.951 | 14.33 | CPPHN b | −81.077 | −45.480 | 43.91 |
| CRN a | −83.141 | −83.186 | −0.05 | CRN b | −113.449 | −86.764 | 23.52 | CRN a | −86.995 | −86.975 | 0.02 |
| DahA b | −66.480 | −56.925 | 14.37 | DahA a | −105.255 | −105.328 | −0.07 | DahA b | −81.625 | −62.226 | 23.77 |
| FLR b | −41.383 | −46.911 | −13.36 | FLR a | −67.423 | −67.182 | 0.36 | FLR b | −83.469 | −56.276 | 32.58 |
| FRT a | −49.042 | −48.781 | 0.53 | FRT b | −104.230 | −75.363 | 27.70 | FRT a | −76.780 | −76.825 | −0.06 |
| IcdP a | −65.071 | −65.138 | −0.10 | NAP a | −33.803 | −33.696 | 0.32 | NAP a | −51.637 | −51.298 | 0.66 |
| NAP a | −26.336 | −26.624 | −1.09 | PHE a | −54.440 | −54.451 | −0.02 | PHE a | −64.850 | −65.097 | −0.38 |
| PHE a | −34.993 | −35.103 | −0.31 | PYR b | −92.176 | −75.580 | 18.00 | PRL a | −93.434 | −93.337 | 0.10 |
| PYR b | −48.667 | −53.742 | −10.43 | PYR a | −89.548 | −89.631 | −0.09 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, L.; Zhou, M.; Zhao, Y.; Yang, J.; Pu, Q.; Yang, H.; Wu, Y.; Lyu, C.; Li, Y. Potential Toxicity Risk Assessment and Priority Control Strategy for PAHs Metabolism and Transformation Behaviors in the Environment. Int. J. Environ. Res. Public Health 2022, 19, 10972. https://doi.org/10.3390/ijerph191710972
Zhao L, Zhou M, Zhao Y, Yang J, Pu Q, Yang H, Wu Y, Lyu C, Li Y. Potential Toxicity Risk Assessment and Priority Control Strategy for PAHs Metabolism and Transformation Behaviors in the Environment. International Journal of Environmental Research and Public Health. 2022; 19(17):10972. https://doi.org/10.3390/ijerph191710972
Chicago/Turabian StyleZhao, Lei, Mengying Zhou, Yuanyuan Zhao, Jiawen Yang, Qikun Pu, Hao Yang, Yang Wu, Cong Lyu, and Yu Li. 2022. "Potential Toxicity Risk Assessment and Priority Control Strategy for PAHs Metabolism and Transformation Behaviors in the Environment" International Journal of Environmental Research and Public Health 19, no. 17: 10972. https://doi.org/10.3390/ijerph191710972
APA StyleZhao, L., Zhou, M., Zhao, Y., Yang, J., Pu, Q., Yang, H., Wu, Y., Lyu, C., & Li, Y. (2022). Potential Toxicity Risk Assessment and Priority Control Strategy for PAHs Metabolism and Transformation Behaviors in the Environment. International Journal of Environmental Research and Public Health, 19(17), 10972. https://doi.org/10.3390/ijerph191710972








