Economic Evaluations of Magnetic Resonance Image-Guided Radiotherapy (MRIgRT): A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Information Sources
2.3. Search Strategy
2.4. Selection Process
2.5. Data Collection Process
2.6. Data Items
3. Results
3.1. Study Type
3.2. Adopted Methodologies
3.3. Model Structure
3.4. Uncertainty
3.5. Model Validation
3.6. Model Outcomes
3.7. Quality Assessment
4. Discussion
4.1. Main Considerations
4.2. Recommendations for Future Cost-Effectiveness Models
4.3. Strengths and Limitations of Review
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Delaney, G.; Jacob, S.; Featherstone, C.; Barton, M. The role of radiotherapy in cancer treatment: Estimating optimal utilization from a review of evidence-based clinical guidelines. Cancer 2005, 104, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Tepper, J.E. Radiation therapy-associated toxicity: Etiology, management, and prevention. CA Cancer J. Clin. 2021, 71, 437–454. [Google Scholar] [CrossRef] [PubMed]
- Grégoire, V.; Guckenberger, M.; Haustermans, K.; Lagendijk, J.J.W.; Ménard, C.; Pötter, R.; Slotman, B.J.; Tanderup, K.; Thorwarth, D.; van Herk, M.; et al. Image guidance in radiation therapy for better cure of cancer. Mol. Oncol. 2020, 14, 1470–1491. [Google Scholar] [CrossRef]
- Nonaka, H.; Onishi, H.; Watanabe, M.; Nam, V.H. Assessment of abdominal organ motion using cine magnetic resonance imaging in different gastric motilities: A comparison between fasting and postprandial states. J. Radiat. Res. 2019, 60, 837–843. [Google Scholar] [CrossRef]
- Keall, P.J.; Mageras, G.S.; Balter, J.M.; Emery, R.S.; Forster, K.M.; Jiang, S.B.; Kapatoes, J.M.; Low, D.A.; Murphy, M.J.; Murray, B.R.; et al. The management of respiratory motion in radiation oncology report of AAPM Task Group 76. Med. Phys. 2006, 33, 3874–3900. [Google Scholar] [CrossRef]
- O’Neill, A.G.M.; Jain, S.; Hounsell, A.R.; O’Sullivan, J.M. Fiducial marker guided prostate radiotherapy: A review. Br. J. Radiol. 2016, 89, 20160296. [Google Scholar] [CrossRef]
- Alaei, P.; Spezi, E. Imaging dose from cone beam computed tomography in radiation therapy. Phys. Med. 2015, 31, 647–658. [Google Scholar] [CrossRef]
- Hall, W.A.; Paulson, E.; Li, X.A.; Erickson, B.; Schultz, C.; Tree, A.; Awan, M.; Low, D.A.; McDonald, B.A.; Salzillo, T.; et al. Magnetic resonance linear accelerator technology and adaptive radiation therapy: An overview for clinicians. CA Cancer J. Clin. 2022, 72, 34–56. [Google Scholar] [CrossRef]
- Acharya, S.; Fischer-Valuck, B.W.; Kashani, R.; Parikh, P.; Yang, D.; Zhao, T.; Green, O.; Wooten, O.; Li, H.H.; Hu, Y.; et al. Online Magnetic Resonance Image Guided Adaptive Radiation Therapy: First Clinical Applications. Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 394–403. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Mandrik, O.L.; Severens, J.L.H.; Bardach, A.; Ghabri, S.; Hamel, C.; Mathes, T.; Vale, L.; Wisløff, T.; Goldhaber-Fiebert, J.D. Critical Appraisal of Systematic Reviews With Costs and Cost-Effectiveness Outcomes: An ISPOR Good Practices Task Force Report. Value Health J. Int. Soc. Pharm. Outcomes Res. 2021, 24, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Rethlefsen, M.L.; Kirtley, S.; Waffenschmidt, S.; Ayala, A.P.; Moher, D.; Page, M.J.; Koffel, J.B.; Blunt, H.; Brigham, T.; Chang, S.; et al. PRISMA-S: An extension to the PRISMA Statement for Reporting Literature Searches in Systematic Reviews. Syst. Rev. 2021, 10, 39. [Google Scholar] [CrossRef] [PubMed]
- Wallace, B.C.; Small, K.; Brodley, C.E.; Lau, J.; Trikalinos, T.A. Deploying an interactive machine learning system in an evidence-based practice center: Abstrackr. In Proceedings of the 2nd ACM International Health Informatics Symposium (IHI), Miami, FL, USA, 28–30 January 2012; pp. 819–824. [Google Scholar]
- Medical Services Advisory Committee (MSAC) Australian Government. The Use of Image Guided Radiation Therapy (IGRT) in the Treatment of Cancer; Australian Government: Canberra, ACT, Australia, 2015.
- Johnstone, P.A.S.; Kerstiens, J.; Wasserman, S.; Rosenberg, S.A. MRI-Linac Economics II: Rationalizing Schedules. J. Clin. Med. 2022, 11, 869. [Google Scholar] [CrossRef]
- Parikh, N.R.; Lee, P.P.; Raman, S.S.; Cao, M.; Lamb, J.; Tyran, M.; Chin, W.; Gilchrist, T.; Agazaryan, N.; Mittauer, K.; et al. Time-Driven Activity-Based Costing Comparison of CT-Guided Versus MR-Guided SBRT. JCO Oncol. Pract. 2020, 16, e1378–e1385. [Google Scholar] [CrossRef] [PubMed]
- Parikh, N.R.; Clark, M.A.; Patel, P.; Kafka-Peterson, K.; Zaide, L.; Ma, T.M.; Steinberg, M.L.; Cao, M.; Raldow, A.C.; Lamb, J.; et al. Time-Driven Activity-Based Costing of CT-Guided vs. MR-Guided Prostate SBRT. Appl. Radiat. Oncol. 2021, 10, 33–40. [Google Scholar]
- Berber, S.; Blaya Novakova, V.; Agresta, B.; Shah, K.; Fox, N.; Raichand, S. Magnetic Resonance Image Guided Radiation Therapy; MSAC Application 1620, Assessment Report; Commonwealth of Australia: Canberra, ACT, Australia, 2020. [Google Scholar]
- Schumacher, L.-E.D.; Dal Pra, A.; Hoffe, S.E.; Mellon, E.A. Toxicity reduction required for MRI-guided radiotherapy to be cost-effective in the treatment of localized prostate cancer. Br. J. Radiol. 2020, 93, 20200028. [Google Scholar] [CrossRef]
- Hehakaya, C.; van der Voort van Zyp, J.R.N.; Vanneste, B.G.; Grutters, J.P.; Grobbee, D.E.; Verkooijen, H.M.; Frederix, G.W. Early health economic analysis of 1.5 T MRI-guided radiotherapy for localized prostate cancer: Decision analytic modelling. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2021, 161, 74–82. [Google Scholar] [CrossRef]
- Nederland Zorginstituut Richtlijn Voor Het Uitvoeren van Economische Evaluaties in de Gezondheidszorg; The Netherlands National Health Care Institute: Diemen, The Netherlands, 2016.
- Stewart, S.T.; Lenert, L.; Bhatnagar, V.; Kaplan, R.M. Utilities for prostate cancer health states in men aged 60 and older. Med. Care 2005, 43, 347–355. [Google Scholar] [CrossRef]
- Alongi, F.; Rigo, M.; Figlia, V.; Cuccia, F.; Giaj-Levra, N.; Nicosia, L.; Ricchetti, F.; Sicignano, G.; De Simone, A.; Naccarato, S.; et al. 1.5 T MR-guided and daily adapted SBRT for prostate cancer: Feasibility, preliminary clinical tolerability, quality of life and patient-reported outcomes during treatment. Radiat. Oncol. 2020, 15, 69. [Google Scholar] [CrossRef]
- Bruynzeel, A.M.; Tetar, S.U.; Oei, S.S.; Senan, S.; Haasbeek, C.J.; Spoelstra, F.O.; Piet, A.H.; Meijnen, P.; Bakker van der Jagt, M.A.B.; Fraikin, T.; et al. A Prospective Single-Arm Phase 2 Study of Stereotactic Magnetic Resonance Guided Adaptive Radiation Therapy for Prostate Cancer: Early Toxicity Results. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 1086–1094. [Google Scholar] [CrossRef]
- Kaplan, R.S.; Anderson, S.R. Time-driven activity-based costing. Harv. Bus. Rev. 2004, 82, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Campbell and Cochrane Economics Methods Group—CCEMG. Evidence for Policy and Practice Information and Coordinating Centre–EPPI-Centre CCEMG–EPPI-Centre Cost Converter 2019. Available online: https://eppi.ioe.ac.uk/costconversion/ (accessed on 25 July 2022).
- Shemilt, I.; Thomas, J.; Morciano, M. A web-based tool for adjusting costs to a specific target currency and price year. Évid. Policy 2010, 6, 51–59. [Google Scholar] [CrossRef]
- Caro, J.J.; Eddy, D.M.; Kan, H.; Kaltz, C.; Patel, B.; Eldessouki, R.; Briggs, A.H. Questionnaire to Assess Relevance and Credibility of Modeling Studies for Informing Health Care Decision Making: An ISPOR-AMCP-NPC Good Practice Task Force Report. Value Health J. Int. Soc. Pharm. Outcomes Res. 2014, 17, 174–182. [Google Scholar] [CrossRef]
- Evers, S.; Goossens, M.; de Vet, H.; van Tulder, M.; Ament, A. Criteria list for assessment of methodological quality of economic evaluations: Consensus on Health Economic Criteria. Int. J. Technol. Assess. Health Care 2005, 21, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Claxton, K.; Sculpher, M.; Drummond, M. A rational framework for decision making by the National Institute For Clinical Excellence (NICE). Lancet 2002, 360, 711–715. [Google Scholar] [CrossRef]
- Grutters, J.P.; Govers, T.; Nijboer, J.; Tummers, M.; van der Wilt, G.J.; Rovers, M.M. Problems and Promises of Health Technologies: The Role of Early Health Economic Modeling. Int. J. Health Policy Manag. 2019, 8, 575–582. [Google Scholar] [CrossRef]
- Ijzerman, M.J.; Koffijberg, H.; Fenwick, E.; Krahn, M. Emerging Use of Early Health Technology Assessment in Medical Product Development: A Scoping Review of the Literature. Pharmacoeconomics 2017, 35, 727–740. [Google Scholar] [CrossRef]
- Sorenson, C.; Drummond, M.; Khan, B.B. Medical technology as a key driver of rising health expenditure: Disentangling the relationship. Clin. Outcomes Res. 2013, 5, 223–234. [Google Scholar] [CrossRef]
- Kim, J.-I.; Park, J.M.; Choi, C.H.; An, H.J.; Kim, Y.-J. Retrospective study comparing MR-guided radiation therapy (MRgRT) setup strategies for prostate treatment: Repositioning vs. replanning. Radiat. Oncol. 2019, 14, 139. [Google Scholar] [CrossRef]
- Boer, D.D.; Veldman, J.K.; van Tienhoven, G.; Bel, A.; van Kesteren, Z. Evaluating differences in respiratory motion estimates during radiotherapy: A single planning 4DMRI versus daily 4DMRI. Radiat. Oncol. 2021, 16, 188. [Google Scholar] [CrossRef] [PubMed]
- Raaijmakers, A.J.E.; Raaymakers, B.W.; Lagendijk, J.J.W. Integrating a MRI scanner with a 6 MV radiotherapy accelerator: Dose increase at tissue–air interfaces in a lateral magnetic field due to returning electrons. Phys. Med. Biol. 2005, 50, 1363–1376. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Zhang, K.; Li, M.; Tian, Y.; Men, K.; Wang, J.; Yi, J.; Li, Y.; Dai, J. Impact of Magnetic Field on Dose Distribution in MR-Guided Radiotherapy of Head and Neck Cancer. Front. Oncol. 2020, 10, 1739. [Google Scholar] [CrossRef] [PubMed]
- Claxton, K.; Paulden, M.; Gravelle, H.; Brouwer, W.; Culyer, A.J. Discounting and decision making in the economic evaluation of health-care technologies. Health Econ. 2011, 20, 2–15. [Google Scholar] [CrossRef]
- Khorasani, E.; Davari, M.; Kebriaeezadeh, A.; Fatemi, F.; Sari, A.A.; Varahrami, V. A comprehensive review of official discount rates in guidelines of health economic evaluations over time: The trends and roots. Eur. J. Health Econ. 2022. [Google Scholar] [CrossRef]
- Newall, A.; Chaiyakunapruk, N.; Lambach, P.; Hutubessy, R.C.W. Who guide on the economic evaluation of influenza vaccination. Influ. Other Respir. Viruses 2018, 12, 211–219. [Google Scholar] [CrossRef]
- Ferket, B.S.; Oxman, J.M.; Iribarne, A.; Gelijns, A.C.; Moskowitz, A.J. Cost-effectiveness analysis in cardiac surgery: A review of its concepts and methodologies. J. Thorac. Cardiovasc. Surg. 2018, 155, 1671–1681. [Google Scholar] [CrossRef]
- Bhagat, N.; Fidelman, N.; Durack, J.C.; Collins, J.; Gordon, R.L.; LaBerge, J.M.; Kerlan, R.K. Complications Associated with the Percutaneous Insertion of Fiducial Markers in the Thorax. Cardiovasc. Interv. Radiol. 2010, 33, 1186–1191. [Google Scholar] [CrossRef]
- Kord, M.; Kluge, A.; Kufeld, M.; Kalinauskaite, G.; Loebel, F.; Stromberger, C.; Budach, V.; Gebauer, B.; Acker, G.; Senger, C. Risks and Benefits of Fiducial Marker Placement in Tumor Lesions for Robotic Radiosurgery: Technical Outcomes of 357 Implantations. Cancers 2021, 13, 4838. [Google Scholar] [CrossRef]
- Morgan, S.C.; Hoffman, K.; Loblaw, D.A.; Buyyounouski, M.K.; Patton, C.; Barocas, D.; Bentzen, S.; Chang, M.; Efstathiou, J.; Greany, P.; et al. Hypofractionated Radiation Therapy for Localized Prostate Cancer: Executive Summary of an ASTRO, ASCO, and AUA Evidence-Based Guideline. Pract. Radiat. Oncol. 2018, 8, 354–360. [Google Scholar] [CrossRef]
- Torvinen, S.; Bergius, S.; Roine, R.; Lodenius, L.; Sintonen, H.; Taari, K. Use of Patient Assessed Health-Related Quality of Life Instruments in Prostate Cancer Research: A Systematic Review of the Literature 2002–15. Int. J. Technol. Assess. Health Care 2016, 32, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Philips, Z.; Ginnelly, L.; Sculpher, M.; Claxton, K.; Golder, S.; Riemsma, R.; Woolacoot, N.; Glanville, J. Review of guidelines for good practice in decision-analytic modelling in health technology assessment. Health Technol. Assess. 2004, 8, iii–iv, ix–xi, 1–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Study | Aim | Target Audience | Type of Economic Analysis | Country | Population | Compared Technologies | Study Design | |
|---|---|---|---|---|---|---|---|---|
| MRIgRT | Other RT Modalities | |||||||
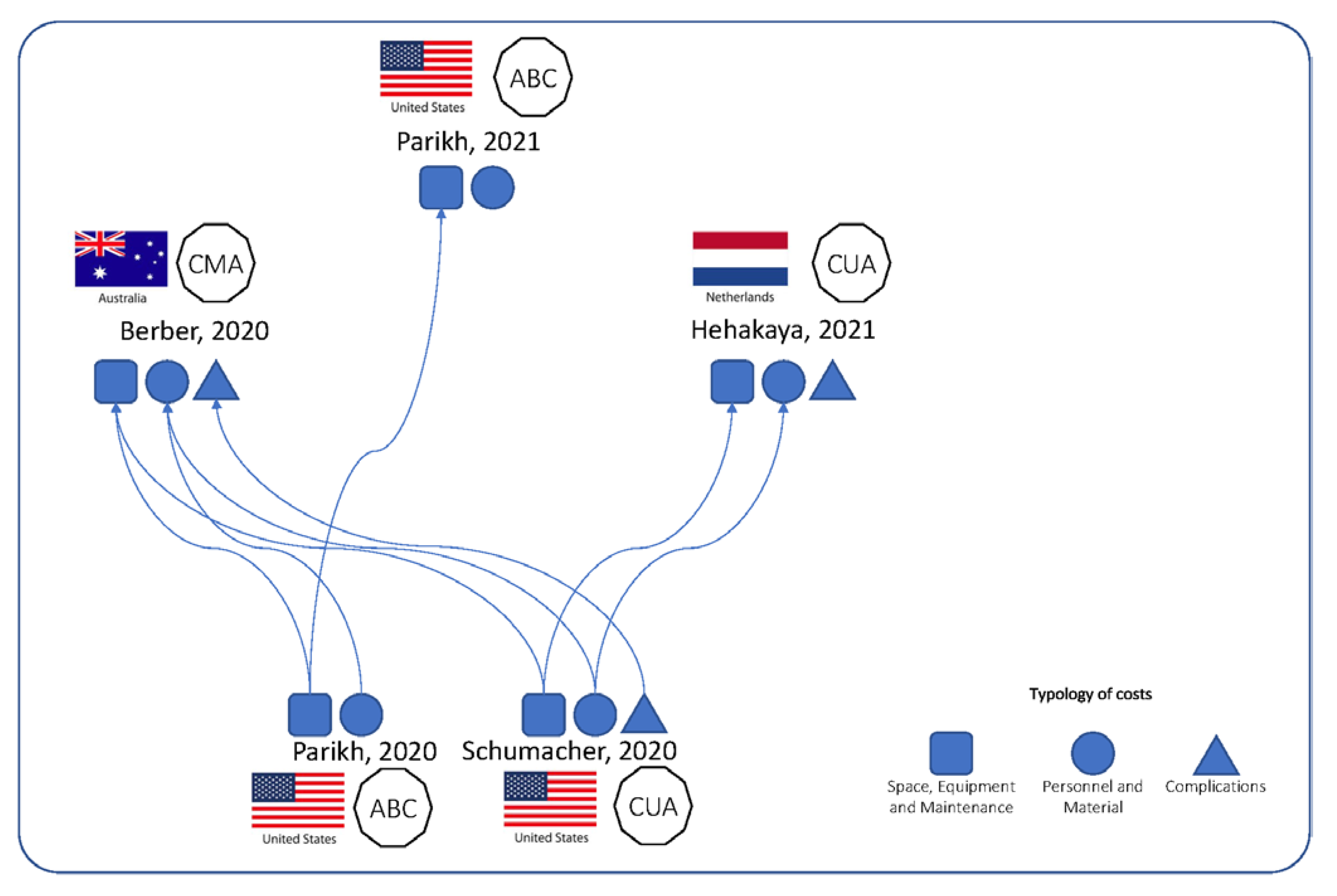
| Parikh, 2020 [17] | To determine and compare the direct cost of treatment of SBRT using CTIgRT and MRIgRT | Decision makers for investment choices | TDABC | California, USA | Subjects with localized unresectable HCC | 0.35 T 5f-MRIgRT SBRT | 5f-CTIgRT SBRT | Empirical (expert opinion through interviews) |
| Parikh, 2021 [18] | To determine the difference of direct cost of treatment of SBRT using CTIgRT and MRIgRT | Decision makers for investment choices | TDABC | California, USA | Subjects with localized PCa eligible for SBRT | 0.35 T 5f-MRIgRT SBRT | 5f-CTIgRT SBRT | Empirical (interviews/surveys with departmental personnel); CTgRT and MRgRT treatment times measured from local patients undergoing prostate SBRT |
| Berber, 2020 [19] | To determine the costs of two compared image-guided radiotherapies (MR and CT) | Decision makers for investment choices | CMA | Australia | Subjects with PCa undergoing EBRT | 0.35 T 5f-MRIgRT SBRT | 5f-CTIgRT SBRT | Model-based |
| Schumacher, 2020 [20] | To determine the toxicity reduction required to justify the added costs of MRIgRT over CTIgRT for the treatment of localized prostate cancer | Decision makers for investment choices | CUA | Florida, USA | Subjects with PCa (median age of prostate cancer diagnosis is 66 years old) |
|
| Model-based (Markov model). TDABC to determine the costs. Threshold analysis for CUA |
| Hehakaya, 2021 [21] | To estimate the relative minimally required reduction in grade ≥2 urinary, grade ≥2 bowel, and sexual complications in patients with low- and intermediate-risk localized PCa, and the maximum price of 5-fraction MRIgRT to be cost-effective, compared to current radiotherapy regimens | Decision makers for investment choices; researchers for future studies on prospective cost- effectiveness analysis | CUA | The Netherlands | Hypothetical cohort of 1000 men with low-/intermediate-risk localized PCa and no other severe comorbidities, treated at age 65 years | 1.5 T 5f-MRIgRT SBRT | 5f-, 20f- or 39f-EBRT | Model-based (state transition model, threshold analysis) |
| Study | Time Horizon | Annual Discount Rate | Type of Costs | Sources for Calculation of Costs | Health Outcomes | Sources for Calculation of Health Outcomes | Treatment of Uncertainty | Model Validation | Conflicts of Interest and Sources of Funding | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Direct Medical | Direct Non-Medical | Information Presented in Natural Units | |||||||||
| Parikh, 2020 [17] | N.A. | N.A. | (1) Space, Equipment and Maintenance; (2) Materials; (3) Personnel | No | Details provided in terms of time/patient in each process phase (new patient, simulation, planning, treatment, on treatment visit, follow-up visit, quality assurance) and for each personnel category (attending interventional radiologist, attending radiation oncologist, dosimetrist, environmental services staff, front desk staff, imaging technologist, medical/hospital assistant, nurse, physicist, radiation therapist, technician, transporter) | Inputs from:
| N.A. | N.A. | One-way deterministic sensitivity analyses (input parameters changed with ± 20%). Additional sensitivity analyses: 3 and 7 fractions instead of 5 for MRIgRT | Process flow maps and their various subcomponents, including the probability of time spent during the activity and specific resources used by each activity, were formulated and validated on the basis of input from nurses, dosimetrists, physicists, attending physicians from radiation oncology and interventional radiology, front office personnel, and radiation therapists. In addition, treatment times for MRIgRT and CTIgRT were further validated with patient-level data. | Several authors received research support or honoraria from ViewRay or Varian |
| Parikh, 2021 [18] | N.A. | N.A. | (1) Space, Equipment and Maintenance; (2) Materials; (3) Personnel | No | No detail provided | Inputs from healthcare professionals, department chief financial officer, and sales representatives | N.A. | N.A. | One-way deterministic sensitivity analyses (input parameters changed with ± 20%). Additional sensitivity analyses were performed that involved modifications of the number of fractions (1 and 7 instead of 5) for MRIgRT and CTIgRT | N.R. | Several authors are consultants, employees, or shareholders of ViewRay or Varian. The study received a grant from ViewRay |
| Berber, 2020 [19] | N.A. | N.A. | (1) Space, Equipment and Maintenance; (2) Materials; (3) Personnel (4) Costs of RT complications (acute and late GI/GU toxicity) | No | Details provided in terms of time/patient in each process phase (patient registration, pre-clinic charting, clinic visit, post-clinic visits, MR clearance process, fiducial marker placement, post-op time (after fiducial placement), simulation, review of images, after simulation, treatment planning, prior treatment, treatment, follow-up visit) and for each personnel category (front desk, radiation oncologist, medical assistant, interventional radiologist, medical physicist, medical assistant, nurse, radiation therapist, dosimetrist, imaging technologist, technician) | [17,20] for cost. Acute and late GI/GU toxicity rates were obtained from a systematic search of literature. | N.A. | N.A. | One-way deterministic sensitivity analyses (input parameters changed by ± 20% and ± 50%). Additional sensitivity analyses were performed that involved modifications of the number of fractions for CTIgRT from 5 fractions to 30 and by removing cost of fiducial marker placement. | N.R. | The authors declared the absence of conflicts of interest. The report was commissioned by the AGDH. |
| Schumacher, 2020 [20] | 15 years | 3% | (1) Space, Equipment and Maintenance; (2) Materials; (3) Personnel (4) Costs of RT complications (acute and late GI/GU toxicity) and BCR | No | Details provided in terms of time/patient in each process phase (consultation, simulation, planning, treatment, on treatment visit, follow-up visit) and for each personnel category (physician, nurse, receptionist, dosimetrist, therapist) | The costs of purchasing and maintaining CTIgRT and MRIgRT units were obtained by reviewing the literature. TDABC was used to determine the cost of all steps of patient care. For each step, personnel time and costs were determined using literature values, and interviews with staff and records of staff salaries of the clinical center involved in the empirical study. Costs of complications and BCR were obtained using previous reports in the literature, databases, and Medicare Physician Fee Schedule. | QALYs | A literature search reporting outcome of daily CTIgRT. The base probabilities of toxicities were then reduced by a relative 1% when using MRIgRT. QALYs were obtained using previous reports in the literature and databases | One-way sensitivity analyses were performed on conventional therapy and SBRT using 50,000 and 100,000 USD/QALY. The ranges for all costs were based on literature values or ±25% of the base estimate, except utilities which were ±0.10 of the base estimate. | N.R. | One of the authors received travel funding from ViewRay. The project was partially supported by the NCI-NIH |
| Hehakaya, 2021 [21] | From 65 years until death | 1.5% for utilities; 4% for costs | Comprehensive cost that includes: (1) Space, Equipment and Maintenance; (2) Materials; (3) Personnel (4) Costs of RT complications (acute and late GU, acute and late GI) and BCR | Travel expenses | No detail provided | Derived from published health economic evaluations in radiotherapy, the Dutch guideline for costing research, and the Dutch online database for medication costs | QALYs | MRIgRT utilities assumed as similar to post-treatment utilities as conventional EBRT (from literature). | One-way deterministic sensitivity analyses (mean input parameters changed with standard deviation or ± 20%) | (1) Model structure, input parameters, and discussion of major model assumptions undertaken with methodological and clinical experts; (2) Model performance appraised by using it similarly by an independent expert and by building it with two different software applications; (3) Model cross-validation through a structured literature search to compare model structure, assumptions, and outcomes of interest with cost-utility models. | The authors declare no personal conflicts of interest. Funds from ZonMw |
| Study | MRIgRT Costs/Patient * | Other RT Modalities Costs/Patient * | Other Considerations | Limitations | Conclusions | ||
|---|---|---|---|---|---|---|---|
| Direct Medical | Direct Non-Medical | Direct Medical | Direct Non-Medical | ||||
| Parikh, 2020 [17] | (1) Space, equipment and maintenance: 4769 2020 USD (4969); (2) Personnel: 3603 (3754); (3) Materials: 250 (260); TOTAL: 8622 (8983) | N.C. | (1) Space, equipment and maintenance: 2912 (3034); (2) Personnel: 3752 (3909); (3) Materials: 642 (669); TOTAL: 7306 (7612) | N.C. | Estimates drawn from a single institution’s processes, salary data, and space and equipment. Data obtained from personnel interviews instead of from measured times for specific patient encounters | The estimated direct costs to treat patients who have localized unresectable HCC with MR-guided SBRT are 18% higher than with CT-guided SBRT, although this difference in cost is sensitive to various assumptions and will vary based on individual practice patterns | |
| Parikh, 2021 [18] | N.R. | N.C. | N.R. | N.C. | Differences in costs between MRIgRT and CTIgRT **** (1) Space, equipment and maintenance: 1542 2021 USD (1571); (2) Personnel: 210 (214); (3) Materials: −255 (−260); TOTAL: 1497 (1526) | Estimates of personnel and material costs drawn from a single institution’s analysis. The equipment costs used in the analysis taken from sales representatives. When accounting for different fractionation regimens (e.g., 1 fraction or 7 fractions vs. 5 fractions), the approximate cost per fraction was kept constant and not explicitly accounted for the variable length of treatment time depending on nominal dose delivered | The base case of the analysis estimates USD 1497 in increased direct costs utilized by delivering prostate SBRT with MRIgRT instead of CTIgRT, although modifications to key model inputs may change this result. |
| Berber, 2020 [19] | (1) Space, equipment and maintenance: 4292.09 2020 AUD (3110); (2–3) Personnel & Material: 1623.33 (1176) (4) Complications: 150.63 (109) TOTAL: 6066.05 ** (4396) | N.C. | (1) Space, equipment and maintenance: 689.5 (500); (2–3) Personnel & Material: 1846.34 (1338); (4) Complications: 1592.95 (1154); TOTAL: 4128.79 *** (2992) | N.C. | The general conclusion of the report was in favor of listing MRIgRT on the Medical Benefits Schedule | ||
| Schumacher, 2020 [20] | (A) Conventional 39f-MRIgRT: (1) Space, equipment and maintenance: 12,406 2019 USD (13,196); (2) Personnel: 6225 (6621) (3) Materials: 205 (218) TOTAL (1 + 2 + 3): 18,836 (20,035) (4) Complications not provided on a per patient basis but detailed for feeding the Markov model. (B) 5f-MRIgRT: (1) Space, equipment and maintenance: 2118 (2253); (2) Personnel: 4664 (4961); (3) Materials: 35 (37); TOTAL (1 + 2 + 3): 6816 (7250) (4) Complications as above | N.C. | (A) Conventional 39f-CTIgRT: (1) Space, equipment and maintenance: 2955 (3143); (2) Personnel: 5752 (6118); (3) Materials: 0 (0) TOTAL (1 + 2 + 3): 8707 (9261) (4) Complications not provided on a per-patient basis but detailed for feeding the Markov model. (B) 5f-CTIgRT: (1) Space, equipment and maintenance: 379 (403); (2) Personnel: 4549 (4839); (3) Materials: 430 (457); TOTAL (1 + 2 + 3): 5357 (5698) (4) Complications as above | N.C. | Percentage reduction in complications to reach cost-efficacy: (i) Target ICER 50,000 USD/QALY (A) 39f-MRIgRT: 94%; (B) 5f-CTIgRT: 14%; (ii) Target ICER 100,000 USD/QALY (A) 39f-MRIgRT: 50%; (B) 5f-CTIgRT: 7% | The lack of technology data is the most important limitation | MRI-IGRT can easily be cost-effective for stereotactic prostate cancer treatment as only a slight reduction in overall side-effects is required (7% using 100,000 USD/QALY). Conventional fractionation would require a greater side-effect reduction (50% using 100,000 USD/QALY), but cost-effectiveness remains possible. A randomized clinical trial comparing MR-IGRT to CT-IGRT would better control for variations in the assumptions required to produce this model |
| Hehakaya, 2021 [21] | (1–3) Space, equipment and maintenance, Personnel and Materials costs all together: 5830 2019 EUR (7789) (4) Complications not provided on a per patient basis but detailed for feeding the Markov model. | 842 (630) | (1–3) Space, equipment and maintenance, Personnel and material costs all together: (A) 5f-EBRT: 1165 (1556); (B) 20f-EBRT: 4660 (6225); (C) 39f-EBRT: 9090 (12,144) (4) Complications not provided on a per patient basis but detailed for feeding the Markov model. | (A) 5f-EBRT: 470 (628); (B) 20f-EBRT: 1870 (2498); (C) 39f-EBRT: 3650 (4876) | Percentage reduction in complications of 5f-MRIgRT to reach cost-efficacy (ICER 80,000 EUR/QALY): (A) 5f-EBRT: 54%; (incremental costs USD 6610 (4948 EUR); incremental QALYs 0.06); (B) 20f-EBRT: 0%; (C) 39f-EBRT: 0% | (1) Lack of technology data (for instance, combined health states and post-treatment utility; some data taken from a similar technology 0.35 T MRIgRT instead of the 1.5 T version). (2) Dutch cost data to estimate cost-effectiveness with a consequent limited applicability in other countries. | MRIgRT is found to be cost-effective compared to 20f- and 39f-EBRT with no further reduction in complications. More challenging scenarios exist for 5f-EBRT in which rates of complications or costs need to be reduced significantly to come to cost-effective out- comes. Cost-effectiveness outcomes are highly sensitive to biochemical progression and utilities of urinary, bowel and sexual complications. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castelluccia, A.; Mincarone, P.; Tumolo, M.R.; Sabina, S.; Colella, R.; Bodini, A.; Tramacere, F.; Portaluri, M.; Leo, C.G. Economic Evaluations of Magnetic Resonance Image-Guided Radiotherapy (MRIgRT): A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 10800. https://doi.org/10.3390/ijerph191710800
Castelluccia A, Mincarone P, Tumolo MR, Sabina S, Colella R, Bodini A, Tramacere F, Portaluri M, Leo CG. Economic Evaluations of Magnetic Resonance Image-Guided Radiotherapy (MRIgRT): A Systematic Review. International Journal of Environmental Research and Public Health. 2022; 19(17):10800. https://doi.org/10.3390/ijerph191710800
Chicago/Turabian StyleCastelluccia, Alessandra, Pierpaolo Mincarone, Maria Rosaria Tumolo, Saverio Sabina, Riccardo Colella, Antonella Bodini, Francesco Tramacere, Maurizio Portaluri, and Carlo Giacomo Leo. 2022. "Economic Evaluations of Magnetic Resonance Image-Guided Radiotherapy (MRIgRT): A Systematic Review" International Journal of Environmental Research and Public Health 19, no. 17: 10800. https://doi.org/10.3390/ijerph191710800
APA StyleCastelluccia, A., Mincarone, P., Tumolo, M. R., Sabina, S., Colella, R., Bodini, A., Tramacere, F., Portaluri, M., & Leo, C. G. (2022). Economic Evaluations of Magnetic Resonance Image-Guided Radiotherapy (MRIgRT): A Systematic Review. International Journal of Environmental Research and Public Health, 19(17), 10800. https://doi.org/10.3390/ijerph191710800