Succession of Microbial Community during the Co-Composting of Food Waste Digestate and Garden Waste
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Source and Composting Operation
2.2. Analytical Methods
2.3. Microbial Community Analysis
2.3.1. Sampling, DNA Extraction and High-Throughput Sequencing
2.3.2. Bioinformatics and Statistical Analyses
3. Results and Discussion
3.1. Evolution of Physicochemical Properties
3.2. Microbial Community Profile in Composting Systems
3.2.1. Bacterial Composition and Diversity
3.2.2. Fungal Composition and Diversity
3.3. Potential Mechanisms of the Co-Composting Efficiency
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, N.; Huang, D.; Shao, M.; Sun, R.; Xu, Q. Use of activated carbon to reduce ammonia emissions and accelerate humification in composting digestate from food waste. Bioresour. Technol. 2022, 347, 126701. [Google Scholar] [CrossRef]
- Jin, C.; Sun, S.; Yang, D.; Sheng, W.; Ma, Y.; He, W.; Li, G. Anaerobic digestion: An alternative resource treatment option for food waste in China. Sci. Total Environ. 2021, 779, 146397. [Google Scholar] [CrossRef]
- Sharma, P.; Gaur, V.K.; Kim, S.-H.; Pandey, A. Microbial strategies for bio-transforming food waste into resources. Bioresour. Technol. 2020, 299, 122580. [Google Scholar] [CrossRef]
- UNEP. Food Waste Index Report 2021; UNEP: Nairobi, Kenya, 2021. [Google Scholar]
- Åsa, S.; Jensen, C.; Quested, T.; Moates, G. Estimates of European Food Waste Levels; IVL Swedish Environmental Research Institute: Stockholm, Sweden, 2016. [Google Scholar]
- Gunders, D. Waste-Free Kitchen Handbook: A Guide to Eating Well and Saving Money by Wasting Less Food; Chronicle Books: San Francisco, CA, USA, 2015. [Google Scholar]
- Li, Y.; Jin, Y.; Borrion, A.; Li, H. Current status of food waste generation and management in China. Bioresour. Technol. 2019, 273, 654–665. [Google Scholar] [CrossRef]
- Feo, G.; Ferrara, C.; Iannone, V.; Parente, P. Improving the efficacy ofmunicipal solid waste collection with a communicative approach based on easily understandable indicators. Sci. Total Environ. 2019, 651, 2380–2390. [Google Scholar] [CrossRef] [PubMed]
- lntergovernmental Panel on Climate Change (IPCC). Climate Change and Land; An IPCC Special Report on Climate Change, Deser Tification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems; IPCC: Geneva, Switzerland, 2020. [Google Scholar]
- Friedlingstein, P.; Jones, M.W.; O’sullivan, M.; Andrew, R.M.; Hauck, J.; Peters, G.P.; Peters, W.; Pongratz, J.; Sitch, S.; Le Quéré, C.; et al. Global Carbon Budget 2019. Earth Syst. Sci. Data 2019, 11, 1783–1838. [Google Scholar] [CrossRef]
- Liu, M.; Ogunmoroti, A.; Liu, W.; Li, M.; Bi, M.; Liu, W.; Cui, Z. Assessment and projection of environmental impacts of food waste treatment in China from life cycle perspectives. Sci. Total Environ. 2022, 807, 150751. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Meng, S.; Chen, G.; Yan, B.; Zhang, Y.; Tao, J.; Li, Y.; Li, J. Co-digestion of garden waste, food waste, and tofu residue: Effects of mixing ratio on methane production and microbial community structure. J. Environ. Chem. Eng. 2021, 9, 105901. [Google Scholar] [CrossRef]
- Xu, Z.; Ma, Y.; Zhang, L.; Han, Y.; Yuan, J.; Li, G.; Luo, W. Relating bacterial dynamics and functions to gaseous emissions during composting of kitchen and garden wastes. Sci. Total Environ. 2021, 767, 144210. [Google Scholar] [CrossRef] [PubMed]
- Qiang, Y.; Chen, L.; Wang, W.; Wang, Q.; Bai, R.; Zhuang, X.; Guo, Y.; Qi, W.; Yuan, Z. Impact of blending on hydrolysis and ethanol fermentation of garden wastes. J. Clean. Prod. 2018, 190, 36–43. [Google Scholar]
- Kim, E.; Lee, J.; Han, G.; Hwang, S. Comprehensive analysis of microbial communities in full-scale mesophilic and thermophilic anaerobic digesters treating food waste-recycling wastewater. Bioresour. Technol. 2018, 259, 442–450. [Google Scholar] [CrossRef]
- Troschinetz, A.M.; Mihelcic, J.R. Sustainable recycling of municipal solid waste in developing countries. Waste Manag. 2009, 29, 915–923. [Google Scholar] [CrossRef]
- Ravindran, B.; Nguyen, D.D.; Chaudhary, D.K.; Chang, S.W.; Kim, J.; Lee, S.-R.; Shin, J.; Jeon, B.-H.; Chung, S.; Lee, J. Influence of biochar on physico-chemical and microbial community during swine manure composting process. J. Environ. Manag. 2019, 232, 592–599. [Google Scholar] [CrossRef]
- Wei, H.; Wang, L.; Hassan, M.; Xie, B. Succession of the functional microbial communities and the metabolic functions in maize straw composting process. Bioresour. Technol. 2018, 256, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, Y.; Peng, Y.; Mironov, V.; Chen, J.; Jin, H.; Zhang, S. Feasibility of sewage sludge and food waste aerobic co-composting: Physicochemical properties, microbial community structures, and contradiction between microbial metabolic activity and safety risks. Sci. Total Environ. 2022, 825, 154047. [Google Scholar] [CrossRef] [PubMed]
- Manu, M.K.; Wang, C.; Li, D.; Varjani, S.; Xu, Y.; Ladumor, N.; Lui, M.; Zhou, J.; Wong, J.W. Biodegradation kinetics of ammonium enriched food waste digestate compost with biochar amendment. Bioresour. Technol. 2021, 341, 125871. [Google Scholar] [CrossRef] [PubMed]
- Arab, G.; Razaviarani, V.; Sheng, Z.; Liu, Y.; McCartney, D. Benefits to decomposition rates when using digestate as compost co-feedstock: Part II—Focus on microbial community dynamics. Waste Manag. 2017, 68, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Agriculture and Rural Affairs of the People’s Republic of China. Organic Fertilizer; Standards Press of China: Beijing, China, 2021. [Google Scholar]
- Song, C.; Li, M.; Jia, X.; Wei, Z.; Zhao, Y.; Xi, B.; Zhu, C.; Liu, D. Comparison of bacterial community structure and dynamics during the thermophilic composting of different types of solid wastes: Anaerobic digestion residue, pig manure and chicken manure. Microb. Biotechnol. 2014, 7, 424–433. [Google Scholar] [CrossRef]
- Yu, Z.; Morrison, M. Comparisons of different hypervariable regions of rrs genes for use in fingerprinting of microbial communities by PCR-Denaturing Gradient Gel Electrophoresis. Appl. Environ. Microbiol. 2004, 70, 4800–4806. [Google Scholar] [CrossRef]
- Manu, M.K.; Kumar, R.; Garg, A. Decentralized composting of household wet biodegradable waste in plastic drums: Effect of waste turning, microbial inoculum and bulking agent on product quality. J. Clean. Prod. 2019, 226, 233–241. [Google Scholar] [CrossRef]
- Song, B.; Manu, M.; Li, D.; Wang, C.; Varjani, S.; Ladumor, N.; Michael, L.; Xu, Y.; Wong, J.W. Food waste digestate composting: Feedstock optimization with sawdust and mature compost. Bioresour. Technol. 2021, 341, 125759. [Google Scholar] [CrossRef]
- Xie, D.; Gao, M.; Yang, M.; Xu, M.; Meng, J.; Wu, C.; Wang, Q.; Liu, S.; Sun, X. Composting—A solution of eliminating a nitrite-rich wastewater by reusing it as a moisture conditioning agent. Chemosphere 2021, 284, 131365. [Google Scholar] [CrossRef] [PubMed]
- HKORC. Compost and Soil Conditioner Quality Standards; Hong Kong Organic Resource Centre: Hongkong, China, 2021. [Google Scholar]
- AS 4454-2012; Composts, Soil Conditioners and Mulches. Standards Australia: Sydney, Australia, 2012.
- Liao, P.H.; Jones, L.; Lau, A.K.; Walkemeyer, S.; Egan, B.; Holbek, N. Composting of fish wastes in a full-scale in vessel system. Bioresour. Technol. 1997, 59, 163–168. [Google Scholar] [CrossRef]
- Petric, I.; Helic, A.; Avdic, E.A. Evolution of process parameters and determination of kinetics for co-composting of organic fraction of municipal solid waste with poultry manure. Bioresour. Technol. 2012, 117, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Selvam, A.; Wong, J.W. Influence of lime on struvite formation and nitrogen conservation during food waste composting. Bioresour. Technol. 2016, 217, 227–232. [Google Scholar] [CrossRef]
- Wang, T.-T.; Wang, S.; Zhong, X.-Z.; Sun, Z.-Y.; Huang, Y.-L.; Tan, L.; Tang, Y.-Q.; Kida, K. Converting digested residue eluted from dry anaerobic digestion of distilled grain waste into value-added fertilizer by aerobic composting. J. Clean. Prod. 2017, 166, 530–536. [Google Scholar] [CrossRef]
- Wang, K.; Mao, H.; Li, X. Functional characteristics and influence factors of microbial community in sewage sludge composting with inorganic bulking agent. Bioresour. Technol. 2018, 249, 527–535. [Google Scholar] [CrossRef]
- De Gannes, V.; Eudoxie, G.; Hickey, W.J. Prokaryotic successions and diversity in composts as revealed by 454-pyrosequencing. Bioresour. Technol. 2013, 133, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Xu, X.; Qu, J.; Zhu, L.; Wang, T. Evaluation of microbial population dynamics in the co-composting of cow manure and rice straw using high throughput sequencing analysis. World J. Microbiol. Biotechnol. 2016, 32, 101. [Google Scholar] [CrossRef] [PubMed]
- Akyol, C.; Ince, O.; Ince, B. Ince, Crop-based composting of lignocellulosic digestates: Focus on bacterial and fungal diversity. Bioresour. Technol. 2019, 288, 121549. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ma, H.; Zhang, H.; Xun, L.; Chen, G.; Wang, L. Thermomyces lanuginosus is the dominant fungus in maize straw composts. Bioresour. Technol. 2015, 197, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Pan, Y.; Yang, X.; Liu, J.; Miao, H.; Ren, Y.; Zhang, C.; Yan, G.; Lv, J.; Li, Y. Beneficial influences of pelelith and dicyandiamide on gaseous emissions and the fungal community during sewage sludge composting. Environ. Sci. Pollut. Res. 2019, 26, 8928–8938. [Google Scholar] [CrossRef]
- Greiner, K.; Peršoh, D.; Weig, A.; Rambold, G. Phialosimplex salinarum, a new species of Eurotiomycetes from a hypersaline habitat. IMA Fungus 2014, 5, 161–172. [Google Scholar] [CrossRef]
- Xiao, Y.; Zeng, G.-M.; Yang, Z.-H.; Shi, W.-J.; Huang, C.; Fan, C.-Z.; Xu, Z.-Y. Continuous thermophilic composting (CTC) for rapid biodegradation and maturation of organic municipal solid waste. Bioresour. Technol. 2009, 100, 4807–4813. [Google Scholar] [CrossRef]
- Wang, X.; Cui, H.; Shi, J.; Zhao, X.; Zhao, Y.; Wei, Z. Relationship between bacterial diversity and environmental parameters during composting of different raw materials. Bioresour. Technol. 2015, 198, 395–402. [Google Scholar] [CrossRef]
- Qiao, C.; Penton, C.R.; Liu, C.; Shen, Z.; Ou, Y.; Liu, Z.; Xu, X.; Li, R.; Shen, Q. Key extracellular enzymes triggered high-efficiency composting associated with bacterial community succession. Bioresour. Technol. 2019, 288, 121576. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cheng, D.; Xue, J.; Weaver, L.; Wakelin, S.A.; Feng, Y.; Li, Z. Changes in microbial community structure during pig manure composting and its relationship to the fate of antibiotics and antibiotic resistance genes. J. Hazard. Mater. 2020, 389, 122082. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, Z.; Xia, J.; Chen, Y. Effect of microbial inoculation on physicochemical properties and bacterial community structure of citrus peel composting. Bioresour. Technol. 2019, 291, 121843. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.P.; Wang, L.; Sun, Z.Y.; Wang, S.T.; Shen, C.H.; Tang, Y.Q.; Kida, K. Biochar addition reduces nitrogen loss and accelerates composting process by affecting the core microbial community during distilled grain waste composting. Bioresour. Technol. 2021, 337, 125492. [Google Scholar] [CrossRef]
- Zeng, Y.; de Guardia, A.; Daumoin, M.; Benoist, J.-C. Characterizing the transformation and transfer of nitrogen during the aerobic treatment of organic wastes and digestates. Waste Manag. 2012, 32, 2239–2247. [Google Scholar] [CrossRef]
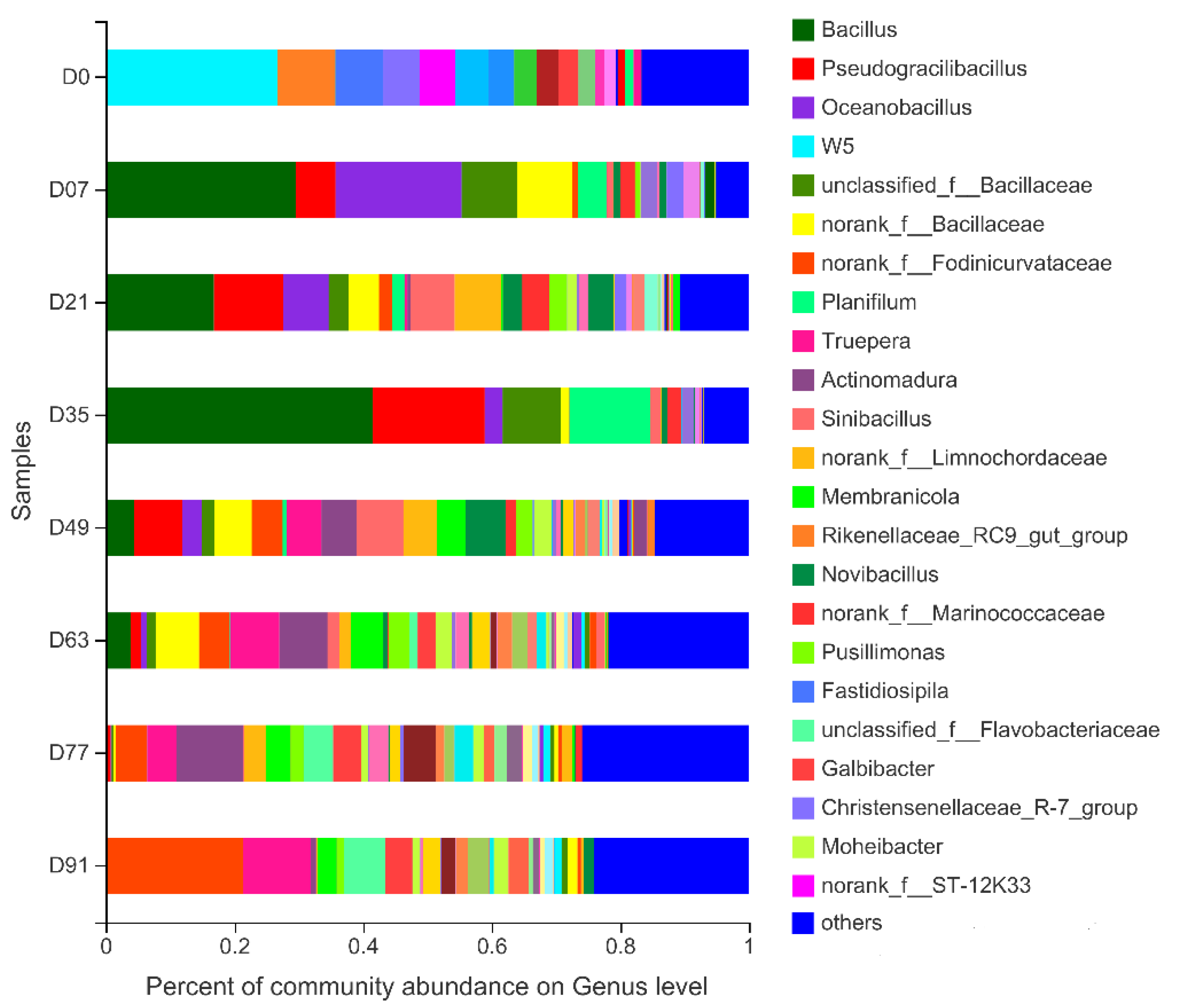
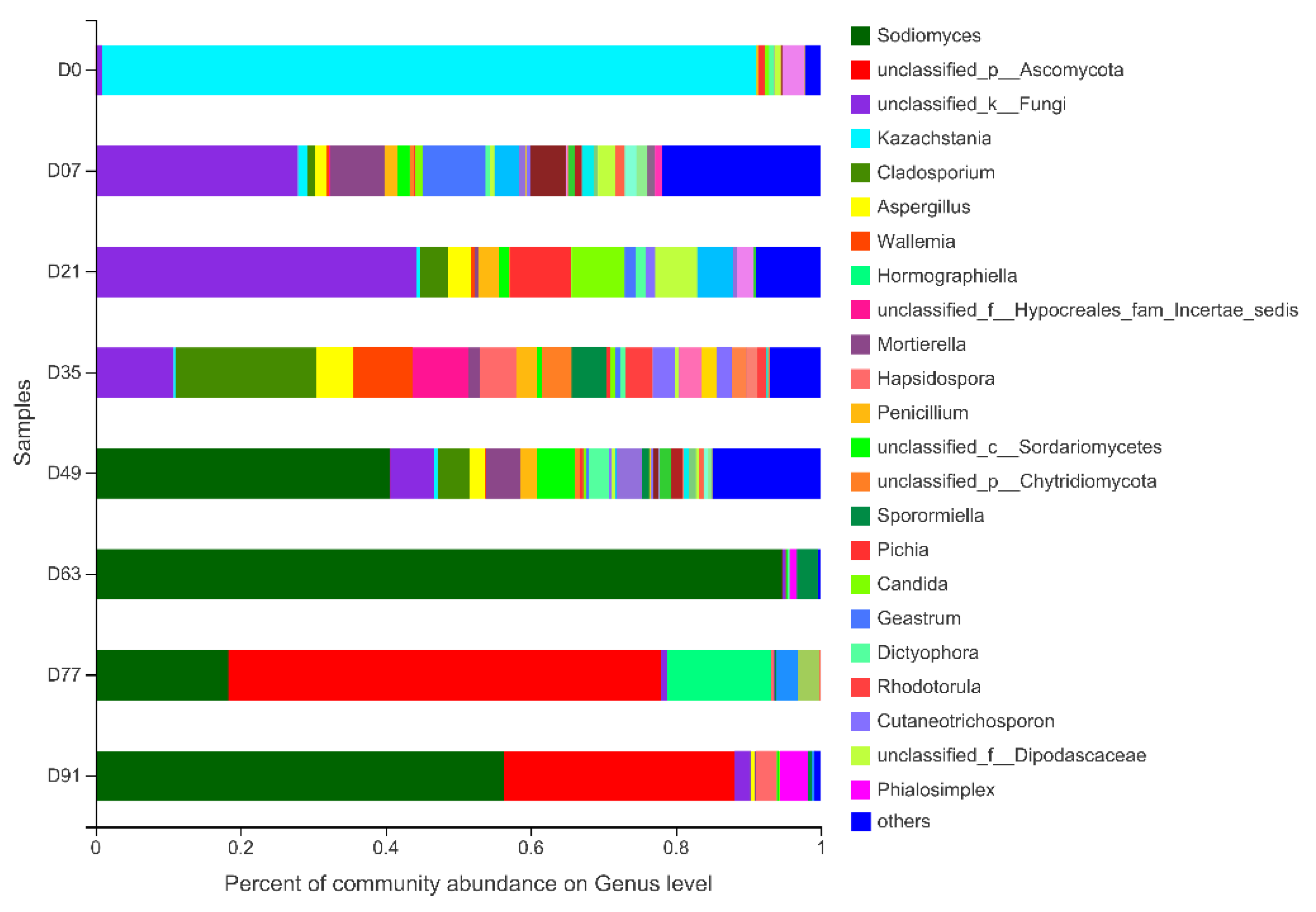
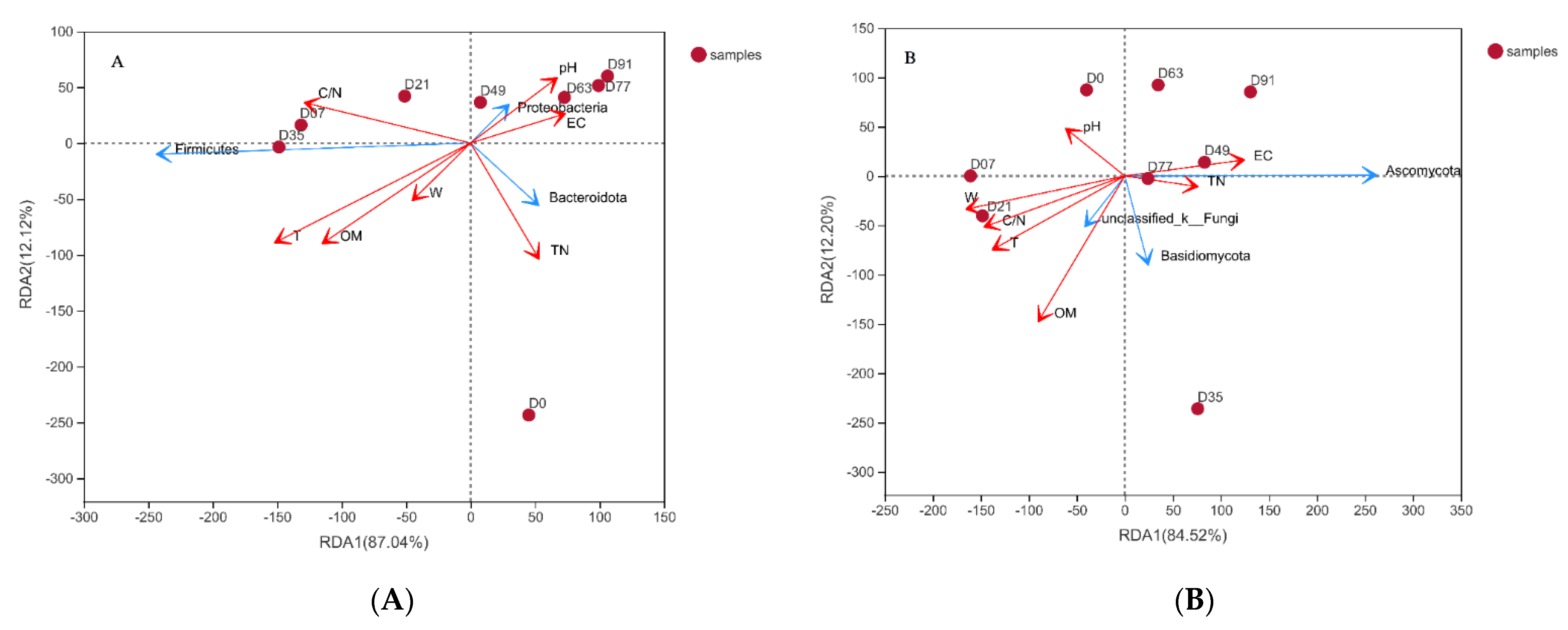
| Materials | PH | EC (mS cm−1) | TN (%) | C/N | TOM (%) |
|---|---|---|---|---|---|
| GW | 7.69 | 0.83 | 1.63 | 35.14 | 98.80 |
| FW digestate | 9.13 | 3.11 | 3.57 | 7.20 | 44.33 |
| Parameters | D0 | D7 | D21 | D35 | D49 | D63 | D77 | D91 |
|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | 60.8 | 68.2 | 54 | 62.5 | 45.6 | 56.2 | 47.1 | 43.2 |
| pH | 8.8 | 8.7 | 9.2 | 8.7 | 8.8 | 8.9 | 8.9 | 8.9 |
| Moisture (%) | 39.5 | 39.0 | 42.8 | 33.5 | 32.7 | 32.7 | 43.5 | 24.8 |
| EC (mS·cm−1) | 3.4 | 3.5 | 2.7 | 3.6 | 3.4 | 3.1 | 4 | 4.2 |
| TOM (%) | 44.2 | 38.7 | 46.0 | 46.4 | 37.5 | 35.1 | 40.2 | 34.8 |
| C/N | 8.27 | 15.91 | 9.85 | 10.19 | 7.88 | 9.04 | 10.23 | 8.43 |
| TN (g·kg−1-TS) | 31.0 | 14.1 | 27.1 | 26.4 | 27.6 | 22.5 | 22.8 | 23.9 |
| GI (%) | 0 | 14.2 | 7.0 | 10.6 | 10.9 | 20.6 | 56.0 | 62.2 |
| Sampling ID | Richness (Chao1) Bacteria/Fungi | Evenness (Shannon Evenness) Bacteria/Fungi | Shannon Bacteria/Fungi | Simpson Index Bacteria/Fungi |
|---|---|---|---|---|
| D0 | 463.25/184.78 | 0.58/0.16 | 3.43/0.85 | 0.09/0.74 |
| D7 | 493.42/209 | 0.52/0.69 | 3.10/3.68 | 0.09/0.09 |
| D21 | 612.86/260 | 0.60/0.63 | 3.72/2.57 | 0.05/0.21 |
| D35 | 561.21/267 | 0.45/0.63 | 2.67/3.38 | 0.13/0.06 |
| D49 | 614.55/217 | 0.65/0.59 | 4.12/3.18 | 0.03/0.18 |
| D63 | 584.03/86.80 | 0.73/0.37 | 4.51/1.32 | 0.02/0.90 |
| D77 | 597.53/38.43 | 0.72/0.33 | 4.46/1.21 | 0.03/0.41 |
| D91 | 525.60/36.00 | 0.66/0.33 | 4.08/1.19 | 0.04/0.42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; He, X.; Liang, J. Succession of Microbial Community during the Co-Composting of Food Waste Digestate and Garden Waste. Int. J. Environ. Res. Public Health 2022, 19, 9945. https://doi.org/10.3390/ijerph19169945
Wang X, He X, Liang J. Succession of Microbial Community during the Co-Composting of Food Waste Digestate and Garden Waste. International Journal of Environmental Research and Public Health. 2022; 19(16):9945. https://doi.org/10.3390/ijerph19169945
Chicago/Turabian StyleWang, Xiaohan, Xiaoli He, and Jing Liang. 2022. "Succession of Microbial Community during the Co-Composting of Food Waste Digestate and Garden Waste" International Journal of Environmental Research and Public Health 19, no. 16: 9945. https://doi.org/10.3390/ijerph19169945
APA StyleWang, X., He, X., & Liang, J. (2022). Succession of Microbial Community during the Co-Composting of Food Waste Digestate and Garden Waste. International Journal of Environmental Research and Public Health, 19(16), 9945. https://doi.org/10.3390/ijerph19169945





