Implications of Adipose Tissue Content for Changes in Serum Levels of Exercise-Induced Adipokines: A Quasi-Experimental Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Overview
2.2. Participants
2.3. Measurement of Anaerobic and Aerobic Fitness Level
2.3.1. Maximal Anaerobic Effort
2.3.2. Maximal Aerobic Effort
2.4. Blood Sample Collection and Measurements of Selected Markers
2.5. Statistical Analysis
3. Results
3.1. Maximal Anaerobic Effort
3.2. Maximal Aerobic Effort
4. Discussion
4.1. Limitations
4.2. Practical Application
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stanford, K.I.; Goodyear, L.J. Exercise regulation of adipose tissue. Adipocyte 2016, 5, 153–162. [Google Scholar] [CrossRef]
- Mika, A.; Macaluso, F.; Barone, R.; Di Felice, V.; Sledzinski, T. Effect of Exercise on Fatty Acid Metabolism and Adipokine Secretion in Adipose Tissue. Front. Physiol. 2019, 10, 26. [Google Scholar] [CrossRef]
- Coelho, M.; Oliveira, T.; Fernandes, R. Biochemistry of adipose tissue: An endocrine organ. Arch. Med. Sci. 2013, 9, 191–200. [Google Scholar] [CrossRef]
- Makki, K.; Froguel, P.; Wolowczuk, I. Adipose tissue in obesity-related inflammation and insulin resistance: Cells, cytokines, and chemokines. ISRN Inflamm. 2013, 2013, 139239. [Google Scholar] [CrossRef] [PubMed]
- Scherer, P.E.; Williams, S.; Fogliano, M.; Baldini, G.; Lodish, H.F. A Novel Serum Protein Similar to C1q, Produced Exclusively in Adipocytes. J. Biol. Chem. 1995, 270, 26746–26749. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, K.; Ouchi, N.; Matsuzawa, Y. Anti-inflammatory and anti-atherogenic properties of adiponectin. Biochimie 2012, 94, 2137–2142. [Google Scholar] [CrossRef] [PubMed]
- Kuryszko, J.; Sławuta, P.; Sapikowski, G. Secretory function of adipose tissue. Pol. J. Vet. Sci. 2016, 19, 441–446. [Google Scholar] [CrossRef]
- Wang, Z.V.; Scherer, P.E. Adiponectin, the past two decades. J. Mol. Cell Biol. 2016, 8, 93–100. [Google Scholar] [CrossRef]
- Zhang, Y.; Proenca, R.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional cloning of the mouse obese gene and its human homologue. Nature 1991, 372, 425–432. [Google Scholar] [CrossRef]
- Cammisotto, P.G.; Bukowiecki, L.J. Mechanisms of leptin secretion from white adipocytes. Am. J. Physiol. Cell Physiol. 2002, 283, 244–250. [Google Scholar] [CrossRef]
- Klok, M.D.; Jakobsdottir, S.; Drent, M.L. The role of leptin and ghrelin in the regulation of food intake and body weight in humans: A review. Obes. Rev. 2007, 8, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Acquarone, E.; Monacelli, F.; Borghi, R.; Nencioni, A.; Odetti, P. Resistin: A reappraisal. Mech. Ageing Dev. 2019, 178, 46–63. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, D.; Kant, S.; Pandey, S.; Ehtesham, N.Z. Resistin in metabolism, inflammation, and disease. FEBS J. 2020, 287, 3141–3149. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Kamon, J.; Minokoshi, Y.; Ito, Y.; Waki, H.; Uchida, S.; Yamashita, S.; Noda, M.; Kita, S.; Ueki, K.; et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat. Med. 2002, 8, 1288–1295. [Google Scholar] [CrossRef]
- Steppan, C.M.; Bailey, S.T.; Bhat, S.; Brown, E.J.; Banerjee, R.R.; Wright, C.M.; Patel, H.R.; Ahima, R.S.; Lazar, M.A. The hormone resistin links obesity to diabetes. Nature 2001, 409, 307–312. [Google Scholar] [CrossRef]
- Matzuk, M.M.; Lu, N.; Vogel, H.; Sellheyer, K.; Roop, D.R.; Bradley, A. Multiple defects and perinatal death in mice deficient in follistatin. Nature 1995, 374, 360–363. [Google Scholar] [CrossRef]
- Lee, S.-J.; Lee, Y.-S.; Zimmers, T.A.; Soleimani, A.; Matzuk, M.M.; Tsuchida, K.; Cohn, R.D.; Barton, E.R. Regulation of Muscle Mass by Follistatin and Activins. Front. Cell Dev. Biol. 2010, 24, 1998–2008. [Google Scholar] [CrossRef]
- Singh, R.; Braga, M.; Reddy, S.T.; Lee, S.-J.; Parveen, M.; Grijalva, V.; Vergnes, L.; Pervin, S. Follistatin Targets Distinct Pathways to Promote Brown Adipocyte Characteristics in Brown and White Adipose Tissues. Endocrinology 2017, 158, 1217–1230. [Google Scholar] [CrossRef]
- Sánchez-Infantes, D.; White, U.A.; Elks, C.M.; Morrison, R.F.; Gimble, J.M.; Considine, R.V.; Ferrante, A.W.; Ravussin, E.; Stephens, J.M. Oncostatin M Is Produced in Adipose Tissue and Is Regulated in Conditions of Obesity and Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2014, 99, E217–E225. [Google Scholar] [CrossRef]
- Elks, C.M.; Zhao, P.; Grant, R.W.; Hang, H.; Bailey, J.L.; Burk, D.H.; McNulty, M.A.; Mynatt, R.L.; Stephens, J.M. Loss of Oncostatin M Signaling in Adipocytes Induces Insulin Resistance and Adipose Tissue Inflammation in Vivo. J. Biol. Chem. 2016, 291, 17066–17076. [Google Scholar] [CrossRef]
- Stephens, J.M.; Bailey, J.L.; Hang, H.; Rittell, V.; Dietrich, M.A.; Mynatt, R.L.; Elks, C.M. Adipose Tissue Dysfunction Occurs Independently of Obesity in Adipocyte-Specific Oncostatin Receptor Knockout Mice. Obesity 2018, 26, 1439–1447. [Google Scholar] [CrossRef]
- Fain, J.N.; Madan, A.K.; Hiler, M.L.; Cheema, P.; Bahouth, S.W. Comparison of the Release of Adipokines by Adipose Tissue, Adipose Tissue Matrix, and Adipocytes from Visceral and Subcutaneous Abdominal Adipose Tissues of Obese Humans. Endocrinology 2004, 145, 2273–2282. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose Expression of Tumor Necrosis Factor-α: Direct Role in Obesity-Linked Insulin Resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S.; Arner, P.; Caro, J.F.; Atkinson, R.L.; Spiegelman, B.M. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J. Clin. Investig. 1995, 95, 2409–2415. [Google Scholar] [CrossRef]
- Schetz, M.; De Jong, A.; Deane, A.M.; Druml, W.; Hemelaar, P.; Pelosi, P.; Pickkers, P.; Reintam-Blaser, A.; Roberts, J.; Sakr, Y.; et al. Obesity in the critically ill: A narrative review. Intensiv. Care Med. 2019, 45, 757–769. [Google Scholar] [CrossRef] [PubMed]
- Pogodziński, D.; Ostrowska, L.; Smarkusz-Zarzecka, J.; Zyśk, B. Secretome of Adipose Tissue as the Key to Understanding the Endocrine Function of Adipose Tissue. Int. J. Mol. Sci. 2022, 23, 2309. [Google Scholar] [CrossRef] [PubMed]
- Bobbert, T.; Wegewitz, U.; Brechtel, L.; Freudenberg, M.; Mai, K.; Möhlig, M.; Diederich, S.; Ristow, M.; Rochlitz, H.; Pfeiffer, A.; et al. Adiponectin Oligomers in Human Serum during Acute and Chronic Exercise: Relation to Lipid Metabolism and Insulin Sensitivity. Int. J. Sports Med. 2007, 28, 1–8. [Google Scholar] [CrossRef]
- Bouassida, A.; Lakhdar, N.; Benaissa, N.; Mejri, S.; Zaouali, M.; Zbidi, A.; Tabka, Z. Adiponectin responses to acute moderate and heavy exercises in overweight middle aged subjects. J. Sports Med. Phys. Fit. 2010, 50, 330–335. [Google Scholar]
- Numao, S.; Katayama, Y.; Hayashi, Y.; Matsuo, T.; Tanaka, K. Influence of acute aerobic exercise on adiponectin oligomer concentrations in middle-aged abdominally obese men. Metabolism 2011, 60, 186–194. [Google Scholar] [CrossRef]
- Fatouros, I.G.; Tournis, S.; Leontsini, D.; Jamurtas, A.Z.; Sxina, M.; Thomakos, P.; Manousaki, M.; Douroudos, I.; Taxildaris, K.; Mitrakou, A. Leptin and Adiponectin Responses in Overweight Inactive Elderly following Resistance Training and Detraining Are Intensity Related. J. Clin. Endocrinol. Metab. 2005, 90, 5970–5977. [Google Scholar] [CrossRef]
- Elias, A.; Pandian, M.; Wang, L.; Suarez, E.; James, N.; Wilson, A. Leptin and IGF-I levels in unconditioned male volunteers after short-term exercise. Psychoneuroendocrinology 2000, 25, 453–461. [Google Scholar] [CrossRef]
- Fisher, J.S.; Van Pelt, R.; Zinder, O.; Landt, M.; Kohrt, W.M. Acute exercise effect on postabsorptive serum leptin. J. Appl. Physiol. 2001, 91, 680–686. [Google Scholar] [CrossRef]
- Zhang, M.H.; Na, B.; Schiller, N.B.; Whooley, M.A. Resistin, exercise capacity, and inducible ischemia in patients with stable coronary heart disease: Data from the Heart and Soul study. Atherosclerosis 2010, 213, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Jamurtas, A.Z.; Theocharis, V.; Koukoulis, G.; Stakias, N.; Fatouros, I.G.; Kouretas, D.; Koutedakis, Y. The effects of acute exercise on serum adiponectin and resistin levels and their relation to insulin sensitivity in overweight males. Eur. J. Appl. Physiol. 2006, 97, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Højbjerre, L.; Rosenzweig, M.; Dela, F.; Bruun, J.; Stallknecht, B.M.; Verkauskiene, R.; Beltrand, J.; Claris, O.; Chevenne, D.; Deghmoun, S.; et al. Acute exercise increases adipose tissue interstitial adiponectin concentration in healthy overweight and lean subjects. Eur. J. Endocrinol. 2007, 157, 613–623. [Google Scholar] [CrossRef]
- Hansen, J.; Brandt, C.; Nielsen, A.R.; Hojman, P.; Whitham, M.; Febbraio, M.A.; Pedersen, B.K.; Plomgaard, P. Exercise Induces a Marked Increase in Plasma Follistatin: Evidence That Follistatin Is a Contraction-Induced Hepatokine. Endocrinology 2011, 152, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, M.; Schober-Halper, B.; Oesen, S.; Franzke, B.; Tschan, H.; Bachl, N.; Strasser, E.-M.; Quittan, M.; Wagner, K.-H.; Wessner, B. Effects of elastic band resistance training and nutritional supplementation on muscle quality and circulating muscle growth and degradation factors of institutionalized elderly women: The Vienna Active Ageing Study (VAAS). Eur. J. Appl. Physiol. 2016, 116, 885–897. [Google Scholar] [CrossRef]
- Hwang, J.H.; McGovern, J.; Minett, G.M.; Della Gatta, P.A.; Roberts, L.; Harris, J.M.; Thompson, E.W.; Parker, T.J.; Peake, J.M.; Neubauer, O. Mobilizing serum factors and immune cells through exercise to counteract age-related changes in cancer risk. Exerc. Immunol. Rev. 2020, 26, 80–99. [Google Scholar]
- Hojman, P.; Dethlefsen, C.; Brandt, C.; Hansen, J.; Pedersen, L.; Pedersen, B.K. Exercise-induced muscle-derived cytokines inhibit mammary cancer cell growth. Am. J. Physiol. Endocrinol. Metab. 2011, 301, 504–510. [Google Scholar] [CrossRef]
- Fischer, C.P. Interleukin-6 in Acute Exercise and Training: What Is the Biological Relevance? Exerc. Immunol. Rev. 2006, 12, 6–33. [Google Scholar]
- Papanicolaou, D.A.; Petrides, J.S.; Tsigos, C.; Bina, S.; Kalogeras, K.T.; Wilder, R.; Gold, P.W.; Deuster, P.; Chrousos, G.P. Exercise stimulates interleukin-6 secretion: Inhibition by glucocorticoids and correlation with catecholamines. Am. J. Physiol. Metab. 1996, 271, E601–E605. [Google Scholar] [CrossRef]
- Orban, Z.; Remaley, A.T.; Sampson, M.; Trajanoski, Z.; Chrousos, G.P. The Differential Effect of Food Intake and β-Adrenergic Stimulation on Adipose-Derived Hormones and Cytokines in Man. J. Clin. Endocrinol. Metab. 1999, 84, 2126–2133. [Google Scholar] [CrossRef]
- Lyngsø, D.; Simonsen, L.; Bülow, J. Interleukin-6 production in human subcutaneous abdominal adipose tissue: The effect of exercise. J. Physiol. 2002, 543, 373–378. [Google Scholar] [CrossRef]
- Sarkar, S.; Karmakar, S.C.; Dey, S.K. Comparison of body composition, physical fitness parameters and skeletal muscle damage indices among young Indian male soccer & hockey players. Balt. J. Health Phys. Act. 2019, 11, 1–10. [Google Scholar] [CrossRef]
- Maciejewska-Skrendo, A.; Mieszkowski, J.; Kochanowicz, A.; Stankiewicz, B.; Cieszczyk, P.; Switala, K.; Gomes de Assis, G.; Kecler, K.; Tarnowski, M.; Sawczuk, M. TNFA expression level changes observed in response to the Wingate Anaerobic Test in non-trained and trained individuals. Balt. J. Health Phys. Act. 2019, 11, 1–9. [Google Scholar] [CrossRef]
- Gallagher, D.; Heymsfield, S.B.; Heo, M.; Jebb, S.A.; Murgatroyd, P.R.; Sakamoto, Y. Healthy percentage body fat ranges: An approach for developing guidelines based on body mass index. Am. J. Clin. Nutr. 2000, 72, 694–701. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Obesity: Preventing and Managing the Global Epidemic. In Report of a WHO Consultation on Obesity, Geneva, 3–5 June 1997; World Health Organization: Geneva, Switzerland, 1998. [Google Scholar]
- Skurk, T.; Alberti-Huber, C.; Herder, C.; Hauner, H. Relationship between Adipocyte Size and Adipokine Expression and Secretion. J. Clin. Endocrinol. Metab. 2007, 92, 1023–1033. [Google Scholar] [CrossRef]
- Konigorski, S.; Janke, J.; Drogan, D.; Bergmann, M.M.; Hierholzer, J.; Kaaks, R.; Boeing, H.; Pischon, T. Prediction of Circulating Adipokine Levels Based on Body Fat Compartments and Adipose Tissue Gene Expression. Obes. Facts 2019, 12, 590–605. [Google Scholar] [CrossRef]
- Humińska-Lisowska, K.; Mieszkowski, J.; Kochanowicz, A.; Stankiewicz, B.; Niespodziński, B.; Brzezińska, P.; Ficek, K.; Kemerytė-Ivanauskienė, E.; Cięszczyk, P. cfDNA Changes in Maximal Exercises as a Sport Adaptation Predictor. Genes 2021, 12, 1238. [Google Scholar] [CrossRef] [PubMed]
- Kochanowicz, A.; Sawczyn, S.; Niespodziński, B.; Mieszkowski, J.; Kochanowicz, K.; Żychowska, M. Cellular Stress Response Gene Expression during Upper and Lower Body High Intensity Exercises. PLoS ONE 2017, 12, e0171247. [Google Scholar] [CrossRef]
- Bar-Or, O. The Wingate Anaerobic Test. An Update on Methodology, Reliability and Validity. Sports Med. 1987, 4, 381–394. [Google Scholar] [CrossRef]
- Mieszkowski, J.; Borkowska, A.; Stankiewicz, B.; Kochanowicz, A.; Niespodziński, B.; Surmiak, M.; Waldziński, T.; Rola, R.; Petr, M.; Antosiewicz, J. Single High-Dose Vitamin D Supplementation as an Approach for Reducing Ultramarathon-Induced Inflammation: A Double-Blind Randomized Controlled Trial. Nutrients 2021, 13, 1280. [Google Scholar] [CrossRef] [PubMed]
- Bouassida, A.; Chamari, K.; Zaouali, M.; Feki, Y.; Zbidi, A.; Tabka, Z. Review on leptin and adiponectin responses and adaptations to acute and chronic exercise. Br. J. Sports Med. 2010, 44, 620–630. [Google Scholar] [CrossRef] [PubMed]
- Saeidi, A.; Haghighi, M.M.; Kolahdouzi, S.; Daraei, A.; Ben Abderrahmane, A.; Essop, M.F.; Laher, I.; Hackney, A.C.; Zouhal, H. The effects of physical activity on adipokines in individuals with overweight/obesity across the lifespan: A narrative review. Obes. Rev. 2021, 22, e13090. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.; Ruan, Y.; Gao, X.; Sun, J. Systematic Review and Meta-Analysis of Randomized, Controlled Trials on the Effect of Exercise on Serum Leptin and Adiponectin in Overweight and Obese Individuals. Horm. Metab. Res. 2017, 49, 164–173. [Google Scholar] [CrossRef]
- Cruz, I.S.; Rosa, G.; Valle, V.; De Mello, D.B.; Fortes, M.; Dantas, E.H. Acute Effects of Concurrent Training on Serum Leptin and Cortisol in Overweighed Young Adults. Rev. Bras. Med. Esporte 2012, 18, 81–86. [Google Scholar] [CrossRef]
- Weltman, A.; Pritzlaff, C.J.; Wideman, L.; Considine, R.V.; Fryburg, D.A.; Gutgesell, M.E.; Hartman, M.L.; Veldhuis, J.D. Intensity of acute exercise does not affect serum leptin concentrations in young men. Med. Sci. Sports Exerc. 2000, 32, 1556–1561. [Google Scholar] [CrossRef]
- Racette, S.B.; Coppack, S.W.; Landt, M.; Klein, S. Leptin Production during Moderate-Intensity Aerobic Exercise. J. Clin. Endocrinol. Metab. 1997, 82, 2275–2277. [Google Scholar] [CrossRef]
- Middelbeek, R.J.W.; Motiani, P.; Brandt, N.; Nigro, P.; Zheng, J.; Virtanen, K.A.; Kalliokoski, K.K.; Hannukainen, J.C.; Goodyear, L.J. Exercise intensity regulates cytokine and klotho responses in men. Nutr. Diabetes 2021, 11, 1–11. [Google Scholar] [CrossRef]
- Bilski, J.; Mazur-Bialy, A.I.; Surmiak, M.; Hubalewska-Mazgaj, M.; Pokorski, J.; Nitecki, J.; Nitecka, E.; Pokorska, J.; Targosz, A.; Ptak-Belowska, A.; et al. Effect of Acute Sprint Exercise on Myokines and Food Intake Hormones in Young Healthy Men. Int. J. Mol. Sci. 2020, 21, 8848. [Google Scholar] [CrossRef]
- Dimitrow, P.P.; Undas, A.; Cheng, T.O. Exercise modulates circulating adipokine levels in hypertrophic cardiomyopathy. Pol. Arch. Intern. Med. 2011, 121, 384–390. [Google Scholar] [CrossRef]
- Zaccaria, M.; Ermolao, A.; Brugin, E.; Bergamin, M. Plasma leptin and energy expenditure during prolonged, moderate intensity, treadmill exercise. J. Endocrinol. Investig. 2013, 36, 396–401. [Google Scholar] [CrossRef]
- Duclos, M.; Corcuff, J.-B.; Ruffie, A.; Roger, P.; Manier, G. Rapid leptin decrease in immediate post-exercise recovery. Clin. Endocrinol. 1999, 50, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Bilski, J.; Jaworek, J.; Pokorski, J.; Nitecki, J.; Nitecka, E.; Pokorska, J.; Mazur-Bialy, A.; Szklarczyk, J. Effects of time of day and the wingate test on appetite perceptions, food intake and plasma levels of adipokines. J. Physiol. Pharmacol. 2016, 67, 667–676. [Google Scholar]
- Guerra, B.; Olmedillas, H.; Guadalupe-Grau, A.; Ponce-González, J.G.; Morales-Alamo, D.; Fuentes, T.; Chapinal, E.; Fernández-Pérez, L.; De Pablos-Velasco, P.; Santana, A.; et al. Is sprint exercise a leptin signaling mimetic in human skeletal muscle? J. Appl. Physiol. 2011, 111, 715–725. [Google Scholar] [CrossRef]
- Vardar, S.A.; Karaca, A.; Güldiken, S.; Palabıyık, O.; Süt, N.; Demir, A.M. High-intensity interval training acutely alters plasma adipokine levels in young overweight/obese women. Arch. Physiol. Biochem. 2018, 124, 149–155. [Google Scholar] [CrossRef]
- Duzova, H.; Gullu, E.; Cicek, G.; Koksal, B.K.; Kayhan, B.; Gullu, A.; Sahin, I. The effect of exercise induced weight-loss on myokines and adipokines in overweight sedentary females: Steps-aerobics vs. jogging-walking exercises. J. Sports Med. Phys. Fit. 2018, 58, 295–308. [Google Scholar] [CrossRef]
- Horner, K.; Hopkins, M.; Finlayson, G.; Gibbons, C.; Brennan, L. Biomarkers of appetite: Is there a potential role for metabolomics? Nutr. Res. Rev. 2020, 33, 271–286. [Google Scholar] [CrossRef]
- Mendez-Gutierrez, A.; Aguilera, C.M.; Osuna-Prieto, F.J.; Martinez-Tellez, B.; Prados, M.C.R.; Acosta, F.M.; Llamas-Elvira, J.M.; Ruiz, J.R.; Sanchez-Delgado, G. Exercise-induced changes on exerkines that might influence brown adipose tissue metabolism in young sedentary adults. Eur. J. Sport Sci. 2022, 25, 1–12. [Google Scholar] [CrossRef]
- Essig, D.A.; Alderson, N.L.; Ferguson, M.A.; Bartoli, W.P.; Durstine, J.L. Delayed effects of exercise on the plasma leptin concentration. Metabolism 2000, 49, 395–399. [Google Scholar] [CrossRef]
- Nindl, B.C.; Kraemer, W.J.; Arciero, P.J.; Samatallee, N.; Leone, C.D.; Mayo, M.F.; Hafeman, D.L. Reduction after Resistance Exercise in Men. Med. Sci. Sports Exerc. 2002, 34, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Olive, J.L.; Miller, G.D. Differential effects of maximal- and moderate-intensity runs on plasma leptin in healthy trained subjects. Nutrition 2001, 17, 365–369. [Google Scholar] [CrossRef]
- Roupas, N.; Mamali, I.; Maragkos, S.; Leonidou, L.; Armeni, A.; Markantes, G.; Tsekouras, A.; Sakellaropoulos, G.; Markou, K.; Georgopoulos, N. The effect of prolonged aerobic exercise on serum adipokine levels during an ultra-marathon endurance race. Hormones 2013, 12, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.D.D.J.; Silva, D.d.S.; Pereira, E.V.M.; Pereira, D.D.; Fernandes, M.S.D.S.; Santos, D.F.C.; Oliveira, D.P.M.; Vieira-Souza, L.M.; Aidar, F.J.; de Souza, R.F. Changes in Cytokines Concentration Following Long-Distance Running: A Systematic Review and Meta-Analysis. Front. Physiol. 2022, 13, 203. [Google Scholar] [CrossRef]
- Gökbel, H.; Okudan, N.; Gül, I.; Belviranli, M.; Gergerlioğlu, H.S.; BaŞaral, M.K. Effects of Repeated Bouts of Supramaximal Exercise on Plasma Adiponectin, Interleukin-6, and Tumor Necrosis Factor-α Levels in Sedentary Men. J. Strength Cond. Res. 2012, 26, 1675–1679. [Google Scholar] [CrossRef]
- Galic, S.; Oakhill, J.S.; Steinberg, G.R. Adipose tissue as an endocrine organ. Mol. Cell. Endocrinol. 2010, 316, 129–139. [Google Scholar] [CrossRef]
- Vuolteenaho, K.; Leppänen, T.; Kekkonen, R.; Korpela, R.; Moilanen, E. Running a Marathon Induces Changes in Adipokine Levels and in Markers of Cartilage Degradation–Novel Role for Resistin. PLoS ONE 2014, 9, e110481. [Google Scholar] [CrossRef]
- Czajkowska, A.; Ambroszkiewicz, J.; Mróz, A.; Witek, K.; Nowicki, D.; Małek, Ł. The Effect of the Ultra-Marathon Run at a Distance of 100 Kilometers on the Concentration of Selected Adipokines in Adult Men. Int. J. Environ. Res. Public Health 2020, 17, 4289. [Google Scholar] [CrossRef]
- Steppan, C.M.; Lazar, M.A. Resistin and obesity-associated insulin resistance. Trends Endocrinol. Metab. 2002, 13, 18–23. [Google Scholar] [CrossRef]
- Jamaluddin, S.; Weakley, S.M.; Yao, Q.; Chen, C. Resistin: Functional roles and therapeutic considerations for cardiovascular disease. Br. J. Pharmacol. 2012, 165, 622–632. [Google Scholar] [CrossRef]
- Mieszkowski, J.; Stankiewicz, B.; Kochanowicz, A.; Niespodziński, B.; Borkowska, A.; Antosiewicz, J. Effect of Ischemic Preconditioning on Marathon-Induced Changes in Serum Exerkine Levels and Inflammation. Front. Physiol. 2020, 11, 571220. [Google Scholar] [CrossRef] [PubMed]
- Kon, M.; Ebi, Y.; Nakagaki, K. Effects of acute sprint interval exercise on follistatin-like 1 and apelin secretions. Arch. Physiol. Biochem. 2021, 127, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-S.; Wilson, R.L.; Taaffe, D.R.; Galvão, D.A.; Gray, E.; Newton, R.U. Myokine Expression and Tumor-Suppressive Effect of Serum after 12 wk of Exercise in Prostate Cancer Patients on ADT. Med. Sci. Sports Exerc. 2022, 54, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Coletta, A.M.; Agha, N.H.; Baker, F.L.; Niemiro, G.M.; Mylabathula, P.L.; Brewster, A.M.; Bevers, T.B.; Fuentes-Mattei, E.; Basen-Engquist, K.; Gilchrist, S.C.; et al. The impact of high-intensity interval exercise training on NK-cell function and circulating myokines for breast cancer prevention among women at high risk for breast cancer. Breast Cancer Res. Treat. 2021, 187, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Ellulu, M.S.; Patimah, I.; KhazáAi, H.; Rahmat, A.; Abed, Y. Obesity and inflammation: The linking mechanism and the complications. Arch. Med. Sci. 2017, 13, 851–863. [Google Scholar] [CrossRef]
- Lira, F.S.; Panissa, V.L.G.; Julio, U.; Franchini, E. Differences in metabolic and inflammatory responses in lower and upper body high-intensity intermittent exercise. Eur. J. Appl. Physiol. 2015, 115, 1467–1474. [Google Scholar] [CrossRef]
- Williams, C.B.; Zelt, J.G.; Castellani, L.N.; Little, J.P.; Jung, M.E.; Wright, D.C.; Tschakovsky, M.E.; Gurd, B.J. Changes in mechanisms proposed to mediate fat loss following an acute bout of high-intensity interval and endurance exercise. Appl. Physiol. Nutr. Metab. 2013, 1244, 1236–1244. [Google Scholar] [CrossRef]
- Starkie, R.L.; Arkinstall, M.J.; Koukoulas, I.; Hawley, J.; Febbraio, M.A. Carbohydrate ingestion attenuates the increase in plasma interleukin-6, but not skeletal muscle interleukin-6 mRNA, during exercise in humans. J. Physiol. 2001, 533, 585–591. [Google Scholar] [CrossRef]
- Pedersen, B.K. Muscular Interleukin-6 and Its Role as an Energy Sensor. Med. Sci. Sports Exerc. 2012, 44, 392–396. [Google Scholar] [CrossRef]
- Antosiewicz, J.; Kaczor, J.J.; Kasprowicz, K.; Laskowski, R.; Kujach, S.; Luszczyk, M.; Radziminski, L.; Ziemann, E. Repeated “all out” interval exercise causes an increase in serum hepcidin concentration in both trained and untrained men. Cell. Immunol. 2013, 283, 12–17. [Google Scholar] [CrossRef]
- Cerqueira, É.; Marinho, D.A.; Neiva, H.P.; Lourenço, O. Inflammatory Effects of High and Moderate Intensity Exercise—A Systematic Review. Front. Physiol. 2020, 10, 1550. [Google Scholar] [CrossRef] [PubMed]
- Metsios, G.S.; Moe, R.H.; Kitas, G.D. Exercise and Inflammation. Best Pract. Res. Clin. Rheumatol. 2020, 34, 101504. [Google Scholar] [CrossRef] [PubMed]
- Scheffer, D.D.L.; Latini, A. Exercise-induced immune system response: Anti-inflammatory status on peripheral and central organs. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165823. [Google Scholar] [CrossRef] [PubMed]
- Blanca, M.J.; Alarcón, R.; Arnau, J.; Bono, R.; Bendayan, R. Effect of variance ratio on ANOVA robustness: Might 1.5 be the limit? Behav. Res. Methods 2018, 50, 937–962. [Google Scholar] [CrossRef] [PubMed]
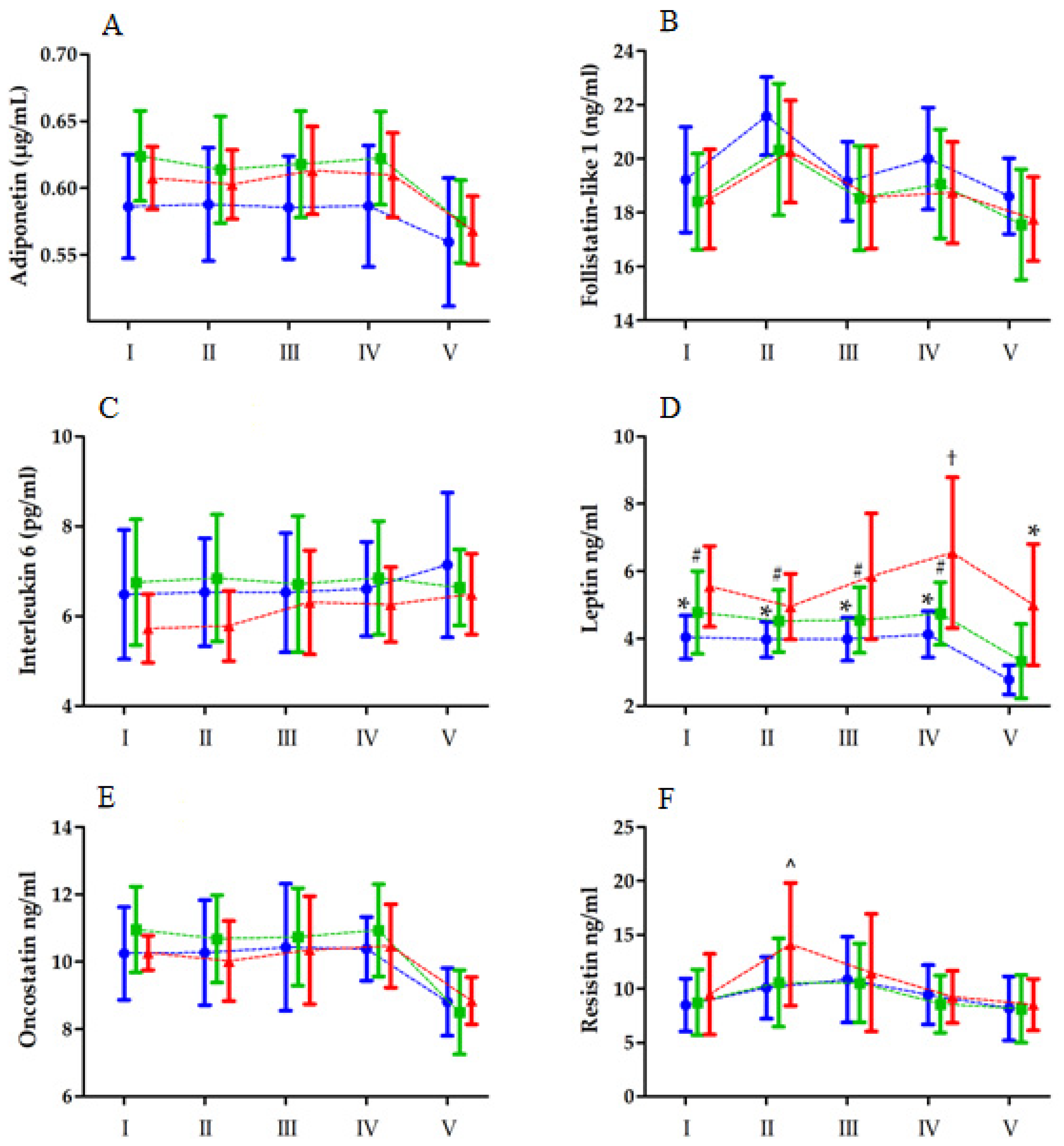
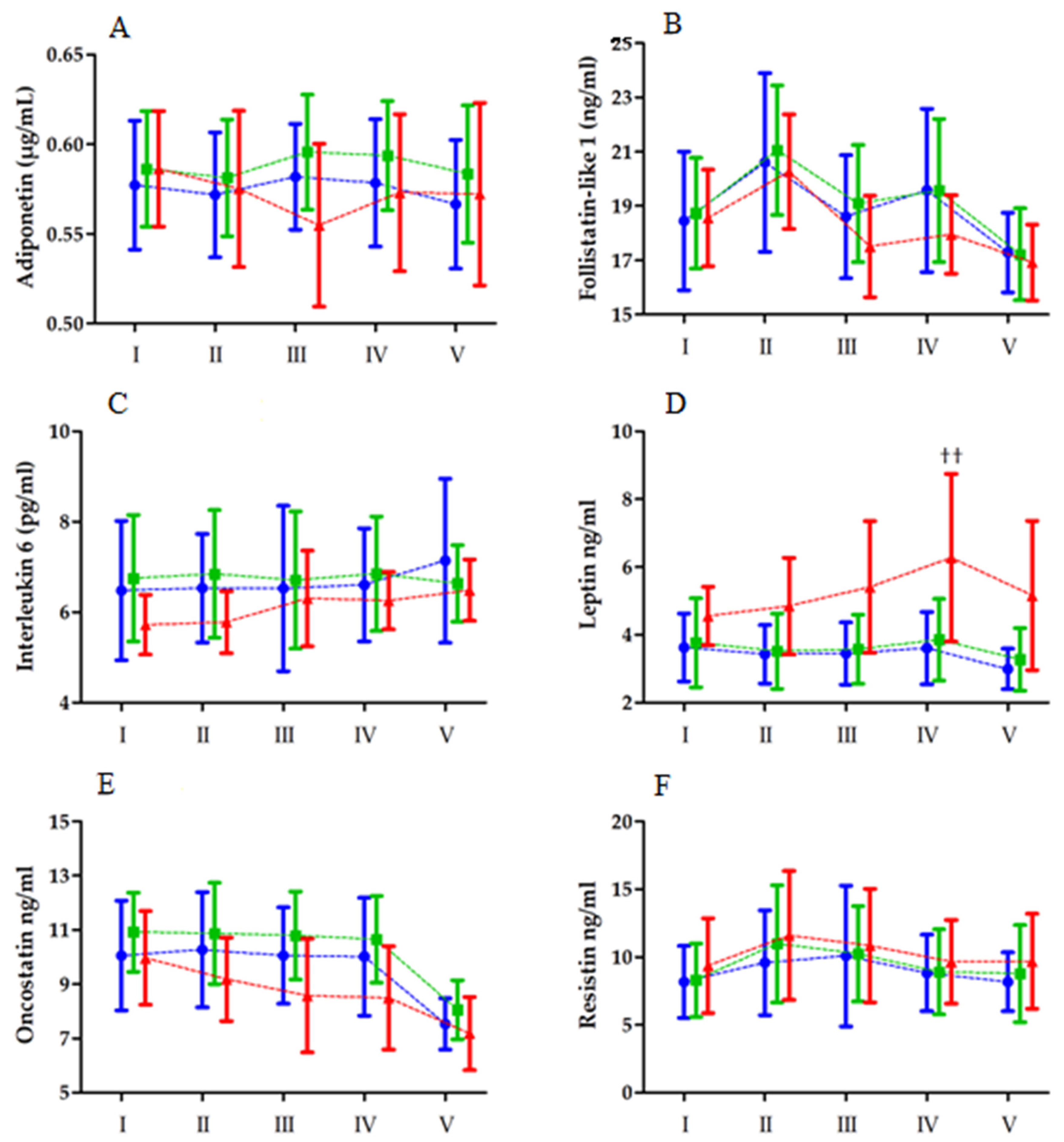
| Variables | Unit | LBF (n = 16) | MBF (n = 19) | HBF (n = 13) |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | ||
| Height | cm | 181.60 ± 3.85 | 179.23 ± 7.27 | 182.26 ± 7.23 |
| Weight | kg | 74.04 ± 8.03 | 76.20 ± 9.11 | 85.37 ± 11.84 *# |
| BMI | kg/m2 | 22.47 ± 2.58 | 23.89 ± 2.33 | 25.61 ± 2.28 * |
| Skeletal muscle mass | kg | 39.87 ± 4.80 | 39.13 ± 4.66 | 40.12 ± 5.32 |
| Body fat mass | kg | 4.56 ± 1.21 | 8.51 ± 1.80 * | 15.06 ± 4.74 *# |
| Percent body fat | % | 6.15 ± 1.48 | 11.12 ± 1.89 * | 17.46 ± 3.86 *# |
| Variables | Unit | LBF (n = 16) | MBF (n = 19) | HBF (n = 13) |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | ||
| Total cholesterol | mg/dL | 137.00 ± 20.71 | 156.04 ± 31.05 * | 156.31 ± 21.50 * |
| High-density lipoprotein (HDL) cholesterol | mg/dL | 49.47 ± 8.49 | 54.79 ± 16.13 | 46.75 ± 7.31 |
| Low-density lipoprotein (LDL) cholesterol | mg/dL | 75.17 ± 16.81 | 86.08 ± 23.14 | 93.75 ± 20.27 * |
| Cholesterol non-HDL | mg/dL | 88.35 ± 18.65 | 98.20 ± 25.61 | 109.68 ± 24.48 * |
| Triglycerides | mg/dL | 60.35 ± 17.94 | 65.70 ± 18.66 | 78.93 ± 35.53 |
| Variables | Unit | LBF (n = 16) | MBF (n = 19) | HBF (n = 13) |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | ||
| Maximal anaerobic effort | ||||
| Relative peak power of the 1st WAnT | W/kg | 10.47 ± 0.99 | 10.57 ± 0.99 | 9.75 ± 0.94 |
| Relative mean power of the 1st WAnT | W/kg | 8.24 ± 0.72 | 8.40 ± 0.66 | 7.87 ± 0.48 |
| Relative peak power of the 2nd WAnT | W/kg | 7.58 ± 0.83 | 7.79 ± 0.70 | 7.56 ± 0.67 |
| Relative mean power of the 2nd WAnT | W/kg | 5.98 ± 0.60 | 5.97 ± 0.54 | 5.53 ± 0.54 |
| Maximal aerobic effort | ||||
| Maximal ventilation | L/min | 148.80 ± 21.83 | 157.41 ± 18.33 | 146.55 ± 26.54 |
| Maximal oxygen uptake | ml/min/kg | 57.72 ± 7.83 | 59.25 ± 5.17 | 55.28 ± 7.97 |
| Maximal heart rate | beats/min | 190.40 ± 9.74 | 188.25 ± 15.55 | 191.33 ± 9.71 |
| Variable | Effect | F | df | p-Value | Effect Size (η2) | Post Hoc Outcome |
|---|---|---|---|---|---|---|
| Adiponectin | Group RM Group × RM | 3. 85 30.64 1.24 | 2, 45 4, 180 8, 180 | 0.02 * <0.01 ** 0.28 | 0.14 0.40 0.05 | LBF < MBF V < I, II, III, IV |
| Follistatin-like 1 | Group RM Group × RM | 1.74 39.63 0.41 | 2, 45 4, 180 8, 180 | 0.18 <0.01 ** 0.91 | 0.07 0.46 0.01 | II > I, III, IV, V; V < I, III, IV |
| Interleukin 6 | Group RM Group × RM | 1.45 1.91 0.88 | 2, 45 4, 180 8, 180 | 0.25 0.31 0.53 | 0.05 0.02 0.03 | |
| Leptin | Group RM Group × RM | 7.22 21.20 2.20 | 2, 45 4, 180 8, 180 | <0.01 ** <0.01 ** 0.02 * | 0.24 0.32 0.10 | LBF < HBF IV < I, II, III, IV; IV > II I-IVLBF > VLBF I-IVMBF > VMBF IIHBF < IVHBF IVLBF < IVHBF VLBF < VHBF |
| Oncostatin | Group RM Group × RM | 0.65 34.22 1.32 | 2, 45 4, 180 8, 180 | 0.52 <0.05 * 0.24 | 0.02 0.43 0.05 | V < I, III, IV |
| Resistin | Group RM Group × RM | 0.66 24.18 2.56 | 2, 45 4, 180 8, 180 | 0.52 <0.01 ** 0.01 * | 0.02 0.34 0.10 | II > I, IV, V; V < III IHBF, IVHBF, VHBF < IIHBF |
| Variable | Effect | F | df | p-Value | Effect Size (η2) | Post Hoc Outcome |
|---|---|---|---|---|---|---|
| Adiponectin | Group RM Group × RM | 1.12 0.77 1.82 | 2, 45 4, 180 8, 180 | 0.33 0.54 0.07 | 0.05 0.01 0.08 | |
| Follistatin-like 1 | Group RM Group × RM | 0.54 28.17 0.84 | 2, 45 4, 180 8, 180 | 0.58 <0.01 ** 0.57 | 0.03 0.41 0.04 | II > V > I, III, IV |
| Interleukin 6 | Group BTT Group × RM | 3.77 1.92 1.66 | 2, 45 4, 180 8, 180 | 0.04 * 0.11 0.53 | 0.05 0.02 0.03 | MBF > HBF |
| Leptin | Group RM Group × RM | 6.32 9.18 2.26 | 2, 45 4, 180 8, 180 | <0.01 ** <0.01 ** 0.02 * | 0.24 0.19 0.10 | LBF, MBF < HBF V < III, IV; IV > II IHBF, IIHBF, IVLBF, IVMBF < IVHBF |
| Oncostatin | Group RM Group × RM | 3.36 6.62 1.25 | 2, 45 4, 180 8, 180 | 0.05 * <0.01 ** 0.27 | 0.13 0.14 0.05 | MBF > HBF V < I, II, III, IV |
| Resistin | Group RM Group × RM | 0.39 12.53 0.58 | 2, 45 4, 180 8, 180 | 0.67 <0.01 ** 0.78 | 0.02 0.23 0.10 | I, IV, V < II, III |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Humińska-Lisowska, K.; Mieszkowski, J.; Kochanowicz, A.; Bojarczuk, A.; Niespodziński, B.; Brzezińska, P.; Stankiewicz, B.; Michałowska-Sawczyn, M.; Grzywacz, A.; Petr, M.; et al. Implications of Adipose Tissue Content for Changes in Serum Levels of Exercise-Induced Adipokines: A Quasi-Experimental Study. Int. J. Environ. Res. Public Health 2022, 19, 8782. https://doi.org/10.3390/ijerph19148782
Humińska-Lisowska K, Mieszkowski J, Kochanowicz A, Bojarczuk A, Niespodziński B, Brzezińska P, Stankiewicz B, Michałowska-Sawczyn M, Grzywacz A, Petr M, et al. Implications of Adipose Tissue Content for Changes in Serum Levels of Exercise-Induced Adipokines: A Quasi-Experimental Study. International Journal of Environmental Research and Public Health. 2022; 19(14):8782. https://doi.org/10.3390/ijerph19148782
Chicago/Turabian StyleHumińska-Lisowska, Kinga, Jan Mieszkowski, Andrzej Kochanowicz, Aleksandra Bojarczuk, Bartłomiej Niespodziński, Paulina Brzezińska, Błażej Stankiewicz, Monika Michałowska-Sawczyn, Anna Grzywacz, Miroslav Petr, and et al. 2022. "Implications of Adipose Tissue Content for Changes in Serum Levels of Exercise-Induced Adipokines: A Quasi-Experimental Study" International Journal of Environmental Research and Public Health 19, no. 14: 8782. https://doi.org/10.3390/ijerph19148782
APA StyleHumińska-Lisowska, K., Mieszkowski, J., Kochanowicz, A., Bojarczuk, A., Niespodziński, B., Brzezińska, P., Stankiewicz, B., Michałowska-Sawczyn, M., Grzywacz, A., Petr, M., & Cięszczyk, P. (2022). Implications of Adipose Tissue Content for Changes in Serum Levels of Exercise-Induced Adipokines: A Quasi-Experimental Study. International Journal of Environmental Research and Public Health, 19(14), 8782. https://doi.org/10.3390/ijerph19148782









