Respiratory Symptoms and Skin Sick Building Syndrome among Office Workers at University Hospital, Chiang Mai, Thailand: Associations with Indoor Air Quality, AIRMED Project
Abstract
:1. Introduction
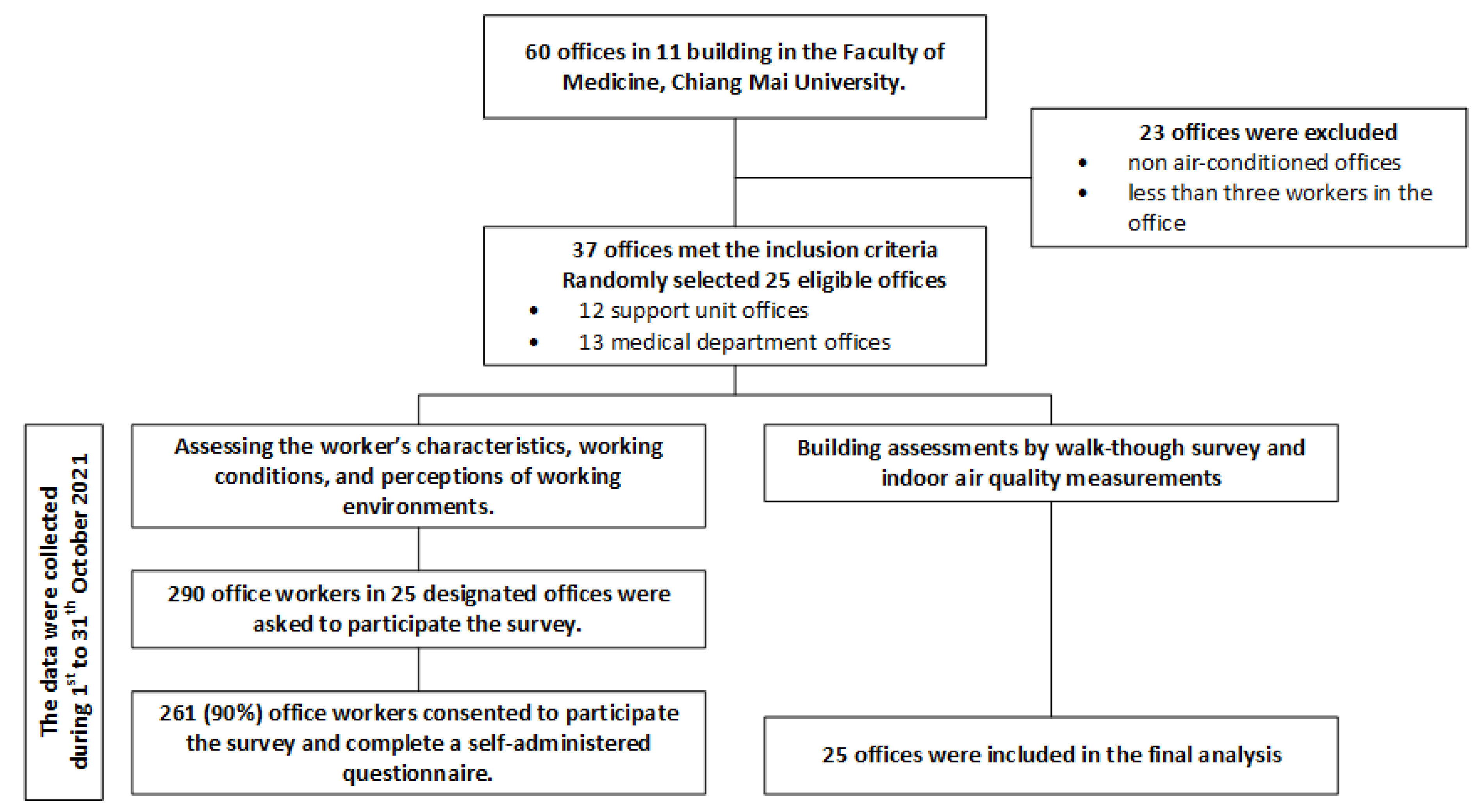
2. Materials and Methods
2.1. Study Design and Participants
2.2. Questionnaire
2.2.1. Worker Characteristics, Working Conditions, and Perceptions of Working Environments
2.2.2. Sick Building Syndromes (SBS)
2.3. Building Assessment and Indoor Air Quality Measurements
2.3.1. Thermal Comfort and Chemical Parameters
2.3.2. Biological Parameters
2.4. Statistical Analysis
2.5. Ethical Considerations
3. Results
3.1. Workers’ Characteristics, Working Conditions, and Perceptions of Working Environment
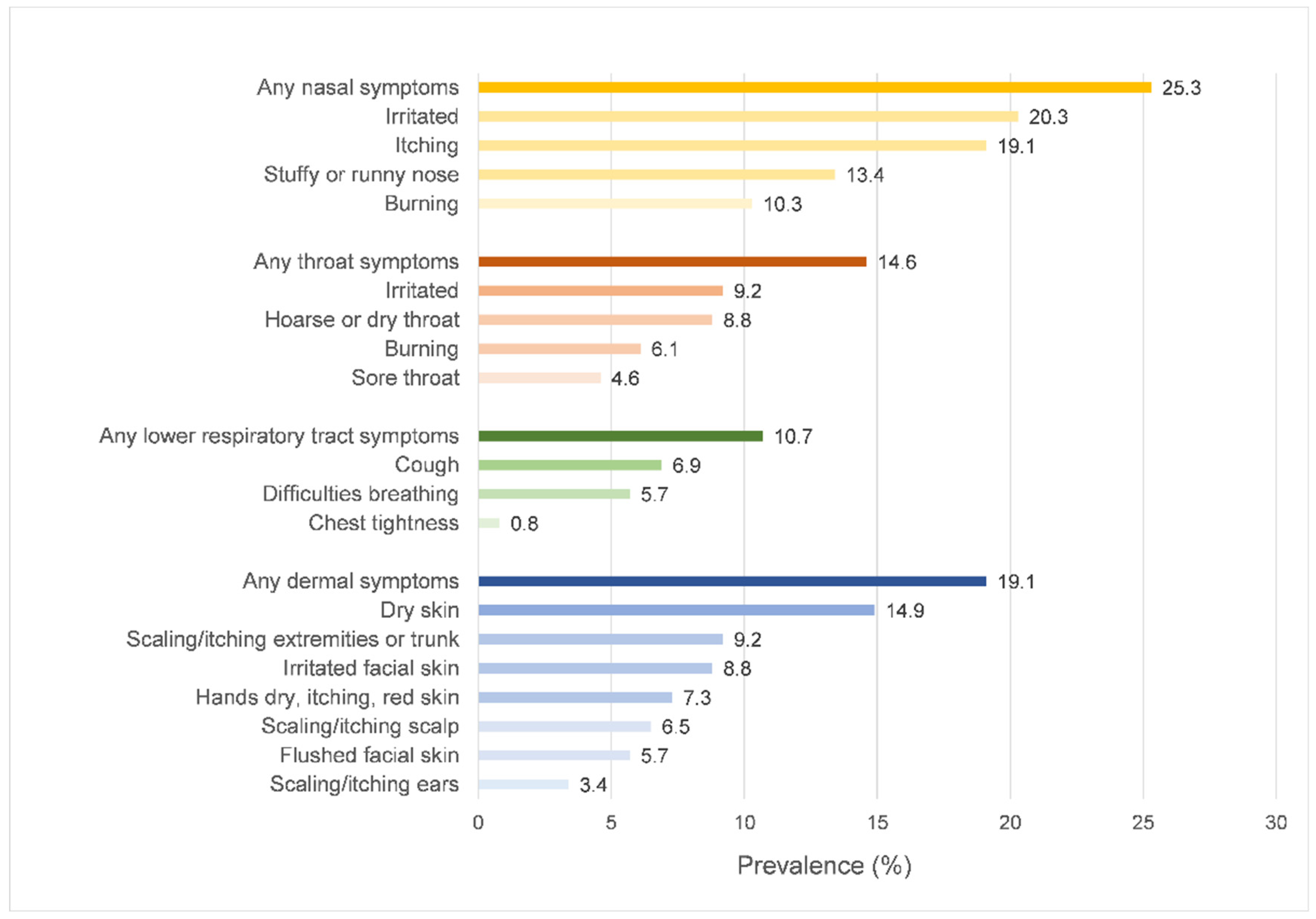
3.2. The Prevalence of SBS Symptoms
3.3. Working Environments
3.4. Factors Related to SBS Symptoms
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dhungana, P.; Chalise, M. Prevalence of sick building syndrome symptoms and its associated factors among bank employees in Pokhara Metropolitan, Nepal. Indoor Air 2020, 30, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.Y.; Tsai, M.C.; Muo, C.H.; Kuo, Y.H.; Sung, F.C.; Wu, C.C. Personal, Psychosocial and Environmental Factors Related to Sick Building Syndrome in Official Employees of Taiwan. Int. J. Environ. Res. Public Health 2017, 15, 7. [Google Scholar] [CrossRef]
- Lu, C.Y.; Lin, J.-M.; Chen, Y.-Y.; Chen, Y.-C. Building-Related Symptoms among Office Employees Associated with Indoor Carbon Dioxide and Total Volatile Organic Compounds. Int. J. Environ. Res. Public Health 2015, 12, 5833–5845. [Google Scholar] [CrossRef]
- Zamani, M.; Jalaludin, J.; Shaharon, M.N. Indoor air quality and prevalence of sick building syndrome among office workers in two different offices in selangor. Am. J. Appl. Sci. 2013, 10, 1040–1047. [Google Scholar] [CrossRef]
- Apte, M.G.; Fisk, W.J.; Daisey, J.M. Associations between indoor CO2 concentrations and sick building syndrome symptoms in U.S. office buildings: An analysis of the 1994–1996 BASE study data. Indoor Air 2000, 10, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hamid, M.A.; Hakim, S.A.; Elokda, E.E.; Mostafa, N.S. Prevalence and risk factors of sick building syndrome among office workers. J. Egypt Public Health Assoc. 2013, 88, 109–114. [Google Scholar] [CrossRef]
- Indoor Air Facts No. 4 (Revised) Sick Building Syndrome. Available online: https://www.epa.gov/sites/default/files/2014-08/documents/sick_building_factsheet.pdf (accessed on 13 June 2022).
- Kalender Smajlović, S.; Kukec, A.; Dovjak, M. Association between Sick Building Syndrome and Indoor Environmental Quality in Slovenian Hospitals: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2019, 16, 3224. [Google Scholar] [CrossRef]
- Akova, İ.; Kiliç, E.; Sümer, H.; Keklikçi, T. Prevalence of sick building syndrome in hospital staff and its relationship with indoor environmental quality. Int. J. Environ. Health Res. 2022, 32, 1204–1219. [Google Scholar] [CrossRef]
- World Health Organization—Regional Office for Europe. Indoor Air Quality: Biological Contaminants: Report on a WHO Meeting, Rautavaara, 29 August–2 September 1988; World Health Organization—Regional Office for Europe: Copenhagen, Denmark, 1990. [Google Scholar]
- Arikan, I.; Tekin, Ö.F.; Erbas, O. Relationship between sick building syndrome and indoor air quality among hospital staff. Med. Lav 2018, 109, 435–443. [Google Scholar] [CrossRef]
- Tsantaki, E.; Smyrnakis, E.; Constantinidis, T.C.; Benos, A. Indoor air quality and sick building syndrome in a university setting: A case study in Greece. Int. J. Environ. Health Res. 2022, 32, 595–615. [Google Scholar] [CrossRef]
- Mendell, M.J.; Lei-Gomez, Q.; Mirer, A.G.; Seppänen, O.; Brunner, G. Risk factors in heating, ventilating, and air-conditioning systems for occupant symptoms in US office buildings: The US EPA BASE study. Indoor Air 2008, 18, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Santamouris, M.; Synnefa, A.; Asssimakopoulos, M.; Livada, I.; Pavlou, K.; Papaglastra, M.; Gaitani, N.; Pavlou, C.; Kolokotsa, D.; Assimakopoulos, V.D. Experimental investigation of the air flow in naturally ventilated classrooms. In Proceedings of the Air Conditioning and the Low Carbon Cooling Challenge, Windsor, UK, 27–29 July 2008. [Google Scholar]
- Vafaeenasab, M.R.; Morowatisharifabad, M.A.; Taghi Ghaneian, M.; Hajhosseini, M.; Ehrampoush, M.H. Assessment of sick building syndrome and its associating factors among nurses in the educational hospitals of Shahid Sadoughi University of Medical Sciences, Yazd, Iran. Glob. J. Health Sci. 2014, 7, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Burge, P.S. Sick building syndrome. Occup. Environ. Med. 2004, 61, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Azuma, K.; Ikeda, K.; Kagi, N.; Yanagi, U.; Osawa, H. Physicochemical risk factors for building-related symptoms in air-conditioned office buildings: Ambient particles and combined exposure to indoor air pollutants. Sci. Total Environ. 2018, 616–617, 1649–1655. [Google Scholar] [CrossRef]
- Norbäck, D.; Hashim, J.H.; Hashim, Z.; Ali, F. Volatile organic compounds (VOC), formaldehyde and nitrogen dioxide (NO2) in schools in Johor Bahru, Malaysia: Associations with rhinitis, ocular, throat and dermal symptoms, headache and fatigue. Sci. Total Environ. 2017, 592, 153–160. [Google Scholar] [CrossRef]
- Nur Fadilah, R.; Jalaludin, J. Indoor Air Quality (IAQ) and Sick Buildings Syndrome (SBS) among Office Workers in New and Old Building in Universiti Putra Malaysia, Serdang. Health Environ. J. 2012, 3, 98–109. [Google Scholar]
- Syazwan, A.; Jalaludin, J.; Norhafizalina, O.; Azman, Z.A.; Jusoff, K. Indoor Air Quality and Sick Building Syndrome in Malaysian Buildings. Glob. J. Health Sci. 2009, 1, 126–135. [Google Scholar] [CrossRef]
- Indoor Air Quality. Available online: https://www.osha.gov/laws-regs/federalregister/1994-04-05 (accessed on 13 June 2022).
- Zainal, Z.A.; Hashim, Z.; Jalaludin, J.; Lim, F.L.; Hashim, J.H. Sick building syndrome among office workers in relation to office environment and indoor air pollutant at an academic institution, Malaysia. Malays. J. Med. Health Sci. 2019, 15, 126–134. [Google Scholar]
- Tsai, D.H.; Lin, J.S.; Chan, C.C. Office workers’ sick building syndrome and indoor carbon dioxide concentrations. J. Occup. Environ. Hyg. 2012, 9, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Apter, A.; Bracker, A.; Hodgson, M.; Sidman, J.; Leung, W.Y. Epidemiology of the sick building syndrome. J. Allergy Clin. Immunol. 1994, 94, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Seppänen, O.A.; Fisk, W.J.; Mendell, M.J. Association of ventilation rates and CO2 concentrations with health and other responses in commercial and institutional buildings. Indoor Air 1999, 9, 226–252. [Google Scholar] [CrossRef] [PubMed]
- Andersson, K. Epidemiological Approach to Indoor Air Problems. Indoor Air 1998, 8, 32–39. [Google Scholar] [CrossRef]
- SS 554-+A1:2016:2021; Code of Practice for Indoor Air Quality for Air-Conditioned Buildings. SSC—Singapore Standard Coucil: Singapore, 2021.
- Lonon, M.K. NIOSH Manual of Analytical Methods 0800: Bioaerosol Sampling (Indoor Air), 4th ed.; National Institute for Occupational Safety and Health: Washington, DC, USA, 1998. Available online: https://www.cdc.gov/niosh/docs/2003-154/pdfs/0800.pdf (accessed on 13 June 2022).
- World Health Organization. WHO Guidelines for Indoor Air Quality: Selected Pollutants; World Health Organization—Regional Office for Europe: Copenhagen, Denmark, 2010. [Google Scholar]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Prev. Med. 2007, 45, 247–251. [Google Scholar] [CrossRef]
- Lim, F.-L.; Hashim, Z.; Md Said, S.; Than, L.T.-L.; Hashim, J.H.; Norbäck, D. Sick building syndrome (SBS) among office workers in a Malaysian university—Associations with atopy, fractional exhaled nitric oxide (FeNO) and the office environment. Sci. Total Environ. 2015, 536, 353–361. [Google Scholar] [CrossRef]
- Azuma, K.; Ikeda, K.; Kagi, N.; Yanagi, U.; Osawa, H. Prevalence and risk factors associated with nonspecific building-related symptoms in office employees in Japan: Relationships between work environment, Indoor Air Quality, and occupational stress. Indoor Air 2015, 25, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Ooi, P.L.; Goh, K.T.; Phoon, M.H.; Foo, S.C.; Yap, H.M. Epidemiology of sick building syndrome and its associated risk factors in Singapore. Occup. Environ. Med. 1998, 55, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-J.; Yang, H.-H.; Wang, Y.-F.; Li, M.-S. Prevalence of Sick Building Syndrome-Related Symptoms among Hospital Workers in Confined and Open Working Spaces. Aerosol Air Qual. Res. 2015, 15, 2378–2384. [Google Scholar] [CrossRef]
- Hoang Quoc, C.; Vu Huong, G.; Nguyen Duc, H. Working Conditions and Sick Building Syndrome among Health Care Workers in Vietnam. Int. J. Environ. Res. Public Health 2020, 17, 3635. [Google Scholar] [CrossRef]
- Kalender-Smajlović, S.; Dovjak, M.; Kukec, A. Sick building syndrome among healthcare workers and healthcare associates at observed general hospital in Slovenia. Cent. Eur. J. Public Health 2021, 29, 28–37. [Google Scholar] [CrossRef]
- Skov, P.; Valbjørn, O.; DISG. The “sick” building syndrome in the office environment: The Danish town hall study. Environ. Int. 1987, 13, 339–349. [Google Scholar] [CrossRef]
- Jaakkola, J.J.K.; Heinonen, O.P.; Seppänen, O. Sick building syndrome, sensation of dryness and thermal comfort in relation to room temperature in an office building: Need for individual control of temperature. Environ. Int. 1989, 15, 163–168. [Google Scholar] [CrossRef]
- Niven, R.M.; Fletcher, A.M.; Pickering, C.A.; Faragher, E.B.; Potter, I.N.; Booth, W.B.; Jones, T.J.; Potter, P.D. Building sickness syndrome in healthy and unhealthy buildings: An epidemiological and environmental assessment with cluster analysis. Occup. Environ. Med. 2000, 57, 627–634. [Google Scholar] [CrossRef] [Green Version]
- Abdul-Wahab, S.A.; Chin Fah En, S.; Elkamel, A.; Ahmadi, L.; Yetilmezsoy, K. A review of standards and guidelines set by international bodies for the parameters of indoor air quality. Atmos. Pollut. Res. 2015, 6, 751–767. [Google Scholar] [CrossRef]
- Nordström, K.; Norbäck, D.; Akselsson, R. Effect of Air Humidification on the Sick Building Syndrome and Perceived Indoor Air Quality in Hospitals: A Four Month Longitudinal Study. Occup. Environ. Med. 1994, 51, 683–688. [Google Scholar] [CrossRef]
- Nevalainen, A.; Morawaska, L. Biological Agents in Indoor Environments. Assessment of Health Risks. Work Conducted by a WHO Expert Group between 2000–2003; World Health Organization: Copenhagen, Denmark, 2009.
- Fromme, H.; Heitmann, D.; Dietrich, S.; Schierl, R.; Körner, W.; Kiranoglu, M.; Zapf, A.; Twardella, D. Air quality in schools—Classroom levels of carbon dioxide (CO2), volatile organic compounds (VOC), aldehydes, endotoxins and cat allergen. Gesundheitswesen 2008, 70, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Bai, Y.; Duong, A.; Smith, M.T.; Li, L.; Zhang, L. Formaldehyde in China: Production, consumption, exposure levels, and health effects. Environ. Int. 2009, 35, 1210–1224. [Google Scholar] [CrossRef]
- Hui, P.S.; Mui, K.W.; Wong, L.T. Influence of indoor air quality (IAQ) objectives on air-conditioned offices in Hong Kong. Environ. Monit. Assess. 2008, 144, 315–322. [Google Scholar] [CrossRef]
- Kotzias, D.; Geiss, O.; Tirendi, S.; Josefa, B.M.; Reina, V.; Gotti, A.; Graziella, C.R.; Casati, B.; Marafante, E.; Sarigiannis, D. Exposure to multiple air contaminants in public buildings, schools and kindergartens-the European indoor air monitoring and exposure assessment (airmex) study. Fresenius Environ. Bull. 2009, 18, 670–681. [Google Scholar]
- Ramaiya, A.; Villalobos, P.; Chipeta, E.; Lopez, J.V.; Maddaleno, M.; Zuo, X.; Mafuta, E.; Lulebo, A.; Green, J.; Richardson, L.; et al. How do adolescents navigate COVID-19 information, and why does it matter? J. Glob. Health 2021, 11, 03110. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risk to Humans. Formaldehyde, 2-butoxyethanol and 1-tert-butoxypropan-2-ol. IARC Monogr. Eval. Carcinog. Risks Hum. 2006, 88, 1–478. [Google Scholar]
- Salthammer, T.; Mentese, S.; Marutzky, R. Formaldehyde in the indoor environment. Chem. Rev. 2010, 110, 2536–2572. [Google Scholar] [CrossRef] [PubMed]
- Haghighat, F.; De Bellis, L. Material emission rates: Literature review, and the impact of indoor air temperature and relative humidity. Build. Environ. 1998, 33, 261–277. [Google Scholar] [CrossRef]
- Clarisse, B.; Laurent, A.M.; Seta, N.; Le Moullec, Y.; El Hasnaoui, A.; Momas, I. Indoor aldehydes: Measurement of contamination levels and identification of their determinants in Paris dwellings. Environ. Res. 2003, 92, 245–253. [Google Scholar] [CrossRef]
- Raw, G.J.; Coward, S.K.; Brown, V.M.; Crump, D.R. Exposure to air pollutants in English homes. J. Expo. Anal. Environ. Epidemiol. 2004, 14 (Suppl. S1), S85–S94. [Google Scholar] [CrossRef] [Green Version]
- Marchand, C.; Bulliot, B.; Le Calvé, S.; Mirabel, P. Aldehyde measurements in indoor environments in Strasbourg (France). Atmos. Environ. 2006, 40, 1336–1345. [Google Scholar] [CrossRef]
- Gilbert, N.L.; Gauvin, D.; Guay, M.; Héroux, M.E.; Dupuis, G.; Legris, M.; Chan, C.C.; Dietz, R.N.; Lévesque, B. Housing characteristics and indoor concentrations of nitrogen dioxide and formaldehyde in Quebec City, Canada. Environ. Res. 2006, 102, 1–8. [Google Scholar] [CrossRef]
- Erdmann, C.A.; Apte, M.G. Mucous membrane and lower respiratory building related symptoms in relation to indoor carbon dioxide concentrations in the 100-building BASE dataset. Indoor Air 2004, 14 (Suppl. S8), 127–134. [Google Scholar] [CrossRef] [Green Version]
| Characteristics | Total (N = 261) | Nasal Symptoms (n = 66) | Throat Symptoms (n = 38) | Lower Respiratory Tract Symptoms (n = 28) | Dermal Symptoms (n = 50) |
|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | n (%) | |
| Age (year), mean ± SD | 40 ± 11 | 38 ± 10 | 40 ± 12 | 37 ± 11 | 38 ± 10 |
| Gender | |||||
| Male | 107 (41.0) | 17 (25.8) | 13 (34.2) | 8 (28.6) | 14 (28.6) |
| Female | 154 (59.0) | 49 (74.2) | 25 (65.8) | 20 (71.4) | 35 (71.4) |
| Working year, median (IQR) | 9 (2–20) | 9 (3–15) | 7 (2–17) | 5 (3–15) | 9 (3–14) |
| Smoking | |||||
| Non-smoker | 225 (86.2) | 61 (92.4) | 34 (89.5) | 26 (92.9) | 45 (91.9) |
| Ex-smoker | 24 (9.2) | 3 (4.6) | 3 (7.9) | 2 (7.1) | 3 (6.1) |
| Current-smoker | 12 (4.6) | 2 (3.0) | 1 (2.6) | - | 1 (2.0) |
| Underlying disease | |||||
| None | 165 (63.2) | 38 (57.6) | 20 (52.6) | 11 (39.3) | 26 (53.1) |
| Allergic rhinitis | 31 (11.9) | 13 (19.7) | 8 (21.1) | 8 (28.6) | 7 (14.3) |
| Sinusitis | 2 (0.8) | 2 (3.0) | 2 (5.41) | - | 1 (2.0) |
| Asthma | 1 (0.4) | 1 (1.5) | 1 (2.7) | - | 1 (2.0) |
| Skin diseases | 5 (1.9) | 2 (3.0) | 1 (2.6) | 1 (3.6) | 3 (6.1) |
| Workers’ perception in working environments | |||||
| Feeling sensitive to smoke | 237 (90.8) | 62 (93.9) | 35 (92.1) | 25 (89.3) | 45 (91.8) |
| Feeling sensitive to chemical agents | 190 (80.3) | 53 (80.3) | 28 (73.7) | 22 (78.6) | 37 (75.5) |
| Working conditions | |||||
| Regular working hours per week, mean ± SD | 37 ± 8 | 40 ± 6 | 39 ± 4 | 41 ± 7 | 39 ± 4 |
| Overtime working hours per week, median (IQR) | 2 (0–5) | 2 (0–5) | 3 (0–5) | 3 (0–5) | 2 (1–5) |
| Room sizes (m3), n (%) | |||||
| ≤250 | 89 (34.10) | 26 (39.39) | 10 (26.32) | 10 (35.71) | 16 (32.65) |
| 251–400 | 60 (22.99) | 17 (25.76) | 12 (31.58) | 6 (21.43) | 14 (28.57) |
| 401–600 | 34 (13.03) | 5 (7.58) | 6 (15.79) | 4 (14.29) | 5 (10.20) |
| 601–1000 | 63 (24.14) | 16 (24.24) | 8 (21.05) | 7 (25.00) | 13 (26.53) |
| ≥1000 | 15 (5.75) | 2 (3.03) | 2 (5.26) | 1 (3.57) | 1 (2.04) |
| Number of workers per room (IQR) | 19 (10–28) | 18 (10–28) | 18 (12–28) | 18 (10–29) | 18 (10–31) |
| Parameters | Total Locations (N = 25) | |
|---|---|---|
| Mean ± SD | (Min–Max) | |
| Thermal comfort parameters | ||
| Air temperature (°C) | 23.39 ± 1.00 | (21.40–25.60) |
| Relative humidity (%) | 61.83 ± 5.66 | (50.40–76.60) |
| Air movement (m/s) | 0.11 ± 0.08 | (0.03–0.26) |
| Chemical parameters | ||
| Carbon dioxide (ppm) | 795.75 ± 191.36 | (434.00–1210.00) |
| Formaldehyde (ppm) a | 0.36 (0.28–0.74) | (0.00–2.58) |
| Carbon monoxide (ppm) | ND | ND |
| TVOC (ppb) | ND | ND |
| Particulate matter | ||
| PM2.5 (µg/m3) | 21.0 (13.0–29.0) | (3.0–65.0) |
| Biological parameters | ||
| Total viable bacterial count (CFU/m3) | 40 ± 20 | (6–78) |
| Variables | Nasal Symptoms Model | p-Value | Throat Symptoms Model | p-Value | Lower Respiratory Tract Symptoms Model | p-Value | Dermal Symptoms Model | p-Value |
|---|---|---|---|---|---|---|---|---|
| aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | |||||
| Characteristics | ||||||||
| Age (years) | 0.98 (0.94 to 1.03) | 0.401 | 1.03 (0.98 to 1.08) | 0.240 | 1.00 (0.94 to 1.06) | 0.930 | 1.00 (0.95 to 1.04) | 0.846 |
| Gender | ||||||||
| Male | Ref. | Ref. | Ref. | Ref. | ||||
| Female | 2.38 (1.10 to 5.14) | 0.028 | 1.14 (0.50 to 2.64) | 0.746 | 1.22 (0.42 to 3.59) | 0.715 | 1.81 (0.83 to 3.98) | 0.134 |
| Working year | 1.00 (0.96 to 1.05) | 0.866 | 0.97 (0.92 to 1.02) | 0.184 | 0.97 (0.91 to 1.04) | 0.383 | 0.99 (0.94 to 1.03) | 0.672 |
| Smoking | ||||||||
| Non-smokers | Ref. | Ref. | Ref. | Ref. | ||||
| Current/Ex-smokers | 0.98 (0.43 to 6.76) | 0.987 | 0.78 (0.08 to 7.44) | 0.832 | 1.32 (0.23 to 7.69) | 0.753 | 0.61 (0.06 to 5.78) | 0.669 |
| Underlying disease | ||||||||
| Allergic rhinitis | 2.59 (1.00 to 6.68) | 0.050 | 2.88 (1.06 to 7.85) | 0.038 | 5.03 (1.65 to 15.38) | 0.005 | Not included | |
| Skin diseases | Not included | Not included | Not included | 6.66 (0.93 to 47.60) | 0.059 | |||
| Workers’ perception of working environments | ||||||||
| Feeling sensitive to smoke | 0.93 (0.24 to 3.60) | 0.920 | 1.20 (0.28 to 5.23) | 0.804 | 0.48 (0.10 to 2.43) | 0.376 | 0.76 (0.21 to 2.81) | 0.686 |
| Feeling sensitive to chemical agents | 1.91 (0.83 to 4.43) | 0.134 | 0.89 (0.36 to 2.23) | 0.808 | 1.52 (0.47 to 4.93) | 0.485 | 1.21 (0.52 to 2.78) | 0.660 |
| Working conditions | ||||||||
| Regular working hours per week | 1.08 (1.02 to 1.16) | 0.013 | 1.04 (0.98 to 1.10) | 0.227 | 1.12 (1.03 to 1.22) | 0.008 | 1.05 (0.99 to 1.10) | 0.130 |
| Overtime working > 2 h per week | 0.95 (0.88 to 1.02) | 0.174 | 1.01 (0.95 to 1.07) | 0.798 | 0.94 (0.83 to 1.06) | 0.266 | 0.93 (0.85 to 1.03) | 0.168 |
| Room sizes (m3), increasing 100 m3 | 1.14 (0.99 to 1.32) | 0.067 | 1.11 (0.95 to 1.30) | 0.173 | 1.07 (0.89 to 1.29) | 0.456 | 1.01 (0.88 to 1.16) | 0.867 |
| Thermal comfort parameters | ||||||||
| Air temperature (°C), increasing 1 °C | 2.63 (1.41 to 4.90) | 0.002 | 1.86 (0.91 to 3.83) | 0.088 | 1.87 (0.82 to 4.29) | 0.139 | 1.32 (0.72 to 2.40) | 0.371 |
| Relative humidity (%), increasing 1% | 0.88 (0.80 to 0.97) | 0.007 | 0.87 (0.78 to 0.96) | 0.006 | 0.98 (0.87 to 1.10) | 0.754 | 0.94 (0.86 to 1.02) | 0.144 |
| Chemical parameters | ||||||||
| Carbon dioxide(ppm), increasing 100 ppm | 1.36 (1.03 to 1.78) | 0.027 | 1.16 (0.84 to 1.60) | 0.353 | 1.26 (0.89 to 1.80) | 0.198 | 1.22 (0.93 to 1.61) | 0.158 |
| Formaldehyde (ppm) Q1, <0.28 | Ref. | Ref. | Ref. | Ref. | ||||
| Q2, 0.28 to 0.35 | 0.90 (0.16 to 2.83) | 0.853 | 0.32 (0.08 to 1.39) | 0.128 | 1.19 (0.25 to 5.71) | 0.824 | 0.68 (0.21 to 2.18) | 0.521 |
| Q3, 0.36 to 0.73 | 3.88 (1.38 to 11.09) | 0.010 | 1.44 (0.40 to 5.18) | 0.577 | 1.71 (0.40 to 7.30) | 0.472 | 1.45 (0.52 to 4.03) | 0.478 |
| Q4, ≥0.74 | 5.24 (1.20 to 23.07) | 0.029 | 6.45 (1.07 to 39.01) | 0.042 | 1.31 (0.19 to 8.95) | 0.783 | 1.89 (0.46 to 7.76) | 0.376 |
| PM2.5 ≥ 50 µg/m3 | 0.88 (0.16 to 4.73) | 0.877 | 0.43 (0.06 to 2.90) | 0.384 | 1.98(0.25 to 16.10) | 0.524 | 0.63 (0.10 to 4.00) | 0.621 |
| Biological parameters | ||||||||
| Total viable bacterial count (CFU/m3), increasing 10 CFU/m3 | 1.31 (1.03 to 1.68) | 0.030 | 1.23 (0.89 to 1.69) | 0.204 | 0.96 (0.70 to 1.30) | 0.784 | 1.12 (0.88 to 1.43) | 0.346 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Surawattanasakul, V.; Sirikul, W.; Sapbamrer, R.; Wangsan, K.; Panumasvivat, J.; Assavanopakun, P.; Muangkaew, S. Respiratory Symptoms and Skin Sick Building Syndrome among Office Workers at University Hospital, Chiang Mai, Thailand: Associations with Indoor Air Quality, AIRMED Project. Int. J. Environ. Res. Public Health 2022, 19, 10850. https://doi.org/10.3390/ijerph191710850
Surawattanasakul V, Sirikul W, Sapbamrer R, Wangsan K, Panumasvivat J, Assavanopakun P, Muangkaew S. Respiratory Symptoms and Skin Sick Building Syndrome among Office Workers at University Hospital, Chiang Mai, Thailand: Associations with Indoor Air Quality, AIRMED Project. International Journal of Environmental Research and Public Health. 2022; 19(17):10850. https://doi.org/10.3390/ijerph191710850
Chicago/Turabian StyleSurawattanasakul, Vithawat, Wachiranun Sirikul, Ratana Sapbamrer, Kampanat Wangsan, Jinjuta Panumasvivat, Pheerasak Assavanopakun, and Supang Muangkaew. 2022. "Respiratory Symptoms and Skin Sick Building Syndrome among Office Workers at University Hospital, Chiang Mai, Thailand: Associations with Indoor Air Quality, AIRMED Project" International Journal of Environmental Research and Public Health 19, no. 17: 10850. https://doi.org/10.3390/ijerph191710850
APA StyleSurawattanasakul, V., Sirikul, W., Sapbamrer, R., Wangsan, K., Panumasvivat, J., Assavanopakun, P., & Muangkaew, S. (2022). Respiratory Symptoms and Skin Sick Building Syndrome among Office Workers at University Hospital, Chiang Mai, Thailand: Associations with Indoor Air Quality, AIRMED Project. International Journal of Environmental Research and Public Health, 19(17), 10850. https://doi.org/10.3390/ijerph191710850








