Does 8-Week Resistance Training with Slow Movement Cadenced by Pilates Breathing Affect Muscle Strength and Balance of Older Adults? An Age-Matched Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Balance Assessment
2.3. Dorsiflexion Strength Test
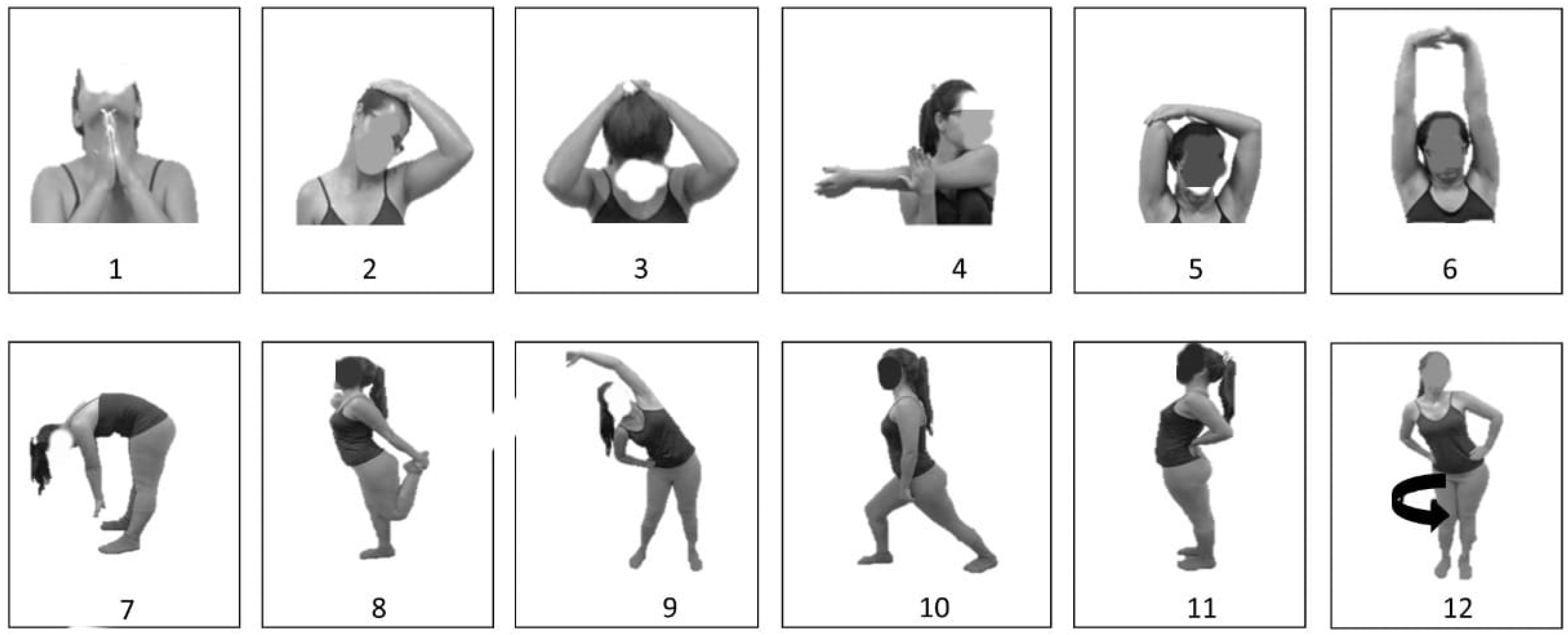
2.4. Intervention Protocol
2.5. Statistical Analysis
3. Results
3.1. RT vs. RT + P
3.2. Baseline vs. Post
4. Discussion
5. Conclusions
6. Supplementary Section
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raj, I.S.; Bird, S.R.; Shield, A.J. Aging and the force-velocity relationship of muscles. Exp. Gerontol. 2010, 45, 81–90. [Google Scholar] [CrossRef]
- United Nations. World Population Ageing 2019; United Nations: Newyork, NY, USA, 2019; ISBN 9789211483260. [Google Scholar]
- Yeung, S.S.Y.; Reijnierse, E.M.; Pham, V.K.; Trappenburg, M.C.; Lim, W.K.; Meskers, C.G.M.; Maier, A.B. Sarcopenia and its association with falls and fractures in older adults: A systematic review and meta-analysis. J. Cachexia. Sarcopenia Muscle 2019, 10, 485–500. [Google Scholar] [CrossRef]
- Dent, E.; Morley, J.E.; Cruz-Jentoft, A.J.; Woodhouse, L.; Rodríguez-Mañas, L.; Fried, L.P.; Woo, J.; Aprahamian, I.; Sanford, A.; Lundy, J.; et al. Physical Frailty: ICFSR International Clinical Practice Guidelines for Identification and Management. J. Nutr. Health Aging 2019, 23, 771–787. [Google Scholar] [CrossRef]
- Trombetti, A.; Reid, K.F.; Hars, M.; Herrmann, F.R.; Pasha, E.; Phillips, E.M.; Fielding, R.A. Age-associated declines in muscle mass, strength, power, and physical performance: Impact on fear of falling and quality of life. Osteoporos. Int. 2016, 27, 463–471. [Google Scholar] [CrossRef]
- McKinnon, N.B.; Connelly, D.M.; Rice, C.L.; Hunter, S.W.; Doherty, T.J. Neuromuscular contributions to the age-related reduction in muscle power: Mechanisms and potential role of high velocity power training. Ageing Res. Rev. 2017, 35, 147–154. [Google Scholar] [CrossRef]
- de Oliveira Moura Abreu, D.R.; Novaes, E.S.; de Oliveira, R.R.; de Freitas Mathias, T.A.; Marcon, S.S. Internação e mortalidade por quedas em idosos no Brasil: Análise de tendência. Cien. Saude Colet. 2018, 23, 1131–1141. [Google Scholar] [CrossRef]
- de Sire, A.; Ammendolia, A.; Invernizzi, M.; Baricich, A.; Lippi, L.; Invernizzi, M.; Grassi, F.A.; Leigheba, M. Optimization of transdisciplinary management of elderly with femur proximal extremity fracture: A patient-tailored plan from orthopaedics to rehabilitation. World J. Orthop. 2021, 12, 456–466. [Google Scholar] [CrossRef]
- Scaglioni, G.; Narici, M.V.; Martin, A. Neural activation during submaximal contractions seems more reflective of neuromuscular ageing than maximal voluntary activation. Front. Aging Neurosci. 2016, 8, 19. [Google Scholar] [CrossRef]
- Hamed, A.; Bohm, S.; Mersmann, F.; Arampatzis, A. Follow-up efficacy of physical exercise interventions on fall incidence and fall risk in healthy older adults: A systematic review and meta-analysis. Sport. Med. Open 2018, 4, 56. [Google Scholar] [CrossRef]
- Lesinski, M.; Hortobágyi, T.; Muehlbauer, T.; Gollhofer, A.; Granacher, U. Effects of Balance Training on Balance Performance in Healthy Older Adults: A Systematic Review and Meta-analysis. Sport. Med. 2015, 45, 1721–1738. [Google Scholar] [CrossRef] [Green Version]
- Kukidome, D.; Nishikawa, T.; Sato, M.; Nishi, Y.; Shimamura, R.; Kawashima, J.; Shimoda, S.; Mizuta, H.; Araki, E. Impaired balance is related to the progression of diabetic complications in both young and older adults. J. Diabetes Complicat. 2017, 31, 1275–1282. [Google Scholar] [CrossRef]
- Oliveira, M.R.; Vieira, E.R.; Gil, A.W.O.; Fernandes, K.B.P.; Teixeira, D.C.; Amorim, C.F.; Da Silva, R.A. One-legged stance sway of older adults with and without falls. PLoS ONE 2018, 13, e0203887. [Google Scholar] [CrossRef]
- Concin, H.; Brozek, W.; Benedetto, K.P.; Häfele, H.; Kopf, J.; Bärenzung, T.; Schnetzer, R.; Schenk, C.; Stimpfl, E.; Waheed-Hutter, U.; et al. Hip fracture incidence 2003–2013 and projected cases until 2050 in Austria: A population-based study. Int. J. Public Health 2016, 61, 1021–1030. [Google Scholar] [CrossRef]
- Thomas, E.; Battaglia, G.; Patti, A.; Brusa, J.; Leonardi, V.; Palma, A.; Bellafiore, M. Physical activity programs for balance and fall prevention in elderly. Medicine 2019, 98, e16218. [Google Scholar] [CrossRef]
- De Souto Barreto, P.; Rolland, Y.; Vellas, B.; Maltais, M. Association of Long-term Exercise Training with Risk of Falls, Fractures, Hospitalizations, and Mortality in Older Adults: A Systematic Review and Meta-analysis. JAMA Intern. Med. 2019, 179, 394–405. [Google Scholar] [CrossRef]
- Eckardt, N. Lower-extremity resistance training on unstable surfaces improves proxies of muscle strength, power and balance in healthy older adults: A randomised control trial. BMC Geriatr. 2016, 16, 191. [Google Scholar] [CrossRef]
- Cadore, E.L.; Rodríguez-Mañas, L.; Sinclair, A.; Izquierdo, M. Effects of different exercise interventions on risk of falls, gait ability, and balance in physically frail older adults: A systematic review. Rejuvenation Res. 2013, 16, 105–114. [Google Scholar] [CrossRef]
- Cancela, J.M.; de Oliveira, I.M.; Rodríguez-Fuentes, G. Effects of Pilates method in physical fitness on older adults. A systematic review. Eur. Rev. Aging Phys. Act. 2014, 11, 81–94. [Google Scholar] [CrossRef]
- Groessl, E.J.; Maiya, M.; Schmalzl, L.; Wing, D.; Jeste, D.V. Yoga to prevent mobility limitations in older adults: Feasibility of a randomized controlled trial. BMC Geriatr. 2018, 18, 306. [Google Scholar] [CrossRef]
- Fragala, M.S.; Cadore, E.L.; Dorgo, S.; Izquierdo, M.; Kraemer, W.J.; Peterson, M.D.; Ryan, E.D. Resistance Training for Older Adults: Position Statement From the National Strength and Conditioning Association. J. Strength Cond. Res. 2019, 33, 2019–2052. [Google Scholar] [CrossRef]
- Olsen, P.O.; Termannsen, A.D.; Bramming, M.; Tully, M.A.; Caserotti, P. Effects of resistance training on self-reported disability in older adults with functional limitations or disability—A systematic review and meta-analysis. Eur. Rev. Aging Phys. Act. 2019, 16, 24. [Google Scholar] [CrossRef] [PubMed]
- Fleck, S.J.; Kraemer, W.J. Designing Resistance Training Programs; Human Kinetics Books: Champaign, IL, USA, 2004. [Google Scholar]
- Morganti, C.M.; Nelson, M.E.; Fiatarone, M.A.; Dallal, G.E.; Economos, C.D.; Crawford, B.M.; Evans, W.J. Strength improvements with 1 yr of progressive resistance training in older women. Med. Sci. Sports Exerc. 1995, 27, 906–912. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Silva, A.; Dutra, M.T.; de Moraes, W.M.A.M.; Funghetto, S.S.; Lopes de Farias, D.; Fernandes dos Santos, P.H.; Vieira, D.C.L.; da Cunha Nascimento, D.; Orsano, V.S.M.; Schoenfeld, B.J.; et al. Resistance training-induced gains in muscle strength, body composition, and functional capacity are attenuated in elderly women with sarcopenic obesity. Clin. Interv. Aging 2018, 13, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.K.; Kirdi, N.; Bozoglu, E.; Meric, A.; Buyukturan, G.; Ozturk, A.; Doruk, H. Effect of low-intensity versus high-intensity resistance training on the functioning of the institutionalized frail elderly. Int. J. Rehabil. Res. 2018, 41, 211–217. [Google Scholar] [CrossRef]
- Syed-Abdul, M.M.; McClellan, C.L.; Parks, E.J.; Ball, S.D. Effects of a resistance training community programme in older adults. Ageing Soc. 2021, 42, 1863–1878. [Google Scholar] [CrossRef]
- Howe, T.; Rochester, L.; Neil, F.; Skelton, D.; Ballinger, C. Exercise for improving balance in older people (Review). Cochrane Database Syst. Rev. 2011, 11, 1465–1858. [Google Scholar] [CrossRef]
- Barbosa, A.C.; Martins, F.M.; Silva, A.F.; Coelho, A.C.; Intelangelo, L.; Vieira, E.R. Activity of lower limb muscles during squat with and without abdominal drawing-in and pilates breathing. J. Strength Cond. Res. 2017, 31, 3018–3023. [Google Scholar] [CrossRef]
- Wells, C.; Kolt, G.S.; Bialocerkowski, A. Defining Pilates exercise: A systematic review. Complement. Ther. Med. 2012, 20, 253–262. [Google Scholar] [CrossRef]
- Bueno de Souza, R.O.; de Faria Marcon, L.; de Arruda, A.S.F.; Pontes Junior, F.L.; de Melo, R.C. Effects of Mat Pilates on Physical Functional Performance of Older Adults: A Meta-analysis of Randomized Controlled Trials. Am. J. Phys. Med. Rehabil. 2018, 97, 414–425. [Google Scholar] [CrossRef]
- Vaquero-Cristóbal, R.; Alacid, F.; Esparza-Ros, F.; López-Plaza, D.; Muyor, J.M.; López-Miñarro, P.A. The effects of a reformer Pilates program on body composition and morphological characteristics in active women after a detraining period. Women Health 2016, 56, 784–806. [Google Scholar] [CrossRef]
- Josephs, S.; Pratt, M.L.; Calk Meadows, E.; Thurmond, S.; Wagner, A. The effectiveness of Pilates on balance and falls in community dwelling older adults. J. Bodyw. Mov. Ther. 2016, 20, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Barker, A.L.; Bird, M.-L.L.; Talevski, J. Effect of pilates exercise for improving balance in older adults: A systematic review with meta-analysis. Arch. Phys. Med. Rehabil. 2015, 96, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Segura, N.; Igual-Camacho, C.; Ballester-Gil, Y.; Blasco-Igual, M.C.; Blasco, J.M. The effects of the pilates training method on balance and falls of older adults: A systematic review and meta-analysis of randomized controlled trials. J. Aging Phys. Act. 2018, 26, 327–344. [Google Scholar] [CrossRef] [PubMed]
- Pucci, G.C.M.F.; Neves, E.B.; Saavedra, F.J.F. Effect of pilates method on physical fitness related to health in the elderly: A systematic review. Rev. Bras. Med. do Esporte 2019, 25, 76–87. [Google Scholar] [CrossRef]
- Barbosa, A.W.C.; Guedes, C.A.; Bonifácio, D.N.; de Fátima Silva, A.; Martins, F.L.M.; Almeida Barbosa, M.C.S. The Pilates breathing technique increases the electromyographic amplitude level of the deep abdominal muscles in untrained people. J. Bodyw. Mov. Ther. 2014, 19, 57–61. [Google Scholar] [CrossRef]
- Ratamess, N.A.; Alvar, B.A.; Evetoch, T.K.; Housh, T.J.; Kibler, W.B.; Kraemer, W.J.; Triplett, N.T. American College of Sports Medicine Progression Models in Resistance Training for Healthy Adults. Med. Sci. Sport. Exerc. 2009, 41, 687–708. [Google Scholar] [CrossRef]
- Tanimoto, M.; Sanada, K.; Yamamoto, K.; Kawano, H.; Gando, Y.; Tabata, I.; Ishii, N.; Miyachi, M. Effects of whole-body low-intensity resistance training with slow movement and tonic force generation on muscular size and strength in young men. J. Strength Cond. Res. 2008, 22, 1926–1938. [Google Scholar] [CrossRef]
- Watanabe, Y.; Madarame, H.; Ogasawara, R.; Nakazato, K.; Ishii, N. Effect of very low-intensity resistance training with slow movement on muscle size and strength in healthy older adults. Clin. Physiol. Funct. Imaging 2013, 34, 463–470. [Google Scholar] [CrossRef]
- Watanabe, Y.; Tanimoto, M.; Ohgane, A.; Sanada, K.; Miyachi, M.; Ishii, N. Increased muscle size and strength from slow-movement, low-intensity resistance exercise and tonic force generation. J. Aging Phys. Act. 2013, 21, 71–84. [Google Scholar] [CrossRef]
- Hackett, D.A.; Davies, T.B.; Orr, R.; Kuang, K.; Halaki, M. Effect of movement velocity during resistance training on muscle-specific hypertrophy: A systematic review. Eur. J. Sport Sci. 2018, 18, 473–482. [Google Scholar] [CrossRef]
- Snejdrlova, M.; Kalvach, Z.; Topinkova, E.; Vrablik, M.; Prochazkova, R.; Kvasilova, M.; Lanska, V.; Zlatohlavek, L.; Prusikova, M.; Ceska, R. APOE polymorphism as a potential determinant of functional fitness in the elderly regardless of nutritional status. Neuro Endocrinol. Lett. 2011, 32 (Suppl. S2), 51–54. [Google Scholar] [PubMed]
- Caramelli, P.; Nitrini, R. Como avaliar de forma breve e objetiva o estado mental de um paciente? Rev. Assoc. Med. Bras. 2000, 46, 301. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Mancia, G.; Spiering, W.; Rosei, E.A.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; De Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESHGuidelines for themanagement of arterial hypertension. J. Hypertens. 2018, 36, 1956–2041. [Google Scholar] [CrossRef]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-Country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef] [PubMed]
- Patil, R.; Uusi-Rasi, K.; Kannus, P.; Karinkanta, S.; Sievänen, H. Concern about falling in older women with a history of falls: Associations with health, functional ability, physical activity and quality of life. Gerontology 2013, 60, 22–30. [Google Scholar] [CrossRef]
- Irez, G.B.; Ozdemir, R.A.; Evin, R.; Irez, S.G.; Korkusuz, F. Integrating pilates exercise into an exercise program for 65+ year-old women to reduce falls. J. Sport. Sci. Med. 2011, 10, 105–111. [Google Scholar]
- O’Connor, S.M.; Baweja, H.S.; Goble, D.J. Validating the BTrackS Balance Plate as a low cost alternative for the measurement of sway-induced center of pressure. J. Biomech. 2016, 49, 4142–4145. [Google Scholar] [CrossRef]
- Moraux, A.; Canal, A.; Ollivier, G.; Ledoux, I.; Doppler, V.; Payan, C.; Hogrel, J.Y. Ankle dorsi- and plantar-flexion torques measured by dynamometry in healthy subjects from 5 to 80 years. BMC Musculoskelet. Disord. 2013, 14, 104. [Google Scholar] [CrossRef]
- Sawilowsky, S.S. New Effect Size Rules of Thumb. J. Mod. Appl. Stat. Methods 2009, 8, 597–599. [Google Scholar] [CrossRef]
- Watanabe, Y.; Tanimoto, M.; Oba, N.; Sanada, K.; Miyachi, M.; Ishii, N. Effect of resistance training using bodyweight in the elderly: Comparison of resistance exercise movement between slow and normal speed movement. Geriatr. Gerontol. Int. 2015, 15, 1270–1277. [Google Scholar] [CrossRef]
- Billot, M.; Simoneau, E.M.; Van Hoecke, J.; Martin, A. Age-related relative increases in electromyography activity and torque according to the maximal capacity during upright standing. Eur. J. Appl. Physiol. 2010, 109, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Spink, M.J.; Fotoohabadi, M.R.; Menz, H.B. Foot and ankle strength assessment using hand-held dynamometry: Reliability and age-related differences. Gerontology 2010, 56, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, F.V.; Gavin, J.P.; Wainwright, T.; McConnell, A. The effects of 8 weeks of inspiratory muscle training on the balance of healthy older adults: A randomized, double-blind, placebo-controlled study. Physiol. Rep. 2019, 7, e14076. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, F.V.; Gavin, J.P.; Wainwright, T.W.; McConnell, A.K. Comparison of balance changes after inspiratory muscle or Otago exercise training. PLoS ONE 2020, 15, e0227379. [Google Scholar] [CrossRef]
- Keating, C.J.; Cabrera-Linares, J.C.; Párraga-Montilla, J.A.; Latorre-Román, P.A.; del Castillo, R.M.; García-Pinillos, F. Influence of resistance training on gait & balance parameters in older adults: A systematic review. Int. J. Environ. Res. Public Health 2021, 18, 1759. [Google Scholar] [CrossRef] [PubMed]
- El-kader, S.M.A.; Ashmawy, E.M. Ankle Dorsiflexors Strength Improves Balance Performance in Elderly: A Corelational Study. Eur. J. Gen. Med. 2014, 11, 60–65. [Google Scholar] [CrossRef]
- Melzer, I.; Benjuya, N.; Kaplanski, J.; Alexander, N. Association between ankle muscle strength and limit of stability in older adults. Age Ageing 2009, 38, 119–123. [Google Scholar] [CrossRef]
- Sherrington, C.; Fairhall, N.J.; Wallbank, G.K.; Tiedemann, A.; Michaleff, Z.A.; Howard, K.; Clemson, L.; Hopewell, S.; Lamb, S.E. Exercise for preventing falls in older people living in the community. Cochrane Database Syst. Rev. 2019, 1, 1465–1858. [Google Scholar] [CrossRef]


| Outcome | Groups | p-Value | |
|---|---|---|---|
| RT | RT + P | ||
| n (%male/%female) | 22 (14/86) | 22 (14/86) | |
| Age (years) | 70 ± 6 | 69 ± 6 | 0.567 |
| BMI (Kg/m2) | 25.4 ± 5.0 | 26.1 ± 4.5 | 0.580 |
| Diabetes (%yes/%no) | 1 (5/95) | 2 (9/91) | 0.305 |
| FES-I | 20.0 ± 2.4 | 21.3 ± 4.3 | 0.217 |
| R-Handgrip (Kgf) | 23.9 ± 5.8 | 23.4 ± 8.0 | 0.830 |
| Falls History (%yes/%no) | 4 (18/82) | 4 (18/82) | 0.689 |
| MiniMental | 25.8 ± 2.7 | 25.8 ± 2.1 | 0.973 |
| SBP (mmHg) | 122.0 ± 13.4 | 125.0 ± 16.6 | 0.510 |
| DBP (mmHg) | 73.5 ± 9.7 | 77.3 ± 10.0 | 0.221 |
| Outcome | RT Group | RT + P Group | Between-Group Pairwise Comparisons (p-Value [ES]) | Within-Group Pairwise Comparisons (p-Value [ES]) | ||
|---|---|---|---|---|---|---|
| Baseline (1) | Post (2) | Baseline (3) | Post (4) | |||
| R-Dorsiflexion (Kgf) | 22.2 ± 4.2 | 29.1 ± 7.7 | 21.2 ± 7.7 | 22.9 ± 5.2 | 1;3;4 < 2 (0.001 [0.96]) | 1 < 2 (0.002 [1.09—very large]) |
| L-Dorsiflexion (Kgf) | 22.4 ± 3.3 | 29.5 ± 6.9 | 21.9 ± 7.3 | 24.0 ± 5.2 | 1;3;4 < 2 (0.001 [0.92]) | 1 < 2 (0.001 [1.06—very large]) |
| Path Length (cm) | 70.8 ± 15.7 | 63.1 ± 11.7 | 71.0 ± 14.3 | 59.7 ± 14.3 | NS | 3 > 4 (0.003 [0.87—large]) |
| Sway Velocity (cm/s) | 3.5 ± 0.8 | 3.1 ± 0.6 | 3.6 ± 0.7 | 2.9 ± 0.7 | NS | 3 > 4 (0.001 [0.90—large]) |
| Sway Area (cm2) | 6.6 ± 2.9 | 5.3 ± 1.7 | 8.9 ± 5.3 | 5.7 ± 2.1 | 3 > 1 (0.01 [0.52]) | 3 > 4 (0.003 [0.71—moderate]) |
| Excursion ML (cm) | 2.6 ± 0.5 | 2.6 ± 0.3 | 3.0 ± 0.7 | 2.6 ± 0.5 | NS | 3 > 4 (0.002 [0.77—moderate]) |
| Excursion AP (cm) | 3.1 ± 0.9 | 2.6 ± 0.6 | 3.6 ± 1.4 | 2.8 ± 0.7 | NS | 3 > 4 (0.010 [0.63—moderate) |
| Outcome | Groups | p-Value | |
|---|---|---|---|
| RT | RT + P | ||
| n | 19 | 19 | |
| Age (years) | 70.5 ± 6.19 | 68.2 ± 5.9 | 0.246 |
| BMI (Kg/m2) | 24.8 ± 4.8 | 26.1 ± 4.8 | 0.413 |
| Diabetes (%yes/%no) | 1 (5/95) | 3 (16/84) | 0.305 |
| FES-I | 20.7 ± 4.4 | 20.3 ± 2.4 | 0.680 |
| R-Handgrip (Kgf) | 22.3 ± 3.5 | 21.6 ± 6 | 0.662 |
| Falls History (%yes/%no) | 4 (21/79) | 2 (11/89) | 0.689 |
| MiniMental | 25.5 ± 2.7 | 25.6 ± 2.1 | 0.944 |
| SBP (mmHg) | 122.0 ± 13.6 | 125.0 ± 17.8 | 0.591 |
| DBP (mmHg) | 73.6 ± 10.1 | 77.8 ± 10.6 | 0.218 |
| Outcome | RT Group | RT + P Group | Between-Group Pairwise Comparisons (p-Value [ES]) | Within-Group Pairwise Comparisons (p-Value [ES]) | ||
|---|---|---|---|---|---|---|
| Baseline (1) | Post (2) | Baseline (3) | Post (4) | |||
| R-Dorsiflexion (Kgf) | 21.4 ± 3.6 | 27.8 ± 7.2 | 19.5 ± 6.2 | 21.9 ± 4.6 | 1;3;4 < 2 (0.002 [0.97]) | 1 < 2 (0.002 [1.02—large]) |
| L-Dorsiflexion (Kgf) | 21.8 ± 2.9 | 28.1 ± 6.0 | 20.0 ± 6.04 | 22.7 ± 4.2 | 1;3;4 < 2 (0.001 [1.04]) | 1 < 2 (0.001 [1.21—very large]) |
| Path Length (cm) | 71.0 ± 16.9 | 63.9 ± 12.2 | 69.6 ± 14.8 | 56.8 ± 12.1 | NS | 3 > 4 (0.003 [0.94—large]) |
| Sway Velocity (cm/s) | 3.55 ± 0.8 | 3.19 ± 0.6 | 3.6 ± 0.7 | 2.8 ± 0.6 | NS | 3 > 4 (0.001 [1.21—large]) |
| Sway Area (cm2) | 6.50 ± 3.0 | 5.17 ± 1.8 | 9.2 ± 6.5 | 5.7 ± 5.2 | 3 > 1 (0.015 [0.53]) | 3 > 4 (0.003 [0.58—moderate]) |
| Excursion ML (cm) | 2.62 ± 0.5 | 2.57 ± 0.3 | 3.0 ± 0.7 | 2.6 ± 0.5 | 3 > 1 (0.020 [0.67]) | 3 > 4 (0.002 [0.64—moderate]) |
| Excursion AP (cm) | 3.08 ± 1.0 | 2.64 ± 0.7 | 3.7 ± 1.5 | 2.8 ± 0.7 | NS | 3 > 4 (0.010 [0.68—moderate]) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, I.G.; Macedo, M.C.G.S.; Souza, M.A.; Silveira-Nunes, G.; Barbosa, M.C.S.A.; Queiroz, A.C.C.; Vieira, E.R.; Barbosa, A.C. Does 8-Week Resistance Training with Slow Movement Cadenced by Pilates Breathing Affect Muscle Strength and Balance of Older Adults? An Age-Matched Controlled Trial. Int. J. Environ. Res. Public Health 2022, 19, 10849. https://doi.org/10.3390/ijerph191710849
Fernandes IG, Macedo MCGS, Souza MA, Silveira-Nunes G, Barbosa MCSA, Queiroz ACC, Vieira ER, Barbosa AC. Does 8-Week Resistance Training with Slow Movement Cadenced by Pilates Breathing Affect Muscle Strength and Balance of Older Adults? An Age-Matched Controlled Trial. International Journal of Environmental Research and Public Health. 2022; 19(17):10849. https://doi.org/10.3390/ijerph191710849
Chicago/Turabian StyleFernandes, Ilha G., Maria C. G. S. Macedo, Matheus A. Souza, Gabriela Silveira-Nunes, Michelle C. S. A. Barbosa, Andreia C. C. Queiroz, Edgar R. Vieira, and Alexandre C. Barbosa. 2022. "Does 8-Week Resistance Training with Slow Movement Cadenced by Pilates Breathing Affect Muscle Strength and Balance of Older Adults? An Age-Matched Controlled Trial" International Journal of Environmental Research and Public Health 19, no. 17: 10849. https://doi.org/10.3390/ijerph191710849
APA StyleFernandes, I. G., Macedo, M. C. G. S., Souza, M. A., Silveira-Nunes, G., Barbosa, M. C. S. A., Queiroz, A. C. C., Vieira, E. R., & Barbosa, A. C. (2022). Does 8-Week Resistance Training with Slow Movement Cadenced by Pilates Breathing Affect Muscle Strength and Balance of Older Adults? An Age-Matched Controlled Trial. International Journal of Environmental Research and Public Health, 19(17), 10849. https://doi.org/10.3390/ijerph191710849







