CCL2 Gene Expression and Protein Level Changes Observed in Response to Wingate Anaerobic Test in High-Trained Athletes and Non-Trained Controls
Abstract
:1. Introduction
2. Material and Methods
2.1. Participants
- gender (males only),
- all participants of the study group were qualified in the lists of Polish sports federations for their discipline, which means that they had a licence to compete,
- all participants had a documented history of systematic sports training and participation in a specific sport for over 10 years,
- all participants had regular medical examinations to allow them to participate in sports and compete,
- good current health status (no concurrent injuries) and a negative medical interview for cardiovascular system disorders, autonomic nervous system disorders, mental disorders, head injuries, and other diseases that could directly affect the experimental procedures,
- no intake of dietary supplements or medications during the study that could directly affect the results obtained.
- gender (males only),
- control participants were identical to those in the study group in terms of age and characteristics of general morphological indicators,
- a low level of physical activity, self-reported by each participant using the Global Physical Activity Questionnaire (according to the World Health Organisation Polish version),
- a good current state of health (no concurrent injuries) and negative medical questioning about disorders of the cardiovascular system, autonomic nervous system, mental disorders, head injuries, and other diseases that could directly affect the experimental procedures,
- no intake of dietary supplements or medications during the study that could directly affect the results obtained.
2.2. Measurement of Anaerobic Capacity by the Wingate Anaerobic Test
2.3. Biological Material Samples Collection
2.4. Isolation of the RNA and cDNA Synthesis
2.5. Application of Quantitative Real-Time PCR
2.6. Enzyme-Linked Immunoassay
2.7. Statistical Analysis
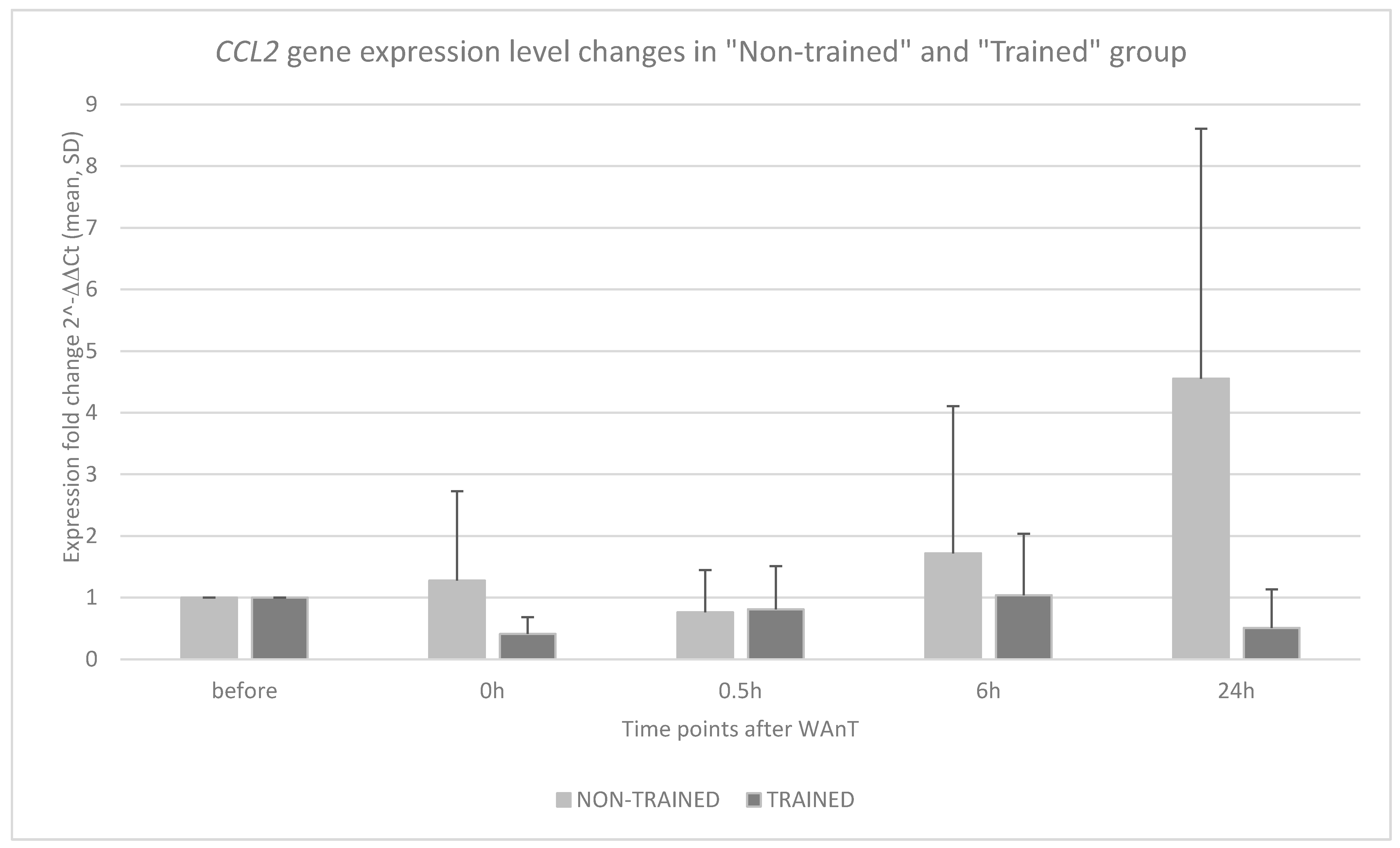
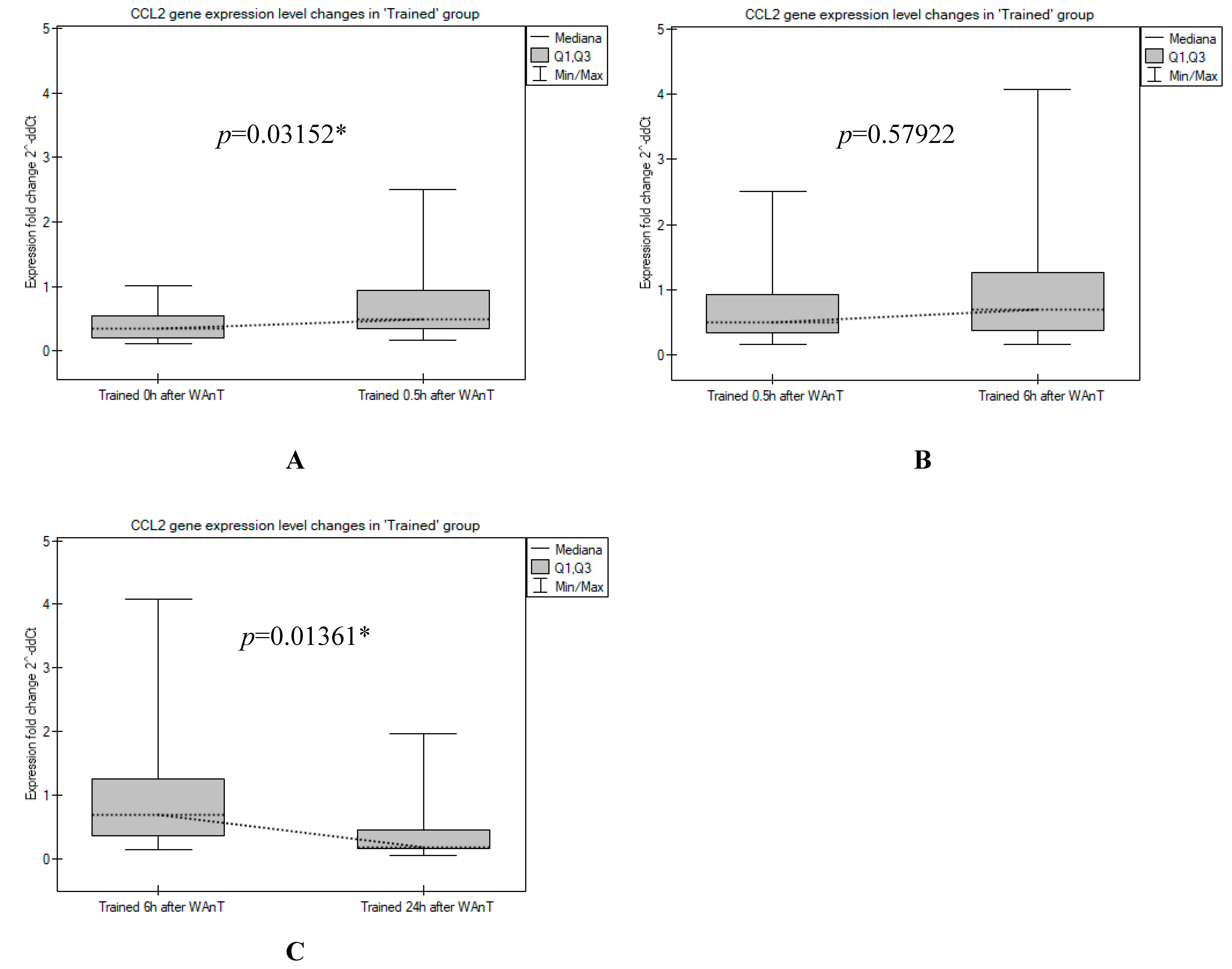
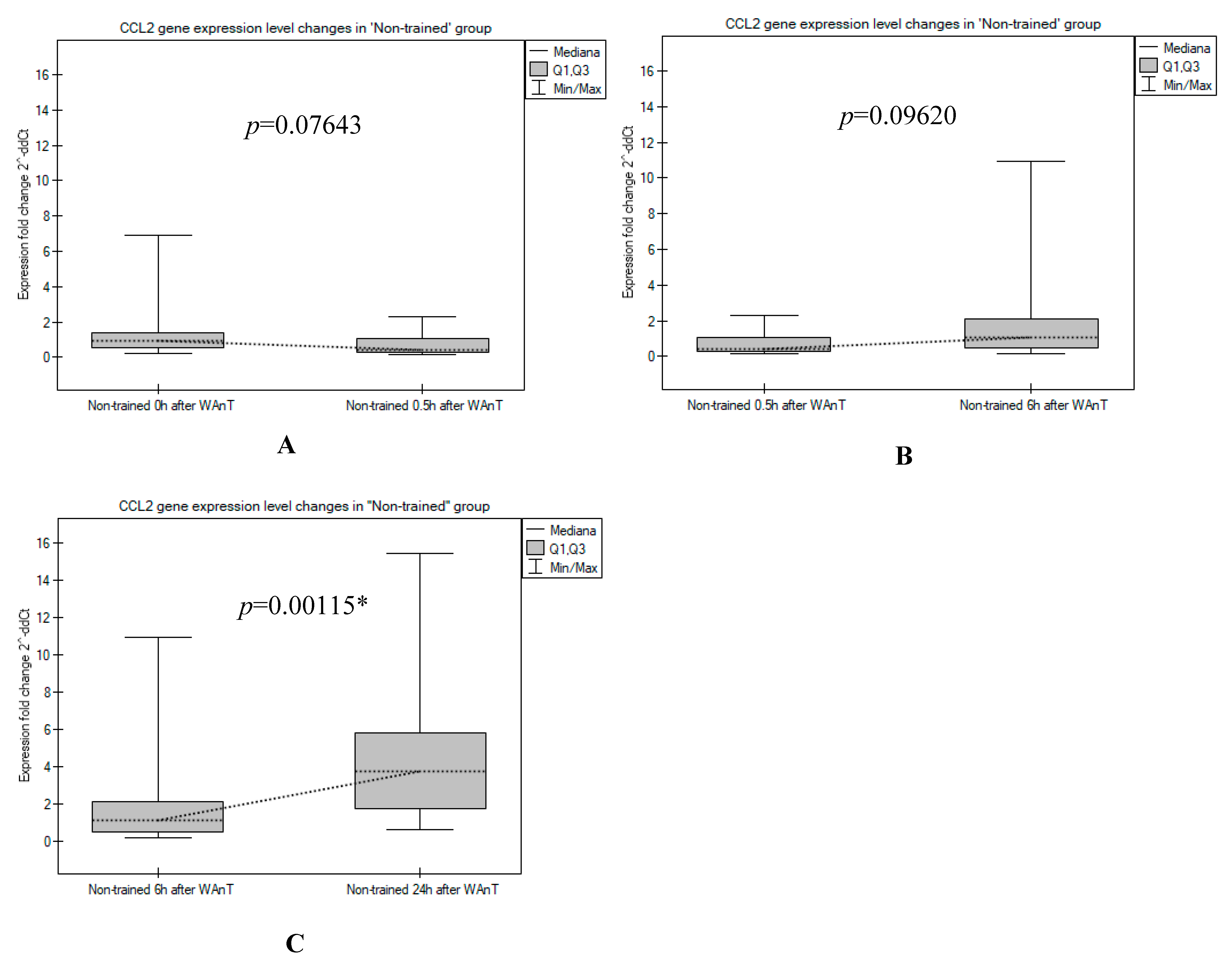
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Anderson, L.; Oldridge, N.; Thompson, D.R.; Zwisler, A.D.; Rees, K.; Martin, N.; Taylor, R.S. Exercise-Based Cardiac Rehabilitation for Coronary Heart Disease: Cochrane Systematic Review and Meta-Analysis. J. Am. Coll. Cardiol. 2016, 67, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hamman, R.F.; Wing, R.R.; Edelstein, S.L.; Lachin, J.M.; Bray, G.A.; Delahanty, L.; Hoskin, M.; Kriska, A.M.; Mayer-Davis, E.J.; Pi-Sunyer, X.; et al. Effect of Weight Loss with Lifestyle Intervention on Risk of Diabetes. Diabetes Care 2006, 29, 2102–2107. [Google Scholar] [CrossRef] [PubMed]
- Warburton, D.E.R.; Nicol, C.W.; Bredin, S.S.D. Health Benefits of Physical Activity: The Evidence. CMAJ 2006, 174, 801–809. [Google Scholar] [CrossRef]
- Gabrys, L.; Soff, J.; Thiel, C.; Schmidt, C.; Swart, E.; Peschke, D. Exercise-Based Cardiac Rehabilitation: Secondary Data Analyses of Mortality and Working Capacity in Germany, 2010–2017. Sports Med.-Open 2021, 7, 88. [Google Scholar] [CrossRef] [PubMed]
- Ades, P.A.; Balady, G.J.; Berra, K. Transforming Exercise-Based Cardiac Rehabilitation Programs into Secondary Prevention Centers: A National Imperative. J. Cardiopulm. Rehabil. 2001, 21, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wei, S.; Shi, Y.; Pang, S.; Qin, Q.; Yin, J.; Deng, Y.; Chen, Q.; Wei, S.; Nie, S.; et al. The Dose-Response Effect of Physical Activity on Cancer Mortality: Findings from 71 Prospective Cohort Studies. Br. J. Sports Med. 2016, 50, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Fogelholm, M. Physical Activity, Fitness and Fatness: Relations to Mortality, Morbidity and Disease Risk Factors. A Systematic Review. Obes. Rev. 2010, 11, 202–221. [Google Scholar] [CrossRef]
- Lancaster, G.I.; Febbraio, M.A. Skeletal Muscle: Not Simply an Organ for Locomotion and Energy Storage. J. Physiol. 2009, 587, 509. [Google Scholar] [CrossRef]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte Chemoattractant Protein-1 (MCP-1): An Overview. J. Interferon Cytokine Res. 2009, 29, 313–325. [Google Scholar] [CrossRef]
- Toumi, H.; Best, T.M. The Inflammatory Response: Friend or Enemy for Muscle Injury? Br. J. Sports Med. 2003, 37, 284–286. [Google Scholar] [CrossRef] [PubMed]
- Deyhle, M.R.; Gier, A.M.; Evans, K.C.; Eggett, D.L.; Nelson, W.B.; Parcell, A.C.; Hyldahl, R.D. Skeletal Muscle Inflammation Following Repeated Bouts of Lengthening Contractions in Humans. Front. Physiol. 2016, 6, 424. [Google Scholar] [CrossRef]
- Catoire, M.; Mensink, M.; Kalkhoven, E.; Schrauwen, P.; Kersten, S. Identification of Human Exercise-Induced Myokines Using Secretome Analysis. Physiol. Genom. 2014, 46, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Wentz, L.M. The Compelling Link between Physical Activity and the Body’s Defense System. J. Sport Health Sci. 2019, 8, 201–217. [Google Scholar] [CrossRef] [PubMed]
- Evans, W.J.; Cannon, J.G. The Metabolic Effects of Exercise-Induced Muscle Damage. Exerc. Sport Sci. Rev. 1991, 19, 99–125. [Google Scholar] [CrossRef]
- Fallon, K.E. The Acute Phase Response and Exercise: The Ultramarathon as Prototype Exercise. Clin. J. Sport Med. 2001, 11, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, É.; Marinho, D.A.; Neiva, H.P.; Lourenço, O. Inflammatory Effects of High and Moderate Intensity Exercise-A Systematic Review. Front. Physiol. 2020, 10, 1550. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Febbraio, M.A. Muscle as an Endocrine Organ: Focus on Muscle-Derived Interleukin-6. Physiol. Rev. 2008, 88, 1379–1406. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.H.; Kim, J.; Song, P.; Lee, T.G.; Suh, P.G.; Ryu, S.H. Secretomics for Skeletal Muscle Cells: A Discovery of Novel Regulators? Adv. Biol. Regul. 2012, 52, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Hu, P. Skeletal Muscle Regeneration Is Modulated by Inflammation. J. Orthop. Transl. 2018, 13, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Gschwandtner, M.; Derler, R.; Midwood, K.S. More Than Just Attractive: How CCL2 Influences Myeloid Cell Behavior Beyond Chemotaxis. Front. Immunol. 2019, 10, 2759. [Google Scholar] [CrossRef] [PubMed]
- Charo, I.F.; Myers, S.J.; Herman, A.; Franci, C.; Connolly, A.J.; Coughlin, S.R. Molecular Cloning and Functional Expression of Two Monocyte Chemoattractant Protein 1 Receptors Reveals Alternative Splicing of the Carboxyl-Terminal Tails. Proc. Natl. Acad. Sci. USA 1994, 91, 2752–2756. [Google Scholar] [CrossRef] [PubMed]
- Thompson, W.L.; Van Eldik, L.J. Inflammatory Cytokines Stimulate the Chemokines CCL2/MCP-1 and CCL7/MCP-3 through NFkB and MAPK Dependent Pathways in Rat Astrocytes. Brain Res. 2009, 1287, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, T.; Itaya-Hironaka, A.; Yamauchi, A.; Makino, M.; Sakuramoto-Tsuchida, S.; Shobatake, R.; Ota, H.; Takeda, M.; Ohbayashi, C.; Takasawa, S. Intermittent Hypoxia Up-Regulates CCL2, RETN, and TNFα MRNAs in Adipocytes via Down-Regulation of MiR-452. Int. J. Mol. Sci. 2019, 20, 1960. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, T. The Chemokine MCP-1 (CCL2) in the Host Interaction with Cancer: A Foe or Ally? Cell. Mol. Immunol. 2018, 15, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Huang, D.; Ransohoff, R.M.; Zhou, L. Acute Skeletal Muscle Injury: CCL2 Expression by Both Monocytes and Injured Muscle Is Required for Repair. FASEB J. 2011, 25, 3344–3355. [Google Scholar] [CrossRef]
- Jiang, Y.; Beller, D.I.; Frendl, G.; Graves, D.T. Monocyte Chemoattractant Protein-1 Regulates Adhesion Molecule Expression and Cytokine Production in Human Monocytes. J. Immunol. 1992, 148, 2423–2428. [Google Scholar]
- Harrington, J.R. The Role of MCP-1 in Atherosclerosis. Stem Cells 2000, 18, 65–66. [Google Scholar] [CrossRef]
- Mahad, D.J.; Ransohoff, R.M. The Role of MCP-1 (CCL2) and CCR2 in Multiple Sclerosis and Experimental Autoimmune Encephalomyelitis (EAE). Semin. Immunol. 2003, 15, 23–32. [Google Scholar] [CrossRef]
- Stankovic, A.; Slavic, V.; Stamenkovic, B.; Kamenov, B.; Bojanovic, M.; Mitrovic, D.R. Serum and Synovial Fluid Concentrations of CCL2 (MCP-1) Chemokine in Patients Suffering Rheumatoid Arthritis and Osteoarthritis Reflect Disease Activity. Bratislava Med. J. 2009, 110, 641–646. [Google Scholar]
- Panee, J. Monocyte Chemoattractant Protein 1 (MCP-1) in Obesity and Diabetes. Cytokine 2012, 60, 1–12. [Google Scholar] [CrossRef]
- Samaan, M.C.; Obeid, J.; Nguyen, T.; Thabane, L.; Timmons, B.W. Chemokine (C-C Motif) Ligand 2 Is a Potential Biomarker of Inflammation & Physical Fitness in Obese Children: A Cross-Sectional Study. BMC Pediatr. 2013, 13, 47. [Google Scholar]
- Roth, C.L.; Kratz, M.; Ralston, M.M.; Reinehr, T. Changes in Adipose-Derived Inflammatory Cytokines and Chemokines after Successful Lifestyle Intervention in Obese Children. Metabolism 2011, 60, 445–452. [Google Scholar] [CrossRef]
- Little, J.; Higgins, J.P.T.; Ioannidis, J.P.A.; Moher, D.; Gagnon, F.; von Elm, E.; Khoury, M.J.; Cohen, B.; Davey-Smith, G.; Grimshaw, J.; et al. Strengthening the Reporting of Genetic Association Studies (STREGA): An Extension of the STROBE Statement. Hum. Genet. 2009, 125, 131–151. [Google Scholar] [CrossRef] [PubMed]
- Maciejewska-Skrendo, A.; Mieszkowski, J.; Kochanowicz, A.; Stankiewicz, B.; Cieszczyk, P.; Switala, K.; Gomes de Assis, G.; Kecler, K.; Tarnowski, M.; Sawczuk, M. TNFA Expression Level Changes Observed in Response to the Wingate Anaerobic Test in Non-Trained and Trained Individuals. Balt. J. Health Phys. Act. 2019, 11, 1–10. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A New Mathematical Model for Relative Quantification in Real-Time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- de Lucas, R.D.; Caputo, F.; de Souza, K.M.; Sigwalt, A.R.; Ghisoni, K.; Silveira, P.C.L.; Remor, A.P.; Scheffer, D.D.L.; Guglielmo, L.G.A.; Latini, A. Increased Platelet Oxidative Metabolism, Blood Oxidative Stress and Neopterin Levels after Ultra-Endurance Exercise. J. Sports Sci. 2014, 32, 22–30. [Google Scholar] [CrossRef]
- Lindsay, A.; Lewis, J.; Scarrott, C.; Draper, N.; Gieseg, S.P. Changes in Acute Biochemical Markers of Inflammatory and Structural Stress in Rugby Union. J. Sports Sci. 2015, 33, 882–891. [Google Scholar] [CrossRef]
- Pinho, R.A.; Silva, L.A.; Pinho, C.A.; Scheffer, D.L.; Souza, C.T.; Benetti, M.; Carvalho, T.; Dal-Pizzol, F. Oxidative Stress and Inflammatory Parameters after an Ironman Race. Clin. J. Sport Med. 2010, 20, 306–311. [Google Scholar] [CrossRef]
- Campbell, J.P.; Turner, J.E. Debunking the Myth of Exercise-Induced Immune Suppression: Redefining the Impact of Exercise on Immunological Health Across the Lifespan. Front. Immunol. 2018, 9, 648. [Google Scholar] [CrossRef]
- Okin, D.; Medzhitov, R. Evolution of Inflammatory Diseases. Curr. Biol. 2012, 22, R733–R740. [Google Scholar] [CrossRef]
- Shireman, P.K.; Contreras-Shannon, V.; Ochoa, O.; Karia, B.P.; Michalek, J.E.; McManus, L.M. MCP-1 Deficiency Causes Altered Inflammation with Impaired Skeletal Muscle Regeneration. J. Leukoc. Biol. 2007, 81, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Hubal, M.J.; Chen, T.C.; Thompson, P.D.; Clarkson, P.M. Inflammatory Gene Changes Associated with the Repeated-Bout Effect. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R1628–R1637. [Google Scholar] [CrossRef] [PubMed]
- Atef, H.; Helmy, Z.; Farghaly, A. Effect of Different Types of Exercise on Sleep Deprivation and Functional Capacity in Middle Aged Patients after Coronary Artery Bypass Grafting. Sleep Sci. 2020, 13, 113–118. [Google Scholar] [PubMed]
- Trøseid, M.; Lappegård, K.T.; Claudi, T.; Damås, J.K.; Mørkrid, L.; Brendberg, R.; Mollnes, T.E. Exercise Reduces Plasma Levels of the Chemokines MCP-1 and IL-8 in Subjects with the Metabolic Syndrome. Eur. Heart J. 2004, 25, 349–355. [Google Scholar] [CrossRef]
- Accattato, F.; Greco, M.; Pullano, S.A.; Caré, I.; Fiorillo, A.S.; Pujia, A.; Montalcini, T.; Foti, D.P.; Brunetti, A.; Gulletta, E. Effects of Acute Physical Exercise on Oxidative Stress and Inflammatory Status in Young, Sedentary Obese Subjects. PLoS ONE 2017, 12, e0178900. [Google Scholar] [CrossRef] [PubMed]
- Wells, A.J.; Hoffman, J.R.; Jajtner, A.R.; Varanoske, A.N.; Church, D.D.; Gonzalez, A.M.; Townsend, J.R.; Boone, C.H.; Baker, K.M.; Beyer, K.S.; et al. Monocyte Recruitment after High-Intensity and High-Volume Resistance Exercise. Med. Sci. Sports Exerc. 2016, 48, 1169–1178. [Google Scholar] [CrossRef]
- Suzuki, K.; Nakaji, S.; Yamada, M.; Liu, Q.; Kurakake, S.; Okamura, N.; Kumae, T.; Umeda, T.; Sugawara, K. Impact of a Competitive Marathon Race on Systemic Cytokine and Neutrophil Responses. Med. Sci. Sports Exerc. 2003, 35, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Jürimäe, J.; Vaiksaar, S.; Purge, P. Circulating Inflammatory Cytokine Responses to Endurance Exercise in Female Rowers. Int. J. Sports Med. 2018, 39, 1041–1048. [Google Scholar] [CrossRef]
- Mooren, F.C.; Krueger, K.; Ringseis, R.; Eder, K.; Liebisch, G.; Conrad, K.; Alack, K.; Maleki, B.H. Combined Effects of Moderate Exercise and Short-Term Fasting on Markers of Immune Function in Healthy Human Subjects. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020, 318, R1103–R1115. [Google Scholar] [CrossRef] [PubMed]
- Zwetsloot, K.A.; John, C.S.; Lawrence, M.M.; Battista, R.A.; Shanely, R.A. High-Intensity Interval Training Induces a Modest Systemic Inflammatory Response in Active, Young Men. J. Inflamm. Res. 2014, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Middelbeek, R.J.W.; Motiani, P.; Brandt, N.; Nigro, P.; Zheng, J.; Virtanen, K.A.; Kalliokoski, K.K.; Hannukainen, J.C.; Goodyear, L.J. Exercise Intensity Regulates Cytokine and Klotho Responses in Men. Nutr. Diabetes 2021, 11, 5. [Google Scholar] [CrossRef] [PubMed]
- Yakeu, G.; Butcher, L.; Isa, S.; Webb, R.; Roberts, A.W.; Thomas, A.W.; Backx, K.; James, P.E.; Morris, K. Low-Intensity Exercise Enhances Expression of Markers of Alternative Activation in Circulating Leukocytes: Roles of PPARγ and Th2 Cytokines. Atherosclerosis 2010, 212, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Gano, L.B.; Donato, A.J.; Pierce, G.L.; Pasha, H.M.; Magerko, K.A.; Roeca, C.; Seals, D.R. Increased Proinflammatory and Oxidant Gene Expression in Circulating Mononuclear Cells in Older Adults: Amelioration by Habitual Exercise. Physiol. Genom. 2011, 43, 895–902. [Google Scholar] [CrossRef]
- Liberman, K.; Njemini, R.; Forti, L.N.; Cools, W.; Debacq-Chainiaux, F.; Kooijman, R.; Beyer, I.; Bautmans, I. Three Months of Strength Training Changes the Gene Expression of Inflammation-Related Genes in PBMC of Older Women: A Randomized Controlled Trial. Cells 2022, 11, 531. [Google Scholar] [CrossRef]
- Gjevestad, G.O.; Hamarsland, H.; Raastad, T.; Ottestad, I.; Christensen, J.J.; Eckardt, K.; Drevon, C.A.; Biong, A.S.; Ulven, S.M.; Holven, K.B. Gene Expression Is Differentially Regulated in Skeletal Muscle and Circulating Immune Cells in Response to an Acute Bout of High-Load Strength Exercise. Genes Nutr. 2017, 12, 8. [Google Scholar] [CrossRef]
- Lescaudron, L.; Peltékian, E.; Fontaine-Pérus, J.; Paulin, D.; Zampieri, M.; Garcia, L.; Parrish, E. Blood Borne Macrophages Are Essential for the Triggering of Muscle Regeneration Following Muscle Transplant. Neuromuscul. Disord. 1999, 9, 72–80. [Google Scholar] [CrossRef]
- Ziemkiewicz, N.; Hilliard, G.; Pullen, N.A.; Garg, K. The Role of Innate and Adaptive Immune Cells in Skeletal Muscle Regeneration. Int. J. Mol. Sci. 2021, 22, 3265. [Google Scholar] [CrossRef] [PubMed]
- Arnold, L.; Henry, A.; Poron, F.; Baba-Amer, Y.; Van Rooijen, N.; Plonquet, A.; Gherardi, R.K.; Chazaud, B. Inflammatory Monocytes Recruited after Skeletal Muscle Injury Switch into Antiinflammatory Macrophages to Support Myogenesis. J. Exp. Med. 2007, 204, 1057–1069. [Google Scholar] [CrossRef] [PubMed]
- Robertson, T.A.; Maley, M.A.L.; Grounds, M.D.; Papadimitriou, J.M. The Role of Macrophages in Skeletal Muscle Regeneration with Particular Reference to Chemotaxis. Exp. Cell Res. 1993, 207, 321–331. [Google Scholar] [CrossRef]
- Tidball, J.G. Inflammatory Cell Response to Acute Muscle Injury. Med. Sci. Sports Exerc. 1995, 27, 1022–1032. [Google Scholar] [CrossRef] [PubMed]
- Merly, F.; Lescaudron, L.; Rouaud, T.; Crossin, F.; Gardahaut, M.F. Macrophages Enhance Muscle Satellite Cell Proliferation and Delay Their Differentiation. Muscle Nerve 1999, 22, 724–732. [Google Scholar] [CrossRef]
- Hawke, T.J.; Garry, D.J. Myogenic Satellite Cells: Physiology to Molecular Biology. J. Appl. Physiol. 2001, 91, 534–551. [Google Scholar] [CrossRef] [PubMed]
- Mukaka, M.M. Statistics Corner: A Guide to Appropriate Use of Correlation Coefficient in Medical Research. Malawi Med. J. 2012, 24, 69–71. [Google Scholar]
- Salcedo, R.; Ponce, M.L.; Young, H.A.; Wasserman, K.; Ward, J.M.; Kleinman, H.K.; Oppenheim, J.J.; Murphy, W.J. Human Endothelial Cells Express CCR2 and Respond to MCP-1: Direct Role of MCP-1 in Angiogenesis and Tumor Progression. Blood 2000, 96, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Živković, L.; Asare, Y.; Bernhagen, J.; Dichgans, M.; Georgakis, M.K. Pharmacological Targeting of the CCL2/CCR2 Axis for Atheroprotection: A Meta-Analysis of Preclinical Studies. Arterioscler. Thromb. Vasc. Biol. 2022, 42, E131–E144. [Google Scholar] [CrossRef]
- Ehling, J.; Bartneck, M.; Wei, X.; Gremse, F.; Fech, V.; Möckel, D.; Baeck, C.; Hittatiya, K.; Eulberg, D.; Luedde, T.; et al. CCL2-Dependent Infiltrating Macrophages Promote Angiogenesis in Progressive Liver Fibrosis. Gut 2014, 63, 1960–1971. [Google Scholar] [CrossRef] [PubMed]
- Gharaee-Kermani, M.; Denholm, E.M.; Phan, S.H. Costimulation of Fibroblast Collagen and Transforming Growth Factor Beta1 Gene Expression by Monocyte Chemoattractant Protein-1 via Specific Receptors. J. Biol. Chem. 1996, 271, 17779–17784. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Eckes, B.; Mauch, C.; Hartmann, K.; Krieg, T. Monocyte Chemoattractant Protein-1 Enhances Gene Expression and Synthesis of Matrix Metalloproteinase-1 in Human Fibroblasts by an Autocrine IL-1 Alpha Loop. J. Immunol. 2000, 164, 6174–6179. [Google Scholar] [CrossRef]

| Group | Time Point | rs | p # | τ | p ^ |
|---|---|---|---|---|---|
| “Trained” | 0 h after WAnT | 0.526316 | 0.017131 * | 0.326316 | 0.044268 * |
| 0.5 h after WAnT | 0.521053 | 0.022161 * | 0.368421 | 0.027518 * | |
| 6 h after WAnT | 0.242105 | 0.303755 | 0.126316 | 0.436178 | |
| 24 h after WAnT | −0.121429 | 0.666401 | −0.12381 | 0.520008 | |
| “Non-trained” | 0 h after WAnT | 0.260641 | 0.281148 | 0.211145 | 0.206525 |
| 0.5 h after WAnT | −0.138596 | 0.571491 | −0.122807 | 0.462524 | |
| 6 h after WAnT | −0.312782 | 0.179364 | −0.252632 | 0.119393 | |
| 24 h after WAnT | −0.283516 | 0.325966 | −0.252747 | 0.207982 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maciejewska-Skrendo, A.; Tarnowski, M.; Kopytko, P.; Kochanowicz, A.; Mieszkowski, J.; Stankiewicz, B.; Sawczuk, M. CCL2 Gene Expression and Protein Level Changes Observed in Response to Wingate Anaerobic Test in High-Trained Athletes and Non-Trained Controls. Int. J. Environ. Res. Public Health 2022, 19, 9947. https://doi.org/10.3390/ijerph19169947
Maciejewska-Skrendo A, Tarnowski M, Kopytko P, Kochanowicz A, Mieszkowski J, Stankiewicz B, Sawczuk M. CCL2 Gene Expression and Protein Level Changes Observed in Response to Wingate Anaerobic Test in High-Trained Athletes and Non-Trained Controls. International Journal of Environmental Research and Public Health. 2022; 19(16):9947. https://doi.org/10.3390/ijerph19169947
Chicago/Turabian StyleMaciejewska-Skrendo, Agnieszka, Maciej Tarnowski, Patrycja Kopytko, Andrzej Kochanowicz, Jan Mieszkowski, Błażej Stankiewicz, and Marek Sawczuk. 2022. "CCL2 Gene Expression and Protein Level Changes Observed in Response to Wingate Anaerobic Test in High-Trained Athletes and Non-Trained Controls" International Journal of Environmental Research and Public Health 19, no. 16: 9947. https://doi.org/10.3390/ijerph19169947
APA StyleMaciejewska-Skrendo, A., Tarnowski, M., Kopytko, P., Kochanowicz, A., Mieszkowski, J., Stankiewicz, B., & Sawczuk, M. (2022). CCL2 Gene Expression and Protein Level Changes Observed in Response to Wingate Anaerobic Test in High-Trained Athletes and Non-Trained Controls. International Journal of Environmental Research and Public Health, 19(16), 9947. https://doi.org/10.3390/ijerph19169947







