Family-Level Multimorbidity among Older Adults in India: Looking through a Syndemic Lens
Abstract
:1. Introduction
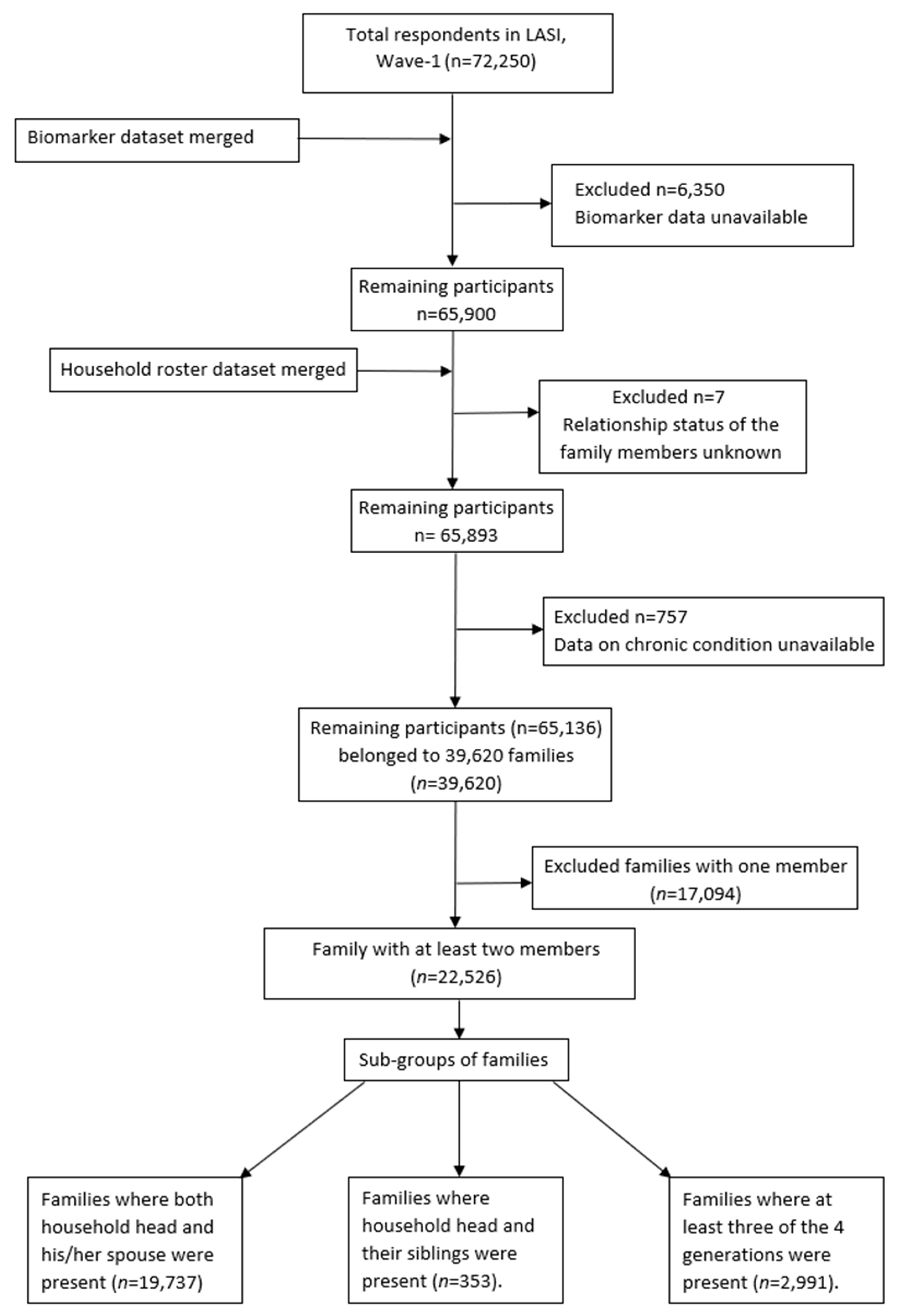
2. Materials and Methods
2.1. Overview of the Data
2.2. Variables
2.2.1. Independent Variables
2.2.2. Outcome Variables
2.3. Statistical Analysis
2.4. Ethical Considerations
3. Results
4. Discussion
4.1. Implications for Policy and Practice
4.2. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bloom, D.E.; Chatterji, S.; Kowal, P.; Lloyd-Sherlock, P.; McKee, M.; Rechel, B.; Rosenberg, L.; Smith, J.P. Macroeconomic implications of population ageing and selected policy responses. Lancet 2014, 385, 649–657. [Google Scholar] [CrossRef] [Green Version]
- Arokiasamy, P. India’s escalating burden of non-communicable diseases. Lancet Glob. Health 2018, 6, e1262-3. [Google Scholar] [CrossRef] [Green Version]
- MacMahon, S.; Calverley, P.; Chaturvedi, N.; Chen, Z.; Corner, L.; Davies, M.; Ezzati, M.; Guthrie, B.; Hanson, K.; Jha, V. Multimorbidity: A Priority for Global Health Research; The Academy of Medical Sciences: London, UK, 2018; p. 127. [Google Scholar]
- Puri, P.; Sinha, A.; Mahapatra, P.; Pati, S. Multimorbidity among midlife women in India: Well-being beyond reproductive age. BMC Women’s Health 2022, 22, 117. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.; Varanasi, R.; Pati, S. Kaleidoscopic use of World Health Organization’s Study on global AGEing and adult health data set to explore multimorbidity and its outcomes in low and middle-income countries: An insider view. J. Fam. Med. Prim. Care 2021, 10, 4623. [Google Scholar]
- Barik, M.; Panda, S.N.; Tripathy, S.S.; Sinha, A.; Ghosal, S.; Acharya, A.S.; Kanungo, S.; Pati, S. Is multimorbidity associated with higher risk of falls among older adults in India? BMC Geriatr. 2022, 22, 486. [Google Scholar] [CrossRef]
- Vetrano, D.L.; Palmer, K.; Marengoni, A.; Marzetti, E.; Lattanzio, F.; Roller-Wirnsberger, R.; Lopez Samaniego, L.; Rodríguez-Mañas, L.; Bernabei, R.; Onder, G. Joint Action ADVANTAGE WP4 Group. Frailty and multimorbidity: A systematic review and me-ta-analysis. J. Gerontol. Ser. A 2019, 74, 659–666. [Google Scholar] [CrossRef] [Green Version]
- Kanungo, S.; Ghosal, S.; Kerketta, S.; Sinha, A.; Mercer, S.W.; Lee, J.T.; Pati, S. Association of Oral Health with Multimorbidity among Older Adults: Findings from the Longitudinal Ageing Study in India, Wave-1, 2017–2019. Int. J. Environ. Res. Public Health 2021, 18, 12853. [Google Scholar] [CrossRef]
- Lugtenberg, M.; Burgers, J.S.; Clancy, C.; Westert, G.P.; Schneider, E.C. Current Guidelines Have Limited Applicability to Patients with Comorbid Conditions: A Systematic Analysis of Evidence-Based Guidelines. PLoS ONE 2011, 6, e25987. [Google Scholar] [CrossRef] [Green Version]
- Mercer, S.; Gunn, J.; Bower, P.; Wyke, S.; Guthrie, B. Managing patients with mental and physical multimorbidity. BMJ 2012, 345, e5559. [Google Scholar] [CrossRef]
- Srivastava, S.; Thalil, M.; Rashmi, R.; Paul, R. Association of family structure with gain and loss of household headship among older adults in India: Analysis of panel data. PLoS ONE 2021, 16, e0252722. [Google Scholar] [CrossRef]
- Rolland, J.S. Parental illness and disability: A family systems framework. J. Fam. Ther. 1999, 21, 242–266. [Google Scholar] [CrossRef]
- Michaelson, V.; Pilato, K.A.; Davison, C.M. Family as a health promotion setting: A scoping review of conceptual models of the health-promoting family. PLoS ONE 2021, 16, e0249707. [Google Scholar] [CrossRef] [PubMed]
- Foster, M.W.; Sharp, R.R. Will Investments in Large-Scale Prospective Cohorts and Biobanks Limit Our Ability to Discover Weaker, Less Common Genetic and Environmental Contributors to Complex Diseases? Environ. Health Perspect. 2005, 113, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Di Castelnuovo, A.; Quacquaruccio, G.; Donati, M.B.; de Gaetano, G.; Iacoviello, L. Spousal concordance for major coronary risk factors: A systematic review and meta-analysis. Am. J. Epidemiol. 2009, 169, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyler, D.; Stimpson, J.; Peek, M.K. Health concordance within couples: A systematic review. Soc. Sci. Med. 2007, 64, 2297–2310. [Google Scholar] [CrossRef]
- Lee, K.E.; Klein, B.E.; Klein, R. Familial aggregation of components of the multiple metabolic syndrome in the Framingham Heart and Offspring Cohorts: Genetic Analysis Workshop Problem 1. BMC Genet. 2003, 4, S94. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Srinivasan, S.R.; Bao, W.; Berenson, G.S. The magnitude of familial associations of cardiovascular risk factor variables between parents and offspring are influenced by age: The Bogalusa Heart Study. Ann. Epidemiol. 2001, 11, 522–528. [Google Scholar] [CrossRef]
- Hoppmann, C.A.; Michalowski, V.; Gerstorf, D. Spousal interrelationships in health across adulthood: Health behaviors and everyday stress as potential underlying mechanisms. In Couple Relationships in the Middle and Later Years: Their Nature, Complexity, and Role in Health and Illness; American Psychological Association: Washington, DC, USA, 2016; pp. 239–257. [Google Scholar]
- Patel, S.A.; Dhillon, P.K.; Kondal, D.; Jeemon, P.; Kahol, K.; Manimunda, S.P.; Purty, A.J.; Deshpande, A.; Negi, P.C.; Ladhani, S.; et al. Chronic disease concordance within Indian households: A cross-sectional study. PLOS Med. 2017, 14, e1002395. [Google Scholar] [CrossRef] [Green Version]
- Bernardes, G.M.; Saulo, H.; Fernandez, R.N.; Lima-Costa, M.F.; de Andrade, F.B. Catastrophic health expenditure and multimorbidity among older adults in Brazil. Rev. Saúde Pública 2020, 54, 125. [Google Scholar] [CrossRef]
- International Institute for Population Sciences (IIPS). Longitudinal Ageing Study in India (LASI). Available online: https://www.iipsindia.ac.in/lasi (accessed on 15 December 2021).
- Agborsangaya, C.B.; Lau, D.; Lahtinen, M.; Cooke, T.; A Johnson, J. Multimorbidity prevalence and patterns across socioeconomic determinants: A cross-sectional survey. BMC Public Health 2012, 12, 201. [Google Scholar] [CrossRef] [Green Version]
- Singer, L.; Green, M.; Rowe, F.; Ben-Shlomo, Y.; Morrissey, K. Social determinants of multimorbidity and multiple functional limita-tions among the ageing population of England, 2002–2015. SSM Popul. Health 2019, 8, 100413. [Google Scholar] [CrossRef] [PubMed]
- Lucyk, K.; McLaren, L. Taking stock of the social determinants of health: A scoping review. PLoS ONE 2017, 12, e0177306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhawan, D.; Sharma, S. Abdominal Obesity, Adipokines and Non-communicable Diseases. J. Steroid Biochem. Mol. Biol. 2020, 203, 105737. [Google Scholar] [CrossRef] [PubMed]
- Van Hecke, O.; Hocking, L.J.; Torrance, N.; Campbell, A.; Padmanabhan, S.; Porteous, D.J.; McIntosh, A.M.; Burri, A.V.; Tanaka, H.; Williams, F.M.; et al. Chronic pain, depression and cardiovascular disease linked through a shared genetic predisposition: Analysis of a family-based cohort and twin study. PLoS ONE. 2017, 12, e0170653. [Google Scholar] [CrossRef] [Green Version]
- Poitras, M.-E.; Maltais, M.-E.; Bestard-Denommé, L.; Stewart, M.; Fortin, M. What are the effective elements in patient-centered and multimorbidity care? A scoping review. BMC Health Serv. Res. 2018, 18, 446. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Women’s Health; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Routray, P.; Torondel, B.; Clasen, T.; Schmidt, W.P. Women’s role in sanitation decision making in rural coastal Odisha, India. PLoS ONE 2017, 12, e0178042. [Google Scholar] [CrossRef] [Green Version]
- Christensen, P. The health-promoting family: A conceptual framework for future research. Soc. Sci. Med. 2004, 59, 377–387. [Google Scholar] [CrossRef]
- Pisciotta, A.; Di Lorenzo, R.; Novara, A.; Laudicina, V.A.; Barone, E.; Santoro, A.; Gristina, L.; Barbagallo, M.G. Cover crop and pruning residue management to reduce nitrogen mineral fertilization in mediterranean vineyards. Agronomy 2021, 11, 164. [Google Scholar] [CrossRef]
- Pati, S.; Sinha, R.; Panda, M.; Puri, P.; Pati, S. Profile of multimorbidity in outpatients attending public healthcare settings: A de-scriptive cross-sectional study from Odisha, India. J. Fam. Med. Prim. Care 2021, 10, 2900. [Google Scholar] [CrossRef]
- Ingram, E.; Ledden, S.; Beardon, S.; Gomes, M.; Hogarth, S.; McDonald, H.; Osborn, D.P.; Sheringham, J. Household and area-level social determinants of multimorbidity: A systematic review. J. Epidemiol. Community Health 2020, 75, 232–241. [Google Scholar] [CrossRef]
- Sinha, A.; Kerketta, S.; Ghosal, S.; Kanungo, S.; Pati, S. Multimorbidity Among Urban Poor in India: Findings From LASI, Wave-1. Front. Public Health 2022, 10, 881967. [Google Scholar] [CrossRef] [PubMed]
- Allen, P.; Sachs, C. Women and food chains: The gendered politics of food. Taking food public: Redefining foodways in a changing world. Int. J. Sociol. Agric. Food 2012, 23, 23–40. [Google Scholar]
- Braveman, P.; Barclay, C. Health Disparities Beginning in Childhood: A Life-Course Perspective. Pediatrics 2009, 124, S163–S175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maguire, K.C. Stress and Coping in Families. In The SAGE Handbook of Family Communication; SAGE Publications: Thousand Oaks, CA, USA, 2014; p. 154. [Google Scholar]
| Household Characteristics | n (%) | |
|---|---|---|
| Household average age (n = 22,526) | Mean: 56.81 (±9.15) years Range: 33–97.5 years | |
| Residence (n = 22,358) | ||
| Rural | 14,781 (66.11) | |
| Urban | 7577 (33.89) | |
| Household average education | Mean: 7.64 (±3.42) years Range: 1–20.5 years | |
| Caste (n = 22,358) | ||
| Scheduled Caste (SC) | 3743 (16.74) | |
| Scheduled Tribe (ST) | 3924 (17.55) | |
| Other Backward Class (OBC) | 8557 (38.27) | |
| None of them | 6134 (27.44) | |
| Region of India (n = 22,358) | ||
| North | 3249 (14.53 | |
| Central | 3822 (17.09) | |
| East | 4227 (18.91) | |
| Northeast | 3038 (13.59) | |
| West | 2771 (12.39) | |
| South | 5251 (23.49) | |
| Household Size (n = 22,358) | ||
| No. of members | 5.12 (±2.52) | |
| Smoking inside household (n = 22,317) | ||
| Yes | 6142 (27.52) | |
| No | 16,175 (72.48) | |
| Cooking fuel (n = 22,358) | ||
| Coal/wood/dung cake | 10,280 (45.98) | |
| LPG†/biogas | 11,776 (52.67) | |
| Other | 302 (1.35) | |
| Source of drinking water (n = 22,317) | ||
| Pipe/tap water | 10,895 (48.82) | |
| Well | 9499 (42.56) | |
| Other sources | 1923 (8.62) | |
| Household-level wealth index (n = 22,358) | ||
| Poorest | 4258 (19.04) | |
| Poorer | 4512 (20.18) | |
| Middle | 4571 (20.44) | |
| Richer | 4615 (20.64) | |
| Richest | 4402 (19.69) | |
| No. of Family Members | Number of Family Members with Multimorbidity | ||||
|---|---|---|---|---|---|
| None | One | Two | Three | >Three | |
| 2 members | 4949 (24.68) | 6718 (33.50) | 8389 (41.83) | - | - |
| 3 members | 280 (13.74) | 478 (23.45) | 640 (31.40) | 640 (31.40) | - |
| 4 members | 20 (5.51) | 56 (15.43) | 87 (23.97) | 94 (25.90) | 106 (29.20) |
| >4 members | 5 (7.25) | 4 (5.80) | 7 (10.14) | 14 (20.29) | 39 (56.52) |
| |
| No. of spouse(s) having multimorbidity | Multimorbidity status n (%) |
| None of the partners | 4969 (25.18) |
| Either of the partners | 6582 (33.35) |
| Both of the partners | 8186 (41.48) |
| |
| No. of sibling(s) having multimorbidity | Multimorbidity status n (%) |
| None | 90 (25.49) |
| One | 116 (32.86) |
| ≥2 | 147 (41.64) |
| |
| No. of member(s) having multimorbidity | Multimorbidity status n (%) |
| None | 531 (17.75) |
| One | 1082 (36.18) |
| ≥2 | 1378 (46.07) |
| Sociodemographic Characteristics | Categories | AOR (95% Confidence Interval) | |||
|---|---|---|---|---|---|
| Family-Level Multimorbidity | Conjugal Multimorbidity | Sibling Multimorbidity | Intergenerational Multimorbidity | ||
| Household average age | 1.05 (1.047–1.055) | 1.04 (1.04–1.05) | 1.03 (0.99–1.06) | 1.01 (1.00–1.03) | |
| Residence | Rural | Reference | |||
| Urban | 1.642 (1.526–1.767) | 1.71 (1.58–1.84) | 1.52 (0.78–2.96) | 1.42 (1.17–1.72) | |
| Household average education | 1.046 (1.038–1.054) | 1.03 (1.02–1.04) | 1.09 (1.01–1.17) | 1.04 (1.02–1.06) | |
| Caste | ST * | Reference | |||
| SC ^ | 1.442 (1.288–1.615) | 1.40 (1.24–1.59) | 0.85 (0.30–2.35) | 1.41 (1.03–1.93) | |
| OBC # | 1.437 (1.302–1.586) | 1.37 (1.23–1.52) | 1.51 (0.67–3.41) | 1.53 (1.16–2.02) | |
| Other | 1.796 (1.612–2.000) | 1.72 (1.53–1.93) | 1.89 (0.77–4.62) | 1.99 (1.48–2.69) | |
| Regions of India | Central | Reference | |||
| North | 2.171 (1.943–2.426) | 2.18 (1.94–2.46) | 2.32 (0.73–7.32) | 1.92 (1.42–2.60) | |
| East | 1.781 (1.609–1.971) | 1.70 (1.52–1.89) | 2.71 (0.96–7.63) | 1.63 (1.24–2.13) | |
| Northeast | 1.142 (1.005–1.296) | 1.12 (0.98–1.29 | 2.79 (0.78–9.92) | 1.12 (0.78–1.62) | |
| West | 1.858 (1.655–2.086) | 1.86 (1.64–2.10) | 1.83 (0.57–5.85) | 1.41 (1.03–1.93) | |
| South | 2.727 (2.467–3.015) | 2.64 (2.37–2.94) | 9.12 (3.29–25.29) | 2.40 (1.82–3.17) | |
| No. of household members | Two | 0.717 (0.653–0.787) | 1.08 (0.96–1.21) | 0.42 (0.14–1.28) | 0.65 (0.40–1.05) |
| Three | 0.730 (0.665–0.801) | 1.04 (0.93–1.16) | 0.91 (0.27–3.07) | 0.73 (0.50–1.06) | |
| Four | 0.742 (0.681–0.807) | 1.21 (1.11–1.33) | 0.65 (0.28–1.49) | 1.04 (0.81–1.35) | |
| ≥Five | Reference | ||||
| Smoking inside household | Yes | Reference | |||
| No | 1.013 (0.945–1.084) | 1.03 (0.96–1.11) | 0.57 (0.30–1.066) | 1.05 (0.87–1.27) | |
| Cooking fuel | Coal/wood/dung cake | Reference | |||
| LPG †/biogas | 1.468 (1.366–1.578) | 1.43 (1.33–1.55) | 2.02 (1.06–3.82) | 1.25 (1.03–1.51) | |
| Other | 1.243 (0.942–1.640) | 1.14 (0.85–1.55) | 3.35 (0.25–44.91) | 1.46 (0.63–3.40) | |
| Source of drinking water | Pipe/tap water | Reference | |||
| Well | 1.030 (0.961–1.105) | 1.08 (1.01–1.17) | 1.44 (0.80–2.59) | 0.85 (0.70–1.02) | |
| Other sources | 1.093 (0.979–1.221) | 1.15 (1.02–1.29) | 1.85 (0.62–5.46) | 1.08 (0.79–1.47) | |
| Household-level wealth index | Poorest | Reference | |||
| Poorer | 1.331 (1.207–1.467) | 1.32 (1.19–1.47) | 2.15 (0.90–5.15) | 1.38 (1.08–1.76) | |
| Middle | 1.457 (1.321–1.607) | 1.45 (1.30–1.61) | 1.93 (0.79–4.67) | 1.42 (1.11–1.83) | |
| Richer | 2.018 (1.826–2.231) | 2.04 (1.83–2.27) | 1.80 (0.72–4.47) | 1.76 (1.36–2.28) | |
| Richest | 2.543 (2.284–2.832) | 2.46 (2.19–2.76) | 1.36 (0.49–3.81) | 2.90 (2.18–3.87) | |
| Household Out-of-Pocket Healthcare-Expenditure | Number of Household Members with Multimorbidity | |||
|---|---|---|---|---|
| None | One | Two | Three or More | |
| Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | |
| IPD cost (INR) | 6900 (2000–22,000) | 9000 (3000–28,000) | 11,750 (3500–34,660) | 20,000 (6100–50,000) |
| OPD cost (INR) | 700 (300–1560) | 900 (400–2000) | 1163 (500–2650) | 1807 (880–4000) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pati, S.; Sinha, A.; Ghosal, S.; Kerketta, S.; Lee, J.T.; Kanungo, S. Family-Level Multimorbidity among Older Adults in India: Looking through a Syndemic Lens. Int. J. Environ. Res. Public Health 2022, 19, 9850. https://doi.org/10.3390/ijerph19169850
Pati S, Sinha A, Ghosal S, Kerketta S, Lee JT, Kanungo S. Family-Level Multimorbidity among Older Adults in India: Looking through a Syndemic Lens. International Journal of Environmental Research and Public Health. 2022; 19(16):9850. https://doi.org/10.3390/ijerph19169850
Chicago/Turabian StylePati, Sanghamitra, Abhinav Sinha, Shishirendu Ghosal, Sushmita Kerketta, John Tayu Lee, and Srikanta Kanungo. 2022. "Family-Level Multimorbidity among Older Adults in India: Looking through a Syndemic Lens" International Journal of Environmental Research and Public Health 19, no. 16: 9850. https://doi.org/10.3390/ijerph19169850
APA StylePati, S., Sinha, A., Ghosal, S., Kerketta, S., Lee, J. T., & Kanungo, S. (2022). Family-Level Multimorbidity among Older Adults in India: Looking through a Syndemic Lens. International Journal of Environmental Research and Public Health, 19(16), 9850. https://doi.org/10.3390/ijerph19169850







