A New Culture Method for the Detection of Non-Tuberculous Mycobacteria in Water Samples from Heater–Cooler Units and Extracorporeal Membrane Oxygenation Machines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microbiological Surveillance in Cardiac Surgery Settings
2.2. Sample Collection
2.3. Microbiology Workflow
2.4. Quality Control
2.5. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gilbert, J.A. Nosocomial Nontuberculous Mycobacteria Infections Associated with Heater-Cooler Devices. Lancet Respir. Med. 2017, 5, 384. [Google Scholar] [CrossRef]
- Stammers, A.H.; Riley, J.B. The Heater Cooler as a Source of Infection from Nontuberculous Mycobacteria. J. Extra Corpor. Technol. 2016, 48, 55–59. [Google Scholar]
- Trudzinski, F.C.; Schlotthauer, U.; Kamp, A.; Hennemann, K.; Muellenbach, R.M.; Reischl, U.; Gärtner, B.; Wilkens, H.; Bals, R.; Herrmann, M.; et al. Clinical Implications of Mycobacterium chimaera Detection in Thermoregulatory Devices Used for Extracorporeal Membrane Oxygenation (ECMO), Germany, 2015 to 2016. Euro Surveill 2016, 21, 1–8. [Google Scholar] [CrossRef] [PubMed]
- van Ingen, J.; Blaak, H.; de Beer, J.; de Roda Husman, A.M.; van Soolingen, D. Rapidly Growing Nontuberculous Mycobacteria Cultured from Home Tap and Shower Water. Appl. Environ. Microbiol. 2010, 76, 6017–6019. [Google Scholar] [CrossRef]
- Fernandes, H.M.Z.; Conceição, E.C.; Gomes, K.M.; da Silva, M.G.; Dias, R.C.S.; Duarte, R.S. Recovery of Non-Tuberculous Mycobacteria from Water Is Influenced by Phenotypic Characteristics and Decontamination Methods. Curr. Microbiol. 2020, 77, 621–631. [Google Scholar] [CrossRef]
- Williams, M.D.; Falkinham, J.O. Effect of Cetylpyridinium Chloride (CPC) on Colony Formation of Common Nontuberculous Mycobacteria. Pathogens 2018, 7, 79. [Google Scholar] [CrossRef] [PubMed]
- Thomson, R.; Carter, R.; Gilpin, C.; Coulter, C.; Hargreaves, M. Comparison of Methods for Processing Drinking Water Samples for the Isolation of Mycobacterium avium and Mycobacterium intracellulare. Appl. Environ. Microbiol. 2008, 74, 3094–3098. [Google Scholar] [CrossRef]
- Rotcheewaphan, S.; Odusanya, O.E.; Henderson, C.M.; Stephenson, D.; Olivier, K.N.; Perry, J.D.; Zelazny, A.M. Performance of RGM Medium for Isolation of Nontuberculous Mycobacteria from Respiratory Specimens from Non-Cystic Fibrosis Patients. J. Clin. Microbiol. 2019, 57, e01519-18. [Google Scholar] [CrossRef]
- EU Protocol for Testing of Mycobacterium Chimaera Infections Potentially Associated with Heater-Cooler Units; ECDC technical document; European Centre for Disease Prevention and Control: Stockholm, Sweden, 2015.
- Peña, J.A.; Ferraro, M.J.; Hoffman, C.G.; Branda, J.A. Growth Detection Failures by the Nonradiometric Bactec MGIT 960 Mycobacterial Culture System. J. Clin. Microbiol. 2012, 50, 2092–2095. [Google Scholar] [CrossRef]
- Piersimoni, C.; Nista, D.; Bornigia, S.; Gherardi, G. Unreliable Detection of Mycobacterium xenopi by the Nonradiometric Bactec MGIT 960 Culture System. J. Clin. Microbiol. 2009, 47, 804–806. [Google Scholar] [CrossRef]
- National Health Service Management and Decontamination of Flexible Endoscopes (HTM 01-06). Available online: https://www.gov.uk/government/publications/management-and-decontamination-of-flexible-endoscopes (accessed on 26 September 2018).
- Parashar, D.; Chauhan, D.S.; Sharma, V.D.; Chauhan, A.; Chauhan, S.V.S.; Katoch, V.M. Optimization of Procedures for Isolation of Mycobacteria from Soil and Water Samples Obtained in Northern India. Appl. Environ. Microbiol. 2004, 70, 3751–3753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- PHE. Protocol for Environmental Sampling, Processing and Culturing of Water and Air Samples for the Isolation of Slow-Growing Mycobacteria Standard Operating Procedure; PHE: London, UK, 2016.
- Radomski, N.; Cambau, E.; Moulin, L.; Haenn, S.; Moilleron, R.; Lucas, F.S. Comparison of Culture Methods for Isolation of Nontuberculous Mycobacteria from Surface Waters. Appl. Environ. Microbiol. 2010, 76, 3514–3520. [Google Scholar] [CrossRef] [PubMed]
- Ulmann, V.; Modrá, H.; Babak, V.; Weston, R.T.; Pavlik, I. Recovery of Mycobacteria from Heavily Contaminated Environmental Matrices. Microorganisms 2021, 9, 2178. [Google Scholar] [CrossRef]
- Asmar, S.; Drancourt, M. Chlorhexidine Decontamination of Sputum for Culturing Mycobacterium tuberculosis. BMC Microbiol. 2015, 15, 155. [Google Scholar] [CrossRef] [PubMed]
- Sattar, A.; Zakaria, Z.; Abu, J.; Aziz, S.A.; Gabriel, R.-P. Evaluation of Six Decontamination Procedures for Isolation of Mycobacterium avium complex from Avian Feces. PLoS ONE 2018, 13, e0202034. [Google Scholar] [CrossRef] [PubMed]
- Alexander, K.J.; Furlong, J.L.; Baron, J.L.; Rihs, J.D.; Stephenson, D.; Perry, J.D.; Stout, J.E. Evaluation of a New Culture Medium for Isolation of Nontuberculous Mycobacteria from Environmental Water Samples. PLoS ONE 2021, 16, e0247166. [Google Scholar] [CrossRef]
- Ditommaso, S.; Giacomuzzi, M.; Memoli, G.; Cavallo, R.; Curtoni, A.; Avolio, M.; Silvestre, C.; Zotti, C.M. Reduction of Turnaround Time for Non-Tuberculous Mycobacteria Detection in Heater-Cooler Units by Propidium Monoazide-Real-Time Polymerase Chain Reaction. J. Hosp. Infect. 2020, 104, 365–373. [Google Scholar] [CrossRef]
- Ditommaso, S.; Giacomuzzi, M.; Memoli, G.; Zotti, C.M. Real-Time PCR, the Best Approaches for Rapid Testing for Mycobacterium chimaera Detection in Heater Cooler Units and Extracorporeal Membrane Oxygenation. Perfusion 2021, 36, 626–629. [Google Scholar] [CrossRef]
- Ditommaso, S.; Giacomuzzi, M.; Memoli, G.; Zotti, C.M. Failure to Eradicate Non-Tuberculous Mycobacteria upon Disinfection of Heater-Cooler Units: Results of a Microbiological Investigation in Northwestern Italy. J. Hosp. Infect. 2020, 106, 585–593. [Google Scholar] [CrossRef]
- Ditommaso, S.; Giacomuzzi, M.; Memoli, G.; Garlasco, J.; Curtoni, A.; Iannaccone, M.; Zotti, C.M. Chemical Susceptibility Testing of Non-Tuberculous Mycobacterium Strains and Other Aquatic Bacteria: Results of a Study for the Development of a More Sensitive and Simple Method for the Detection of NTM in Environmental Samples. J. Microbiol. Methods 2022, 193, 106405. [Google Scholar] [CrossRef]
- ISO 6222; Water Quality—Enumeration of Culturable Micro-Organisms—Colony Count by Inoculation in a Nutrient Agar Culture Medium. International Standards Organization: Geneva, Switzerland, 1999.
- ISO 16266:2006; Water Quality—Detection and Enumeration of Pseudomonas aeruginosa—Method by Membrane Filtration. International Standards Organization: Geneva, Switzerland, 2006.
- Proficiency Testing for Food, Water and Environmental Microbiology: Mycobacterium spp. in Water Scheme. 2016. Available online: https://www.gov.uk/government/publications/mycobacterium-spp-in-water-scheme-sample-schedule (accessed on 20 August 2022).
- R Development Core Team. A Language and Environment for Statistical Computing; R Development Core Team: Vienna, Austria, 2019. [Google Scholar]
- Svensson, E.; Jensen, E.T.; Rasmussen, E.M.; Folkvardsen, D.B.; Norman, A.; Lillebaek, T. Mycobacterium chimaera in Heater-Cooler Units in Denmark Related to Isolates from the United States and United Kingdom. Emerging Infect. Dis. 2017, 23, 507–509. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, P.W.; Sax, H. Mycobacterium chimaera Infections Associated with Heater-Cooler Units in Cardiac Surgery. Curr. Opin. Infect. Dis. 2017, 30, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Marra, A.R.; Diekema, D.J.; Edmond, M.B. Successful Termination of an Outbreak of Mycobacterium chimaera Infections Associated with Contaminated Heater-Cooler Devices. Infect. Control Hosp. Epidemiol. 2021, 42, 471–473. [Google Scholar] [CrossRef] [PubMed]
- Preece, C.L.; Wichelhaus, T.A.; Perry, A.; Jones, A.L.; Cummings, S.P.; Perry, J.D.; Hogardt, M. Evaluation of Various Culture Media for Detection of Rapidly Growing Mycobacteria from Patients with Cystic Fibrosis. J. Clin. Microbiol. 2016, 54, 1797–1803. [Google Scholar] [CrossRef]
- Havelaar, A.H.; During, M.; Delfgou-Van Asch, E.H. Comparative Study of Membrane Filtration and Enrichment Media for the Isolation and Enumeration of Pseudomonas aeruginosa from Sewage, Surface Water, and Swimming Pools. Can. J. Microbiol. 1985, 31, 686–692. [Google Scholar] [CrossRef]
- Torvinen, E.; Suomalainen, S.; Paulin, L.; Kusnetsov, J. Mycobacteria in Finnish Cooling Tower Waters. Apmis 2014, 122, 353–358. [Google Scholar] [CrossRef]
- Kim, B.-J.; Kim, G.-N.; Kim, B.-R.; Jeon, C.O.; Jeong, J.; Lee, S.H.; Lim, J.-H.; Lee, S.-H.; Kim, C.K.; Kook, Y.-H.; et al. Description of Mycobacterium chelonae subsp. bovis subsp. nov., Isolated from Cattle (Bos Taurus Coreanae), Emended Description of Mycobacterium chelonae and Creation of Mycobacterium chelonae subsp. chelonae subsp. nov. Int. J. Syst. Evol. Microbiol. 2017, 67, 3882–3887. [Google Scholar] [CrossRef]
- Kim, B.-J.; Hong, S.-H.; Kook, Y.-H.; Kim, B.-J. Mycobacterium paragordonae sp. nov., a Slowly Growing, Scotochromogenic Species Closely Related to Mycobacterium gordonae. Int. J. Syst. Evol. Microbiol. 2014, 64, 39–45. [Google Scholar] [CrossRef]
- Kim, B.-J.; Kim, B.-R.; Kook, Y.-H.; Kim, B.-J. A Temperature Sensitive Mycobacterium paragordonae Induces Enhanced Protective Immune Responses against Mycobacterial Infections in the Mouse Model. Sci. Rep. 2017, 7, 15230. [Google Scholar] [CrossRef] [Green Version]

| Sampling Source | NTM Positive (%) | NTM Negative (%) | Total |
|---|---|---|---|
| Stockert 3T HCU | 14 (31.1) | 31 (68.9) | 45 |
| Maquet HCU40 | 12 (27.3) | 32 (72.7) | 44 |
| Maquet ECMO | 2 (15.4) | 11(84.6) | 13 |
| Sink | 2 * (33.3) | 4 (66.7) | 6 |
| Total | 30 | 78 | 108 |
| TVCs | P. aeruginosa | ||||||
|---|---|---|---|---|---|---|---|
| <1 CFU/Ml a (%) | 1 ≤ CFU/mL ≤ 100 b (%) | 101 ≤ CFU/mL ≤ 1000 b (%) | 1001 b ≤ CFU/mL ≤3000 b (%) | >3000 a CFU/mL (%) | Positive (%) | Negative (%) | |
| NTM positive samples | 16 (27.6) | 8 (44.4) | 1 (16.7) | 2 (40.0) | 1 (7.7) | 1 (11.1) | 27 (29.7) |
| NTM negative samples | 41 (70.7) | 10 (55.6) | 5 (83.3) | 3 (60.0) | 4 (30.8) | 3 (33.3) | 60 (65.9) |
| Unreadable plates | 1 (1.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 8 (61.5) | 5 (55.6) | 4 (4.4) |
| Total | 58 | 18 | 6 | 5 | 13 | 9 | 91 |
| TVCs | Total | |||||
|---|---|---|---|---|---|---|
| <1 CFU/mL a (%) | 1 ≤ CFU/mL ≤ 100 b (%) | 101 ≤ CFU/mL ≤ 1000 b (%) | 1001 b ≤ CFU/mL ≤3000 b (%) | >3000 a CFU/mL (%) | ||
| Elite NTM negative < 1 CFU/100 mL | 41 (70.7) | 8 (44.4) | 4 (66.7) | 2 (40.0) | 2 (15.4) | 57 |
| Elite NTM negative 1–100 CFU/100 mL | 0 (0.0) | 2 (11.1) | 1 (16.7) | 1 (20.0) | 2 (15.4) | 6 |
| Elite NTM positive < 1 CFU/100 mL | 16 (27.6) | 7 (38.9) | 1 (16.7) | 1 (20.0) | 1 (7.7) | 26 |
| Elite NTM positive 1–50 CFU/100 mL | 0 (0.0) | 1 (5.6) | 0 (0.0) | 1 (20.0) | 0 (0.0) | 2 |
| Unreadable plates | 1 (1.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 8 (61.5) | 9 |
| Total | 58 | 18 | 6 | 5 | 13 | 100 |
| Brand | Type of Samples Collection | NTM ELITE Results | TVCs 22 °C CFU/mL | TVCs 36 °C CFU/mL | P. aeruginosa CFU/250 mL | |
|---|---|---|---|---|---|---|
| 1 | Stockert 3T HCUs | pre-disinfection | non-AFB | >3 × 103 | >3 × 103 | <1 |
| 2 | Stockert 3T HCUs | pre-disinfection | non-AFB | >3 × 103 | >3 × 103 | >150 |
| 3 | Stockert 3T HCUs | post-disinfection | moulds | <1 | <1 | <1 |
| 4 | Stockert 3T HCUs | pre-disinfection | non-AFB | >3 × 103 | >3 × 103 | >150 |
| 5 | Stockert 3T HCUs | pre-disinfection | non-AFB | >3 × 103 | >3 × 103 | >150 |
| 6 | Stockert 3T HCUs | pre-disinfection | non-AFB | >3 × 103 | >3 × 103 | >150 |
| 7 | Stockert 3T HCUs | pre-disinfection | moulds | >3 × 103 | >3 × 103 | <1 |
| 8 | Stockert 3T HCUs | pre-disinfection | non-AFB | >3 × 103 | >3 × 103 | <1 |
| 9 | Stockert 3T HCUs | pre-disinfection | non-AFB | >3 × 103 | >3 × 103 | 15 |
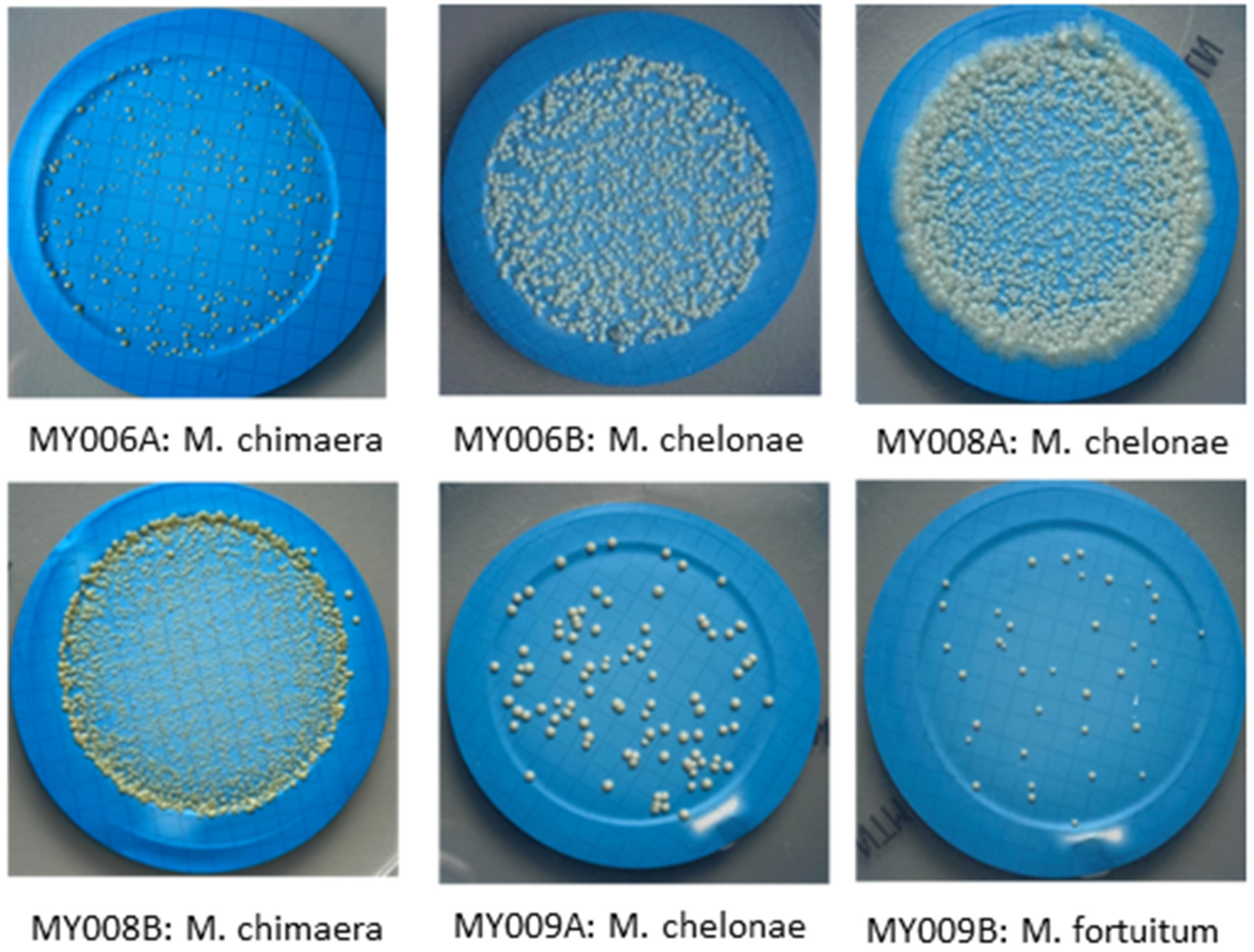
| Number of Distribution | Microorganism Strains by Each Distribution | CFU/100 mL |
|---|---|---|
| MY006 A | M. chimaera | 14 |
| (P. aeruginosa) | (1.1 × 102) | |
| MY006 B | M. chelonae | 2.2 × 102 |
| (B. multivorans) | (1.5 × 102) | |
| MY008 A | M. chelonae | 5.0 × 102 |
| (Microbacterium spp) | (20) | |
| (Staphylococcus xylosus) | (42) | |
| MY008 B | M. chimaera | 8.0 × 102 |
| MY009 A | M. chelonae | 5.8 × 10 |
| (E. coli) | (90) | |
| MY009 B | M. fortuitum | 30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ditommaso, S.; Giacomuzzi, M.; Memoli, G.; Garlasco, J.; Curtoni, A.; Iannaccone, M.; Zotti, C.M. A New Culture Method for the Detection of Non-Tuberculous Mycobacteria in Water Samples from Heater–Cooler Units and Extracorporeal Membrane Oxygenation Machines. Int. J. Environ. Res. Public Health 2022, 19, 10645. https://doi.org/10.3390/ijerph191710645
Ditommaso S, Giacomuzzi M, Memoli G, Garlasco J, Curtoni A, Iannaccone M, Zotti CM. A New Culture Method for the Detection of Non-Tuberculous Mycobacteria in Water Samples from Heater–Cooler Units and Extracorporeal Membrane Oxygenation Machines. International Journal of Environmental Research and Public Health. 2022; 19(17):10645. https://doi.org/10.3390/ijerph191710645
Chicago/Turabian StyleDitommaso, Savina, Monica Giacomuzzi, Gabriele Memoli, Jacopo Garlasco, Antonio Curtoni, Marco Iannaccone, and Carla M. Zotti. 2022. "A New Culture Method for the Detection of Non-Tuberculous Mycobacteria in Water Samples from Heater–Cooler Units and Extracorporeal Membrane Oxygenation Machines" International Journal of Environmental Research and Public Health 19, no. 17: 10645. https://doi.org/10.3390/ijerph191710645
APA StyleDitommaso, S., Giacomuzzi, M., Memoli, G., Garlasco, J., Curtoni, A., Iannaccone, M., & Zotti, C. M. (2022). A New Culture Method for the Detection of Non-Tuberculous Mycobacteria in Water Samples from Heater–Cooler Units and Extracorporeal Membrane Oxygenation Machines. International Journal of Environmental Research and Public Health, 19(17), 10645. https://doi.org/10.3390/ijerph191710645







