Birth Order, Caesarean Section, or Daycare Attendance in Relation to Child- and Adult-Onset Type 1 Diabetes: Results from the German National Cohort
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source and Study Population
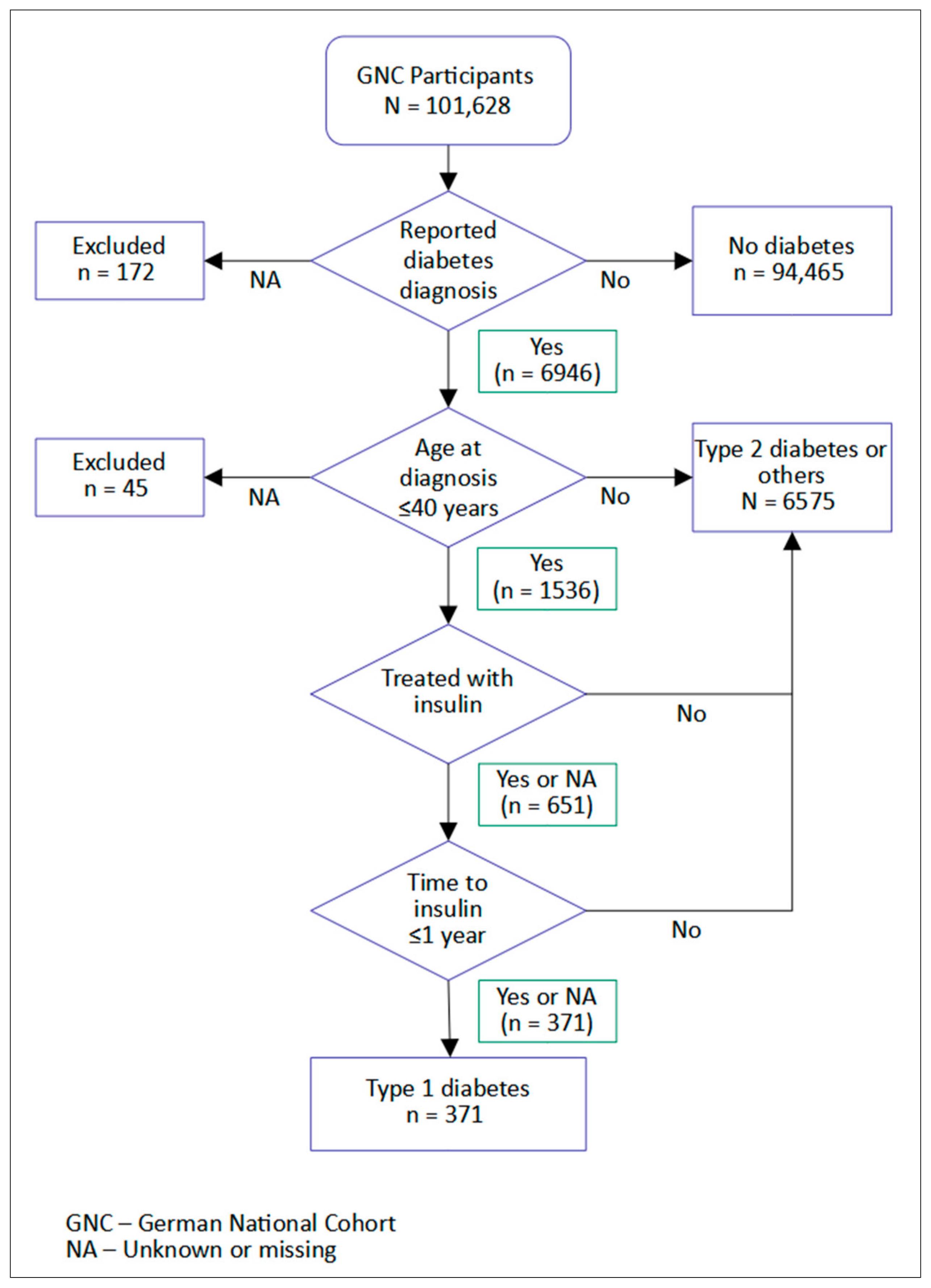
2.2. Type 1 Diabetes Ascertainment
- Age at diagnosis ≤40 years.
- Ongoing insulin therapy at time of recruitment.
- Time from diagnosis to insulin therapy initiation ≤1 year.
2.3. Exposure Variables and Covariables
2.4. Statistical Analysis
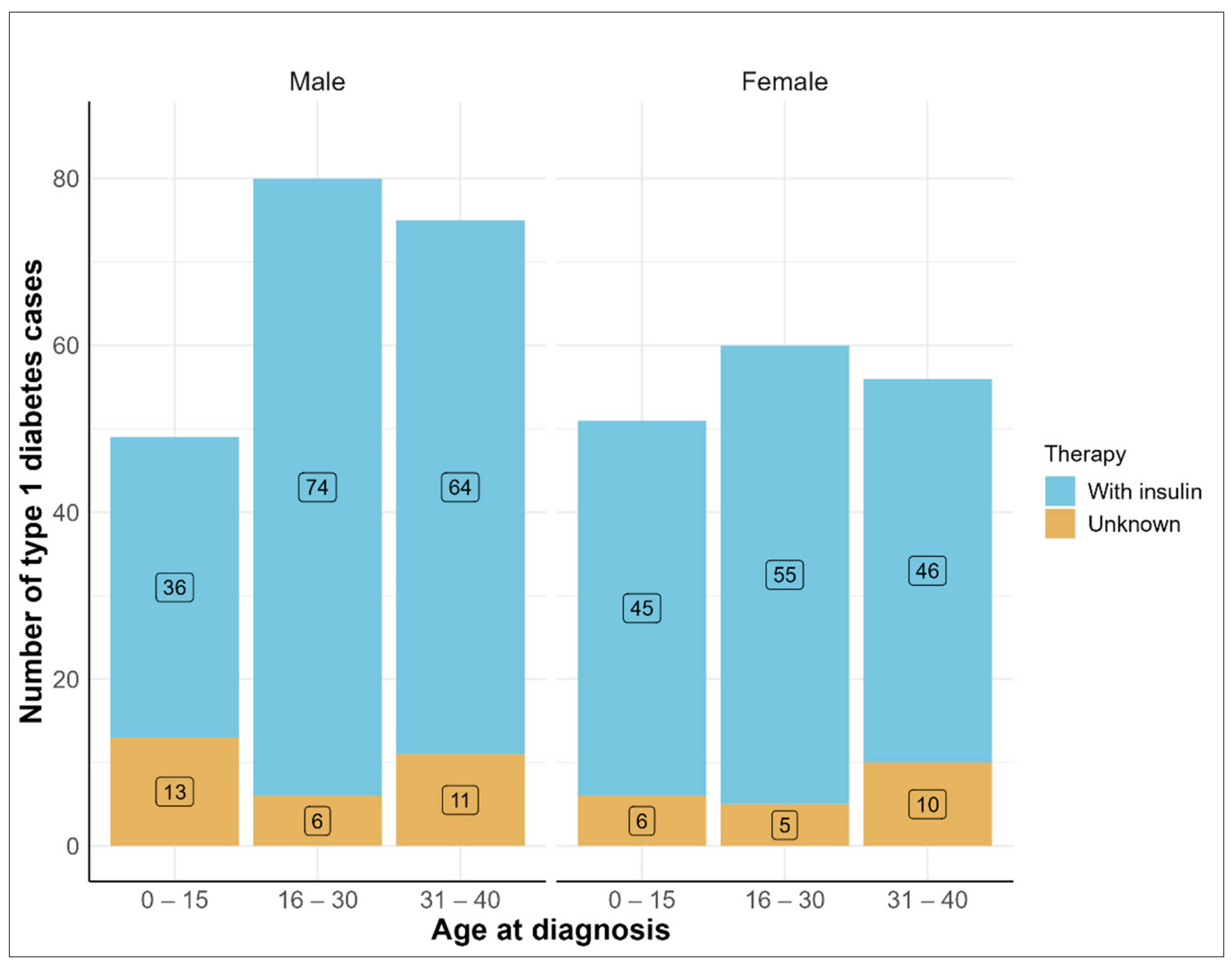
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes—2021. Diabetes Care 2021, 44, S15–S33. [Google Scholar] [CrossRef]
- Chiang, J.L.; Kirkman, M.S.; Laffel, L.M.B.; Peters, A.L. Type 1 diabetes through the life span: A position statement of the American Diabetes Association. Diabetes Care 2014, 37, 2034–2054. [Google Scholar] [CrossRef]
- Rawshani, A.; Sattar, N.; Franzén, S.; Rawshani, A.; Hattersley, A.T.; Svensson, A.M.; Eliasson, B.; Gudbjörnsdottir, S. Excess mortality and cardiovascular disease in young adults with type 1 diabetes in relation to age at onset: A nationwide, register-based cohort study. Lancet 2018, 392, 477–486. [Google Scholar] [CrossRef]
- Patterson, C.C.; Karuranga, S.; Salpea, P.; Saeedi, P.; Dahlquist, G.; Soltesz, G.; Ogle, G.D. Worldwide estimates of incidence, prevalence and mortality of type 1 diabetes in children and adolescents: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107842. [Google Scholar] [CrossRef]
- Diaz-Valencia, P.A.; Bougnères, P.; Valleron, A.J. Global epidemiology of type 1 diabetes in young adults and adults: A systematic review. BMC Public Health 2015, 15, 255. [Google Scholar] [CrossRef]
- Green, A.; Hede, S.M.; Patterson, C.C.; Wild, S.H.; Imperatore, G.; Roglic, G.; Beran, D. Type 1 diabetes in 2017: Global estimates of incident and prevalent cases in children and adults. Diabetologia 2021, 64, 2741–2750. [Google Scholar] [CrossRef]
- Leslie, R.D.; Evans-Molina, C.; Freund-Brown, J.; Buzzetti, R.; Dabelea, D.; Gillespie, K.M.; Goland, R.; Jones, A.G.; Kacher, M.; Phillips, L.S.; et al. Adult-onset type 1 diabetes: Current understanding and challenges. Diabetes Care 2021, 44, 2449–2456. [Google Scholar] [CrossRef]
- Thomas, N.J.; Jones, S.E.; Weedon, M.N.; Shields, B.M.; Oram, R.A.; Hattersley, A.T. Frequency and phenotype of type 1 diabetes in the first six decades of life: A cross-sectional, genetically stratified survival analysis from UK Biobank. Lancet Diabetes Endocrinol 2018, 6, 122–129. [Google Scholar] [CrossRef]
- Wang, Z.; Xie, Z.; Lu, Q.; Chang, C.; Zhou, Z. Beyond genetics: What causes type 1 diabetes. Clin. Rev. Allergy Immunol. 2017, 52, 273–286. [Google Scholar] [CrossRef]
- Redondo, M.J.; Steck, A.K.; Pugliese, A. Genetics of type 1 diabetes. Pediatr. Diabetes 2018, 19, 346–353. [Google Scholar] [CrossRef]
- Patterson, C.C.; Harjutsalo, V.; Rosenbauer, J.; Neu, A.; Cinek, O.; Skrivarhaug, T.; Rami-Merhar, B.; Soltesz, G.; Svensson, J.; Parslow, R.C.; et al. Trends and cyclical variation in the incidence of childhood type 1 diabetes in 26 European centres in the 25 year period 1989–2013: A multicentre prospective registration study. Diabetologia 2019, 62, 408–417. [Google Scholar] [CrossRef]
- Norris, J.M.; Johnson, R.K.; Stene, L.C. Type 1 diabetes-early life origins and changing epidemiology. Lancet Diabetes Endocrinol 2020, 8, 226–238. [Google Scholar] [CrossRef]
- Liu, J.; Ren, Z.-H.; Qiang, H.; Wu, J.; Shen, M.; Zhang, L.; Lyu, J. Trends in the incidence of diabetes mellitus: Results from the Global Burden of Disease Study 2017 and implications for diabetes mellitus prevention. BMC Public Health 2020, 20, 1415. [Google Scholar] [CrossRef] [PubMed]
- Craig, M.E.; Kim, K.W.; Isaacs, S.R.; Penno, M.A.; Hamilton-Williams, E.E.; Couper, J.J.; Rawlinson, W.D. Early-life factors contributing to type 1 diabetes. Diabetologia 2019, 62, 1823–1834. [Google Scholar] [CrossRef] [PubMed]
- Derrien, M.; Alvarez, A.-S.; de Vos, W.M. The gut microbiota in the first decade of life. Trends Microbiol. 2019, 27, 997–1010. [Google Scholar] [CrossRef]
- Moore, R.E.; Townsend, S.D. Temporal development of the infant gut microbiome. Open Biol. 2019, 9, 190128. [Google Scholar] [CrossRef]
- Al Nabhani, Z.; Eberl, G. Imprinting of the immune system by the microbiota early in life. Mucosal Immunol. 2020, 13, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Vaarala, O. Human intestinal microbiota and type 1 diabetes. Curr. Diab. Rep. 2013, 13, 601–607. [Google Scholar] [CrossRef]
- Murri, M.; Leiva, I.; Gomez-Zumaquero, J.M.; Tinahones, F.J.; Cardona, F.; Soriguer, F.; Queipo-Ortuno, M.I. Gut microbiota in children with type 1 diabetes differs from that in healthy children: A case-control study. BMC Med. 2013, 11, 46. [Google Scholar] [CrossRef]
- Munyaka, P.M.; Khafipour, E.; Ghia, J.E. External influence of early childhood establishment of gut microbiota and subsequent health implications. Front. Pediatr. 2014, 2, 109. [Google Scholar] [CrossRef] [Green Version]
- Penders, J.; Thijs, C.; Vink, C.; Stelma, F.F.; Snijders, B.; Kummeling, I.; van den Brandt, P.A.; Stobberingh, E.E. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics 2006, 118, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Bello, M.G.; Costello, E.K.; Contreras, M.; Magris, M.; Hidalgo, G.; Fierer, N.; Knight, R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. USA 2010, 107, 11971–11975. [Google Scholar] [CrossRef]
- Shao, Y.; Forster, S.C.; Tsaliki, E.; Vervier, K.; Strang, A.; Simpson, N.; Kumar, N.; Stares, M.D.; Rodger, A.; Brocklehurst, P.; et al. Stunted microbiota and opportunistic pathogen colonization in caesarean-section birth. Nature 2019, 574, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, L.; Jin, B.; Xu, X.; Zuo, X.; Li, Y.; Li, Z. The effects of delivery mode on the gut microbiota and health: State of art. Front. Microbiol. 2021, 12, 724449. [Google Scholar] [CrossRef]
- Antoine, C.; Young, B.K. Cesarean section one hundred years 1920–2020: The good, the bad and the ugly. J. Perinat. Med. 2021, 49, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Mikolajczyk, R.T.; Schmedt, N.; Zhang, J.; Lindemann, C.; Langner, I.; Garbe, E. Regional variation in caesarean deliveries in Germany and its causes. BMC Pregnancy Childbirth 2013, 13, 99. [Google Scholar] [CrossRef]
- Cardwell, C.R.; Stene, L.C.; Joner, G.; Cinek, O.; Svensson, J.; Goldacre, M.J.; Parslow, R.C.; Pozzilli, P.; Brigis, G.; Stoyanov, D.; et al. Caesarean section is associated with an increased risk of childhood-onset type 1 diabetes mellitus: A meta-analysis of observational studies. Diabetologia 2008, 51, 726–735. [Google Scholar] [CrossRef]
- Tanoey, J.; Gulati, A.; Patterson, C.; Becher, H. Risk of type 1 diabetes in the offspring born through elective or non-elective Caesarean section in comparison to vaginal delivery: A meta-analysis of observational studies. Curr. Diab. Rep. 2019, 19, 124. [Google Scholar] [CrossRef]
- Begum, M.; Pilkington, R.; Chittleborough, C.; Lynch, J.; Penno, M.; Smithers, L. Caesarean section and risk of type 1 diabetes: Whole-of-population study. Diabet. Med. 2019, 36, 1686–1693. [Google Scholar] [CrossRef]
- Frederiksen, B.; Kroehl, M.; Lamb, M.M.; Seifert, J.; Barriga, K.; Eisenbarth, G.S.; Rewers, M.; Norris, J.M. Infant exposures and development of type 1 diabetes mellitus: The Diabetes Autoimmunity Study in the Young (DAISY). JAMA Pediatr. 2013, 167, 808–815. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.Y.; Lu, C.L.; Chen, H.F.; Su, H.F.; Li, C.Y. Perinatal and childhood risk factors for early-onset type 1 diabetes: A population-based case-control study in Taiwan. Eur. J. Public Health 2015, 25, 1024–1029. [Google Scholar] [CrossRef] [PubMed]
- Bach, J.F. The hygiene hypothesis in autoimmunity: The role of pathogens and commensals. Nat. Rev. Immunol. 2018, 18, 105–120. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.J.; Ajami, N.J.; O’Brien, J.L.; Hutchinson, D.S.; Smith, D.P.; Wong, M.C.; Ross, M.C.; Lloyd, R.E.; Doddapaneni, H.; Metcalf, G.A.; et al. Temporal development of the gut microbiome in early childhood from the TEDDY study. Nature 2018, 562, 583–588. [Google Scholar] [CrossRef]
- Kaila, B.; Taback, S.P. The effect of day care exposure on the risk of developing type 1 diabetes: A meta-analysis of case-control studies. Diabetes Care 2001, 24, 1353–1358. [Google Scholar] [CrossRef] [PubMed]
- Hall, K.; Frederiksen, B.; Rewers, M.; Norris, J.M. Daycare attendance, breastfeeding, and the development of type 1 diabetes: The diabetes autoimmunity study in the young. Biomed. Res. Int. 2015, 2015, 203947. [Google Scholar] [CrossRef]
- Amir, A.; Erez-Granat, O.; Braun, T.; Sosnovski, K.; Hadar, R.; BenShoshan, M.; Heiman, S.; Abbas-Egbariya, H.; Glick Saar, E.; Efroni, G.; et al. Gut microbiome development in early childhood is affected by day care attendance. NPJ Biofilms Microbiomes 2022, 8, 2. [Google Scholar] [CrossRef]
- Cardwell, C.R.; Stene, L.C.; Joner, G.; Bulsara, M.K.; Cinek, O.; Rosenbauer, J.; Ludvigsson, J.; Svensson, J.; Goldacre, M.J.; Waldhoer, T.; et al. Birth order and childhood type 1 diabetes risk: A pooled analysis of 31 observational studies. Int. J. Epidemiol. 2011, 40, 363–374. [Google Scholar] [CrossRef]
- Ramachandran, A.; Snehalatha, C.; Joseph, A.; Viswanathan, V.; Viswanathan, M. Maternal age and birth order of young IDDM patients. a study from southern India. Diabetes Care 1993, 16, 636–637. [Google Scholar] [CrossRef]
- D’Angeli, M.A.; Merzon, E.; Valbuena, L.F.; Tirschwell, D.; Paris, C.A.; Mueller, B.A. Environmental factors associated with childhood-onset type 1 diabetes mellitus: An exploration of the hygiene and overload hypotheses. Arch. Pediatr. Adolesc. Med. 2010, 164, 732–738. [Google Scholar] [CrossRef]
- Lammi, N.; Moltchanova, E.; Blomstedt, P.; Eriksson, J.G.; Taskinen, O.; Sarti, C.; Tuomilehto, J.; Karvonen, M. The effect of birth order and parental age on the risk of type 1 and 2 diabetes among young adults. Diabetologia 2007, 50, 2433–2438. [Google Scholar] [CrossRef] [Green Version]
- Diaz-Valencia, P.A.; Bougnères, P.; Valleron, A.-J. Covariation of the Incidence of Type 1 Diabetes with Country Characteristics Available in Public Databases. PLoS ONE 2015, 10, e0118298. [Google Scholar] [CrossRef]
- Harding, J.L.; Wander, P.L.; Zhang, X.; Li, X.; Karuranga, S.; Chen, H.; Sun, H.; Xie, Y.; Oram, R.A.; Magliano, D.J.; et al. The Incidence of Adult-Onset Type 1 Diabetes: A Systematic Review From 32 Countries and Regions. Diabetes Care 2022, 45, 994–1006. [Google Scholar] [CrossRef] [PubMed]
- Gleicher, N.; Barad, D.H. Gender as risk factor for autoimmune diseases. J. Autoimmun. 2007, 28, 1–6. [Google Scholar] [CrossRef]
- Gannon, M.; Kulkarni, R.N.; Tse, H.M.; Mauvais-Jarvis, F. Sex differences underlying pancreatic islet biology and its dysfunction. Mol. Metab. 2018, 15, 82–91. [Google Scholar] [CrossRef] [PubMed]
- German National Cohort, C. The German National Cohort: Aims, study design and organization. Eur. J. Epidemiol. 2014, 29, 371–382. [Google Scholar] [CrossRef]
- NAKO e.V. NAKO Gesundheitstudie. Available online: https://nako.de/ (accessed on 20 July 2021).
- World Health Organization. Classification of Diabetes Mellitus. Available online: https://www.who.int/publications/i/item/classification-of-diabetes-mellitus (accessed on 22 August 2022).
- Royal College of General Practitioners and NHS Diabetes. Coding, Classification and Diagnosis of Diabetes. Available online: https://orchid.phc.ox.ac.uk/wp-content/uploads/2017/02/nhs_diabetes_and_rcgp_cod_final_report.pdf (accessed on 22 January 2022).
- Butler, A.E.; Misselbrook, D. Distinguishing between type 1 and type 2 diabetes. BMJ 2020, 370, m2998. [Google Scholar] [CrossRef] [PubMed]
- Shields, B.M.; Peters, J.L.; Cooper, C.; Lowe, J.; Knight, B.A.; Powell, R.J.; Jones, A.; Hyde, C.J.; Hattersley, A.T. Can clinical features be used to differentiate type 1 from type 2 diabetes? A systematic review of the literature. BMJ Open 2015, 5, e009088. [Google Scholar] [CrossRef]
- Rosenbauer, J.; Neu, A.; Rothe, U.; Seufert, J.; Holl, R.W. Types of diabetes are not limited to age groups: Type 1 diabetes in adults and type 2 diabetes in children and adolescents. J. Health Monit. 2019, 4, 29–49. [Google Scholar] [CrossRef]
- World Health Organization. Body Mass Index—BMI. Available online: https://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi (accessed on 12 January 2022).
- Primavera, M.; Giannini, C.; Chiarelli, F. Prediction and prevention of type 1 diabetes. Front. Endocrinol. (Lausanne) 2020, 11, 248. [Google Scholar] [CrossRef]
- Vaarala, O. Gut microbiota and type 1 diabetes. Rev. Diabet. Stud. 2012, 9, 251–259. [Google Scholar] [CrossRef] [Green Version]
- Khashan, A.S.; Kenny, L.C.; Lundholm, C.; Kearney, P.M.; Gong, T.; Almqvist, C. Mode of obstetrical delivery and type 1 diabetes: A sibling design study. Pediatrics 2014, 134, e806–e813. [Google Scholar] [CrossRef] [PubMed]
- Hoang, D.M.; Levy, E.I.; Vandenplas, Y. The impact of Caesarean section on the infant gut microbiome. Acta Paediatr. 2021, 110, 60–67. [Google Scholar] [CrossRef]
- Endesfelder, D.; zu Castell, W.; Ardissone, A.; Davis-Richardson, A.G.; Achenbach, P.; Hagen, M.; Pflueger, M.; Gano, K.A.; Fagen, J.R.; Drew, J.C.; et al. Compromised gut microbiota networks in children with anti-islet cell autoimmunity. Diabetes 2014, 63, 2006–2014. [Google Scholar] [CrossRef] [PubMed]
- Sadauskaitė-Kuehne, V.; Ludvigsson, J.; Padaiga, Ž.; Jašinskienė, E.; Samuelsson, U. Longer breastfeeding is an independent protective factor against development of type 1 diabetes mellitus in childhood. Diabetes Metab. Res. Rev. 2004, 20, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Francino, M.P. Birth mode-related differences in gut microbiota colonization and immune system development. Ann. Nutr. Metab. 2018, 73 (Suppl. S3), 12–16. [Google Scholar] [CrossRef]
- Romero, R.; Korzeniewski, S.J. Are infants born by elective cesarean delivery without labor at risk for developing immune disorders later in life? Am. J. Obstet. Gynecol. 2013, 208, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.K.; DuBose, S.N.; Haller, M.J.; Miller, K.M.; DiMeglio, L.A.; Bethin, K.E.; Goland, R.S.; Greenberg, E.M.; Liljenquist, D.R.; Ahmann, A.J.; et al. Prevalence of detectable C-Peptide according to age at diagnosis and duration of type 1 diabetes. Diabetes Care 2015, 38, 476–481. [Google Scholar] [CrossRef]
- Quinn, L.M.; Wong, F.S.; Narendran, P. Environmental determinants of type 1 diabetes: From association to proving causality. Front. Immunol. 2021, 12, 737964. [Google Scholar] [CrossRef] [PubMed]
- Geenen, V. Thymus and type 1 diabetes: An update. Diabetes Res. Clin. Pract. 2012, 98, 26–32. [Google Scholar] [CrossRef]
- Nussinovitch, U.; Shoenfeld, Y. The role of gender and organ specific autoimmunity. Autoimmun. Rev. 2012, 11, A377–A385. [Google Scholar] [CrossRef]
- Pugliese, A. The multiple origins of Type 1 diabetes. Diabet. Med. 2013, 30, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Knip, M.; Luopajärvi, K.; Härkönen, T. Early life origin of type 1 diabetes. Semin. Immunopathol. 2017, 39, 653–667. [Google Scholar] [CrossRef] [PubMed]
- Warncke, K.; Lickert, R.; Eitel, S.; Gloning, K.P.; Bonifacio, E.; Sedlmeier, E.M.; Becker, P.; Knoop, J.; Beyerlein, A.; Ziegler, A.G. Thymus growth and fetal immune responses in diabetic pregnancies. Horm. Metab. Res. 2017, 49, 892–898. [Google Scholar] [CrossRef] [PubMed]
- Cardwell, C.R.; Stene, L.C.; Joner, G.; Bulsara, M.K.; Cinek, O.; Rosenbauer, J.; Ludvigsson, J.; Jané, M.; Svensson, J.; Goldacre, M.J.; et al. Maternal age at birth and childhood type 1 diabetes: A pooled analysis of 30 observational studies. Diabetes 2010, 59, 486–494. [Google Scholar] [CrossRef]
- Hussen, H.I.; Persson, M.; Moradi, T. Maternal overweight and obesity are associated with increased risk of type 1 diabetes in offspring of parents without diabetes regardless of ethnicity. Diabetologia 2015, 58, 1464–1473. [Google Scholar] [CrossRef]
- Magnus, M.C.; Olsen, S.F.; Granstrom, C.; Lund-Blix, N.A.; Svensson, J.; Johannesen, J.; Fraser, A.; Skrivarhaug, T.; Joner, G.; Njølstad, P.R.; et al. Paternal and maternal obesity but not gestational weight gain is associated with type 1 diabetes. Int. J. Epidemiol. 2018, 47, 417–426. [Google Scholar] [CrossRef]
- Turtinen, M.; Härkönen, T.; Parkkola, A.; Ilonen, J.; Knip, M.; The Finnish Pediatric Diabetes Register. Characteristics of familial type 1 diabetes: Effects of the relationship to the affected family member on phenotype and genotype at diagnosis. Diabetologia 2019, 62, 2025–2039. [Google Scholar] [CrossRef] [Green Version]
| No Diabetes (n = 94,465) | Diabetes Type 1 (n = 371) | Diabetes Type 2 or Others (n = 6575) | Total (N = 101,411) | |
|---|---|---|---|---|
| Sex | ||||
| - Male | 43,264 (45.8%) | 204 (57.6%) | 3602 (54.6%) | 47,070 (46.4%) |
| - Female | 51,201 (54.2%) | 167 (45.0%) | 2973 (45.2%) | 54,341 (53.6%) |
| Caesarean delivery | ||||
| - No | 72,816 (77.1%) | 259 (69.8%) | 4290 (65.2%) | 77,365 (76.3%) |
| - Yes | 3489 (3.7%) | 21 (5.7%) | 105 (1.6%) | 3615 (3.6%) |
| - Unknown | 18,160 (19.2%) | 91 (24.5%) | 2180 (33.2%) | 20,431 (20.1%) |
| Birth order | ||||
| - Only child | 14,314 (15.2%) | 69 (18.6%) | 918 (14.0%) | 15,301 (15.1%) |
| - First | 24,765 (26.2%) | 99 (26.7%) | 1434 (21.8%) | 26,298 (25.9%) |
| - Second | 24,230 (25.6%) | 81 (21.8%) | 1299 (19.8%) | 25,610 (25.3%) |
| - Third or more | 16,116 (17.1%) | 50 (13.5%) | 1010 (15.4%) | 17,176 (16.9%) |
| - Missing | 15,040 (15.9%) | 72 (19.4%) | 1914 (29.1%) | 17,026 (16.8%) |
| Attended daycare | ||||
| - No | 25,408 (26.9%) | 77 (20.8%) | 2081 (31.7%) | 27,566 (27.2%) |
| - Yes | 54,091 (57.3%) | 222 (59.8%) | 2597 (39.5%) | 56,910 (56.1%) |
| - Unknown | 14,966 (15.8%) | 72 (19.4%) | 1897 (28.9%) | 16,935 (16.7%) |
| Birth year | ||||
| - ≤1955 | 28,978 (30.7%) | 73 (19.7%) | 4142 (63.0%) | 33,193 (32.7%) |
| - 1956–1965 | 27,341 (28.9%) | 118 (31.8%) | 1611 (24.5%) | 29,070 (28.7%) |
| - 1966–1975 | 21,586 (22.9%) | 97 (26.1%) | 620 (9.4%) | 22,303 (22.0%) |
| - 1976–1985 | 9299 (9.8%) | 45 (12.1%) | 175 (2.7%) | 9519 (9.4%) |
| - ≥1986 | 7261 (7.7%) | 38 (10.7%) | 27 (0.4%) | 7326 (7.2%) |
| Paternal diabetes | ||||
| - No | 60,376 (63.9%) | 195 (52.6%) | 2615 (39.8%) | 63,186 (62.3%) |
| - Yes, at age < 40 years | 458 (0.5%) | 12 (3.2%) | 63 (1.0%) | 533 (0.5%) |
| - Yes, at age ≥ 40 years/unknown | 10,635 (11.3%) | 66 (17.8%) | 1201 (18.3%) | 11,902 (11.7%) |
| - Unknown | 22,996 (24.3%) | 98 (26.4%) | 2696 (41.0%) | 25,790 (25.4%) |
| Maternal diabetes | ||||
| - No | 66,019 (69.9%) | 218 (58.8%) | 2621 (39.9%) | 68,858 (67.9%) |
| - Yes, at age < 40 years | 403 (0.4%) | 13 (3.5%) | 94 (1.4%) | 510 (0.5%) |
| - Yes, at age ≥ 40 years/unknown | 11,119 (11.8%) | 63 (17.0%) | 1679 (25.5%) | 12,861 (12.7%) |
| - Unknown | 16,924 (17.9%) | 77 (20.8%) | 2181 (33.2%) | 19,182 (18.9%) |
| Migration background | ||||
| - No migration background | 79,517 (84.2%) | 330 (88.9%) | 5375 (81.8%) | 85,222 (84.0%) |
| - Has migration background | 14,936 (15.8%) | 41 (11.1%) | 1199 (18.2%) | 16,176 (16.0%) |
| - Missing | 12 (0.0%) | 0 (0.0%) | 1 (0.0%) | 13 (0.0%) |
| Premature birth (>4 weeks before due date) | ||||
| - No | 72,203 (76.4%) | 270 (72.8%) | 4142 (63.0%) | 76,615 (75.5%) |
| - Yes | 3179 (3.4%) | 12 (3.2%) | 183 (2.8%) | 3374 (3.3%) |
| - Unknown | 19,083 (20.2%) | 89 (24.0%) | 2250 (34.2%) | 21,422 (21.1%) |
| Birth weight | ||||
| - Light | 8018 (8.5%) | 29 (7.8%) | 511 (7.8%) | 8558 (8.4%) |
| - Average | 47,937 (50.7%) | 176 (47.4%) | 2482 (37.7%) | 50,595 (49.9%) |
| - Heavy | 8102 (8.6%) | 34 (9.2%) | 401 (6.1%) | 8537 (8.4%) |
| - Unknown | 30,408 (32.2%) | 132 (35.6%) | 3181 (48.4%) | 33,721 (33.3%) |
| Ever breastfed | ||||
| - No | 13,260 (14.0%) | 60 (16.2%) | 604 (9.2%) | 13,924 (13.7%) |
| - Yes, >4 months | 18,114 (19.2%) | 66 (17.8%) | 1047 (15.9%) | 19,227 (19.0%) |
| - Yes, until 4 months | 21,351 (22.6%) | 79 (21.3%) | 1200 (18.3%) | 22,630 (22.3%) |
| - Unknown | 41,740 (44.2%) | 166 (44.7%) | 3724 (56.6%) | 45,630 (45.0%) |
| BMI at age 18 | ||||
| - Underweight | 8830 (9.3%) | 24 (6.5%) | 397 (6.0%) | 9251 (9.1%) |
| - Normal weight | 50,140 (53.1%) | 173 (46.6%) | 2519 (38.3%) | 52,832 (52.1%) |
| - Overweight | 5669 (6.0%) | 31 (8.4%) | 567 (16.1%) | 6267 (6.2%) |
| - Obese | 213 (0.2%) | 3 (0.8%) | 25 (0.7%) | 241 (0.2%) |
| - Missing | 29,613 (31.3%) | 140 (37.7%) | 3067 (46.6%) | 32,820 (32.4%) |
| Outcome: Type 1 Diabetes | n a (%) | Hazard Ratio (95% Confidence Interval) | |||||
|---|---|---|---|---|---|---|---|
| Univariable | Multivariable Full Model b | Multivariable Reduced Model b | |||||
| Case Selection | Age at Diagnosis 0–40 Years | Age at Diagnosis 0–15 Years | Age at Diagnosis 16–40 Years | ||||
| Birth order | Only child | 15,301 (15.1) | 1 | 1 | 1 | 1 | 1 |
| First | 26,298 (25.9) | 0.83 (0.61–1.12) | 0.85 (0.63–1.17) | 0.85 (0.62–1.16) | 0.66 (0.38–1.15) | 0.95 (0.65–1.39) | |
| Second | 25,610 (25.3) | 0.69 (0.50–0.96) | 0.70 (0.50–0.96) | 0.69 (0.50–0.96) | 0.54 (0.30–0.95) | 0.78 (0.52–1.15) | |
| ≥Third | 17,176 (16.9) | 0.66 (0.46–0.95) | 0.65 (0.45–0.94) | 0.65 (0.45–0.94) | 0.42 (0.20–0.87) | 0.77 (0.50–1.19) | |
| Unknown | 17,026 (16.8) | 1.07 (0.77–1.49) | 0.61 (0.13–2.91) | 0.63 (0.13–3.01) | 0.74 (0.03–20.23) | 0.62 (0.11–3.66) | |
| C-section delivery | No | 77,365 (76.3) | 1 | 1 | 1 | 1 | 1 |
| Yes | 3615 (3.6) | 1.51 (0.96–2.37) | 1.32 (0.83–2.08) | 1.35 (0.85–2.12) | 1.68 (0.85–3.31) | 1.13 (0.61–2.10) | |
| Unknown | 20,431 (20.1) | 1.48 (1.17–1.89) | 1.46 (0.89–2.42) | 1.43 (0.90–2.29) | 0.97 (0.31–3.02) | 1.58 (0.94–2.65) | |
| Attended daycare | No | 27,566 (27.2) | 1 | 1 | 1 | 1 | 1 |
| Yes | 56,910 (56.1) | 1.12 (0.85–1.49) | 1.05 (0.79–1.39) | 1.04 (0.79–1.38) | 0.84 (0.47–1.48) | 1.11 (0.80–1.54) | |
| Unknown | 16,935 (16.7) | 1.50 (1.08–2.07) | 1.46 (0.31–6.92) | 1.42 (0.30–6.75) | 1.71 (0.06–47.34) | 1.36 (0.23–7.87) | |
| Sex | Male | 47,070 (46.4) | 1 | 1 | 1 | 1 | 1 |
| Female | 54,341 (53.6) | 0.69 (0.56–0.85) | 0.67 (0.54–0.83) | 0.68 (0.55–0.84) | 0.82 (0.55–1.22) | 0.63 (0.50–0.81) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanoey, J.; Baechle, C.; Brenner, H.; Deckert, A.; Fricke, J.; Günther, K.; Karch, A.; Keil, T.; Kluttig, A.; Leitzmann, M.; et al. Birth Order, Caesarean Section, or Daycare Attendance in Relation to Child- and Adult-Onset Type 1 Diabetes: Results from the German National Cohort. Int. J. Environ. Res. Public Health 2022, 19, 10880. https://doi.org/10.3390/ijerph191710880
Tanoey J, Baechle C, Brenner H, Deckert A, Fricke J, Günther K, Karch A, Keil T, Kluttig A, Leitzmann M, et al. Birth Order, Caesarean Section, or Daycare Attendance in Relation to Child- and Adult-Onset Type 1 Diabetes: Results from the German National Cohort. International Journal of Environmental Research and Public Health. 2022; 19(17):10880. https://doi.org/10.3390/ijerph191710880
Chicago/Turabian StyleTanoey, Justine, Christina Baechle, Hermann Brenner, Andreas Deckert, Julia Fricke, Kathrin Günther, André Karch, Thomas Keil, Alexander Kluttig, Michael Leitzmann, and et al. 2022. "Birth Order, Caesarean Section, or Daycare Attendance in Relation to Child- and Adult-Onset Type 1 Diabetes: Results from the German National Cohort" International Journal of Environmental Research and Public Health 19, no. 17: 10880. https://doi.org/10.3390/ijerph191710880
APA StyleTanoey, J., Baechle, C., Brenner, H., Deckert, A., Fricke, J., Günther, K., Karch, A., Keil, T., Kluttig, A., Leitzmann, M., Mikolajczyk, R., Obi, N., Pischon, T., Schikowski, T., Schipf, S. M., Schulze, M. B., Sedlmeier, A., Moreno Velásquez, I., Weber, K. S., ... Becher, H. (2022). Birth Order, Caesarean Section, or Daycare Attendance in Relation to Child- and Adult-Onset Type 1 Diabetes: Results from the German National Cohort. International Journal of Environmental Research and Public Health, 19(17), 10880. https://doi.org/10.3390/ijerph191710880







