Approaches to Peripheral Artery Disease in Diabetes: Are There Any Differences?
Abstract
1. Introduction
2. Is PAD Different in Patients with DM?
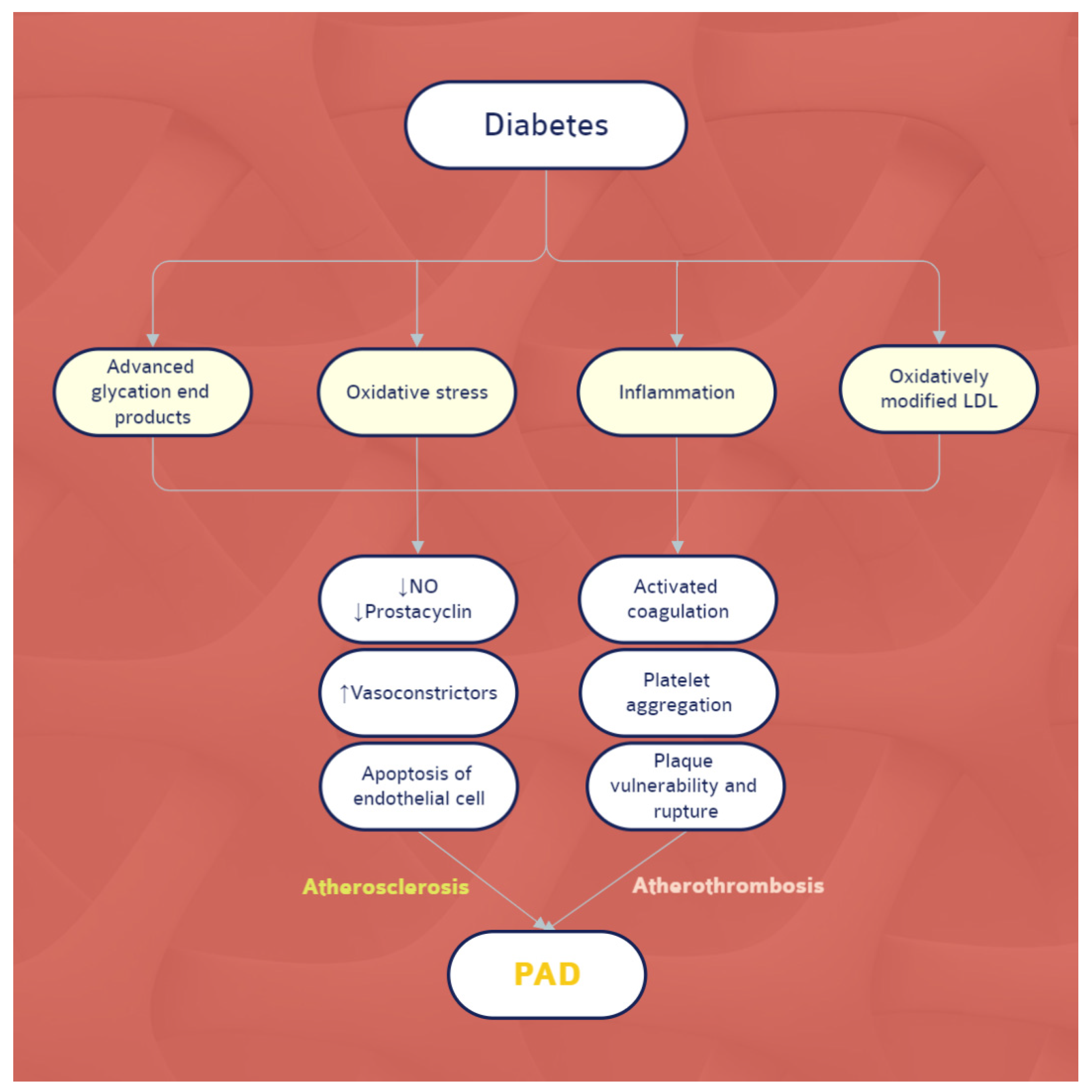
2.1. The Biological Level
2.2. The Clinical Level
3. Diagnosis
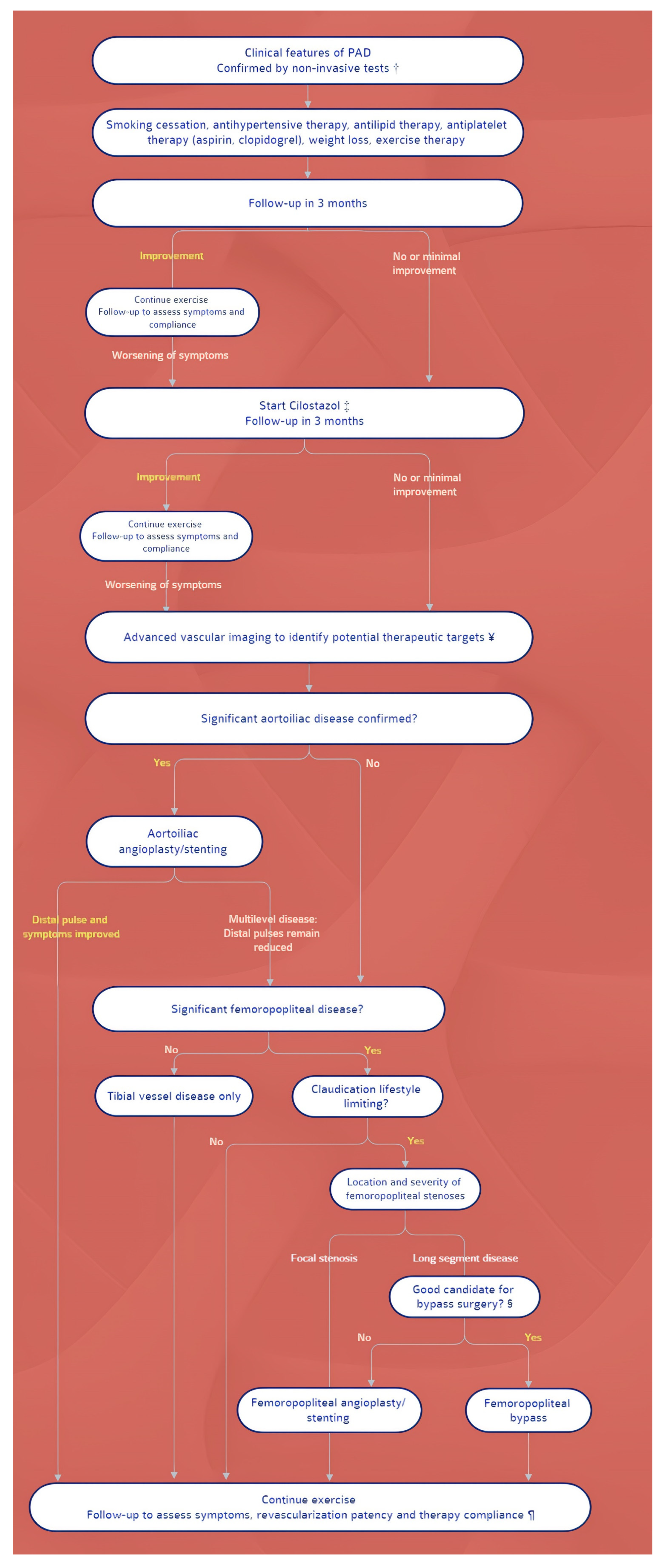
4. Management: Differences between Guidelines
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Wong, N.D.; Gransar Shaw, L.; Polk, D.; Moon, J.H.; Miranda-Peats, R.; Hayes, S.W.; Thomson, L.E.; Rozanski, A.; Friedman, J.D.; Berman, D.S. Thoracic aortic calcium versus coronary artery calcium for the prediction of coronary heart disease and cardiovascular disease events. JACC Cardiovasc. Imaging 2009, 2, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Achim, A.; Kákonyi, K.; Nagy, F.; Jambrik, Z.; Varga, A.; Nemes, A.; Chan, J.S.K.; Toth, G.G.; Ruzsa, Z. Radial Artery Calcification in Predicting Coronary Calcification and Atherosclerosis Burden. Cardiol. Res. Pract. 2022, 2022, 5108389. [Google Scholar] [CrossRef] [PubMed]
- Homorodean, C.; Leucuta, D.C.; Ober, M.; Homorodean, R.; Spinu, M.; Olinic, M.; Tataru, D.; Olinic, D.M. Intravascular ultrasound insights into the unstable features of the coronary atherosclerotic plaques: A systematic review and meta-analysis. Eur. J. Clin. Investig. 2022, 52, e13671. [Google Scholar] [CrossRef]
- Achim, A.; Marc, M.; Ruzsa, Z. Surgical Turned-Downed CHIP Cases-Can PCI Save the Day? Front. Cardiovasc. Med. 2022, 9, 872398. [Google Scholar] [CrossRef] [PubMed]
- The Diabetes Control and Complications Trial (DCCT) Research Group. Effect of intensive diabetes management on macrovascular events and risk factors in the Diabetes Control and Complications Trial. Am. J. Cardiol. 1995, 75, 894–903. [Google Scholar] [CrossRef]
- Jones, W.S.; Baumgartner, I.; Hiatt, W.R.; Heizer, G.; Conte, M.S.; White, C.J.; Berger, J.S.; Held, P.; Katona, B.G.; International Steering Committee and Investigators of the EUCLID Trial; et al. Ticagrelor Compared with Clopidogrel in Patients with Prior Lower Extremity Revascularization for Peripheral Artery Disease. Circulation 2017, 135, 241–250. [Google Scholar] [CrossRef]
- Low Wang, C.C.; Blomster, J.I.; Heizer, G.; Berger, J.S.; Baumgartner, I.; Fowkes, F.G.R.; Held, P.; Katona, B.G.; Norgren, L.; EUCLID Trial Executive Committee and Investigators; et al. Cardiovascular and Limb Outcomes in Patients with Diabetes and Peripheral Artery Disease: The EUCLID Trial. J. Am. Coll. Cardiol. 2018, 72, 3274–3284. [Google Scholar] [CrossRef]
- Wild, S.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care 2004, 27, 1047–1053. [Google Scholar] [CrossRef]
- Fowkes, F.G.; Rudan, D.; Rudan, I.; Aboyans, V.; Denenberg, J.O.; McDermott, M.M.; Norman, P.E.; Sampson, U.K.; Williams, L.J.; Mensah, G.A.; et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: A systematic review and analysis. Lancet 2013, 382, 1329–1340. [Google Scholar] [CrossRef]
- Murabito, J.M.; D’Agostino, R.B.; Silbershatz, H.; Wilson, W.F. Intermittent claudication. A risk profile from The Framingham Heart Study. Circulation 1997, 96, 44–49. [Google Scholar] [CrossRef]
- Hiatt, W.R. Medical treatment of peripheral arterial disease and claudication. N. Engl. J. Med. 2001, 344, 1608–1621. [Google Scholar] [CrossRef] [PubMed]
- Haffner, S.M.; Stern, M.P.; Hazuda, H.P.; Pugh, J.A.; Patterson, J.K. Hyperinsulinemia in a population at high risk for non-insulin-dependent diabetes mellitus. N. Engl. J. Med. 1986, 315, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Rönnemaa, T.; Laakso, M.; Pyörälä, K.; Kallio, V.; Puukka, P. High fasting plasma insulin is an indicator of coronary heart disease in non-insulin-dependent diabetic patients and nondiabetic subjects. Arter. Thromb. 1991, 11, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Saely, C.H.; Rein, P.; Vonbank, A.; Huber, K.; Drexel, H. Type 2 diabetes and the progression of visualized atherosclerosis to clinical cardiovascular events. Int. J. Cardiol. 2013, 167, 776–780. [Google Scholar] [CrossRef]
- Stanek, A.; Fazeli, B.; Bartuś, S.; Sutkowska, E. The Role of Endothelium in Physiological and Pathological States: New Data. BioMed Res. Int. 2018, 2018, 1098039. [Google Scholar] [CrossRef]
- Ridker, P.M.; Cushman, M.; Stampfer, M.J.; Tracy, R.P.; Hennekens, C.H. Plasma concentration of C-reactive protein and risk of developing peripheral vascular disease. Circulation 1998, 97, 425–428. [Google Scholar] [CrossRef]
- Donath, M.Y.; Dalmas, É.; Sauter, N.S.; Böni-Schnetzler, M. Inflammation in obesity and diabetes: Islet dysfunction and therapeutic opportunity. Cell Metab. 2013, 17, 860–872. [Google Scholar] [CrossRef]
- Cermak, J.; Key, N.S.; Bach, R.R.; Balla, J.; Jacob, H.S.; Vercellotti, G.M. C-reactive protein induces human peripheral blood monocytes to synthesize tissue factor. Blood 1993, 82, 513–520. [Google Scholar] [CrossRef]
- Paneni, F.; Beckman, J.A.; Creager, M.A.; Cosentino, F. Diabetes and vascular disease: Pathophysiology, clinical consequences, and medical therapy: Part I. Eur. Heart J. 2013, 34, 2436–2443. [Google Scholar] [CrossRef]
- Geraldes, P.; King, G.L. Activation of protein kinase C isoforms and its impact on diabetic complications. Circul. Res. 2010, 106, 1319–1331. [Google Scholar] [CrossRef]
- Grant, P.J. Diabetes mellitus as a prothrombotic condition. J. Intern. Med. 2007, 262, 157–172. [Google Scholar] [CrossRef]
- Vazzana, N.; Ranalli, P.; Cuccurullo, C.; Davì, G. Diabetes mellitus and thrombosis. Throm. Res. 2012, 129, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Zeiger, F.; Stephan, S.; Hoheisel, G.; Pfeiffer, D.; Ruehlmann, C.; Koksch, M. P-Selectin expression, platelet aggregates, and platelet-derived microparticle formation are increased in peripheral arterial disease. Blood Coagul. Fibrinolysis 2000, 11, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Vinik, A.I.; Erbas, T.; Park, T.S.; Nolan, R.; Pittenger, G.L. Platelet dysfunction in type 2 diabetes. Diabetes Care 2001, 24, 1476–1485. [Google Scholar] [CrossRef] [PubMed]
- Sinning, J.M.; Losch, J.; Walenta, K.; Böhm, M.; Nickenig, G.; Werner, N. Circulating CD31+/Annexin V+ microparticles correlate with cardiovascular outcomes. Eur Heart J. 2011, 32, 2034–2041. [Google Scholar] [CrossRef] [PubMed]
- Tsimerman, G.; Roguin, A.; Bachar, A.; Melamed, E.; Brenner, B.; Aharon, A. Involvement of microparticles in diabetic vascular complications. Thromb. Haemost. 2011, 106, 310–321. [Google Scholar] [CrossRef]
- Aday, A.W.; Matsushita, K. Epidemiology of Peripheral Artery Disease and Polyvascular Disease. Circ Res. 2021, 128, 1818–1832. [Google Scholar] [CrossRef]
- Malyar, N.M.; Freisinger, E.; Meyborg, M.; Lüders, F.; Gebauer, K.; Reinecke, H.; Lawall, H. Amputations and mortality in in-hospital treated patients with peripheral artery disease and diabetic foot syndrome. J. Diabetes Complicat. 2016, 30, 1117–1122. [Google Scholar] [CrossRef]
- Wojtasik-Bakalarz, J.; Ruzsa, Z.; Rakowski, T.; Nyerges, A.; Bartuś, K.; Stanek, A.; Dudek, D.; Surdacki, A.; Kleczyński, P.; Bartuś, S. Impact of Coronary Artery Disease and Diabetes Mellitus on the Long-Term Follow-Up in Patients after Retrograde Recanalization of the Femoropopliteal Arterial Region. J. Diabetes Res. 2019, 2019, 6036359. [Google Scholar] [CrossRef]
- Sherwani, S.I.; Khan, H.A.; Ekhzaimy, A.; Masood, A.; Sakharkar, M.K. Significance of HbA1c Test in Diagnosis and Prognosis of Diabetic Patients. Biomark. Insights 2016, 11, 95–104. [Google Scholar] [CrossRef]
- Obermannova, B.; Petruzelkova, L.; Sulakova, T.; Sumnik, Z. HbA1c but not diabetes duration predicts increased arterial stiffness in adolescents with poorly controlled type 1 diabetes. Pediatr. Diabetes 2017, 18, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Barrett, E.J.; Liu, Z.; Khamaisi, M.; King, G.L.; Klein, R.; Klein, B.E.K.; Hughes, T.M.; Craft, S.; Freedman, B.I.; Bowden, D.W.; et al. Diabetic Microvascular Disease: An Endocrine Society Scientific Statement. J. Clin. Endocrinol. Metab. 2017, 102, 4343–4410. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Sala, R.; Cagliero, E.; Lorenzi, M. Overexpression of fibronectin induced by diabetes or high glucose: Phenomenon with a memory. Proc. Natl. Acad. Sci. USA 1990, 87, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Stratton, I.M.; Adler, A.I.; Neil, H.A.; Matthews, D.R.; Manley, S.E.; Cull, C.A.; Hadden, D.; Turner, R.C.; Holman, R.R. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): Prospective observational study. BMJ 2000, 321, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Ruzsa, Z.; Januszek, R.; Óriás, V.; Chyrchel, M.; Wojtasik-Bakalarz, J.; Bartuś, J.; Arif, S.; Kleczyński, P.; Tokarek, T.; Nyerges, A.; et al. Mortality and chronic obstructive pulmonary disease in patients treated with endovascular revascularization of the infra-inguinal lower limb arteries from retrograde access. Ann. Transl. Med. 2020, 8, 206. [Google Scholar] [CrossRef]
- Achim, A.; Kákonyi, K.; Jambrik, Z.; Nagy, F.; Tóth, J.; Sasi, V.; Hausinger, P.; Nemes, A.; Varga, A.; Bertrand, O.F.; et al. Distal Radial Artery Access for Coronary and Peripheral Procedures: A Multicenter Experience. J. Clin. Med. 2021, 10, 5974. [Google Scholar] [CrossRef]
- Tay, S.; Abdulnabi, S.; Saffaf, O.; Harroun, N.; Yang, C.; Semenkovich, C.F.; Zayed, M.A. Comprehensive Assessment of Current Management Strategies for Patients with Diabetes and Chronic Limb-Threatening Ischemia. Clin. Diabetes 2021, 39, 358–388. [Google Scholar] [CrossRef]
- Latif, K.A.; Freire, A.X.; Kitabchi, A.E.; Umpierrez, G.E.; Qureshi, N. The use of alkali therapy in severe diabetic ketoacidosis. Diabetes Care 2002, 25, 2113–2114. [Google Scholar] [CrossRef][Green Version]
- Jakubiak, G.K.; Pawlas, N.; Cieślar, G.; Stanek, A. Pathogenesis and Clinical Significance of In-Stent Restenosis in Patients with Diabetes. Int. J. Env. Res. Public Health 2021, 18, 11970. [Google Scholar] [CrossRef]
- Wagenknecht, L.E.; Zaccaro, D.; Espeland, M.A.; Karter, A.J.; O’Leary, D.H.; Haffner, S.M. Diabetes and progression of carotid atherosclerosis: The insulin resistance atherosclerosis study. Arter. Thromb. Vasc. Biol. 2003, 23, 1035–1041. [Google Scholar] [CrossRef]
- Achim, A.; Lackó, D.; Hüttl, A.; Csobay-Novák, C.; Csavajda, Á.; Sótonyi, P.; Merkely, B.; Nemes, B.; Ruzsa, Z. Impact of Diabetes Mellitus on Early Clinical Outcome and Stent Restenosis after Carotid Artery Stenting. J. Diabetes Res. 2022, 2022, 4196195. [Google Scholar] [CrossRef] [PubMed]
- Einarson, T.R.; Acs, A.; Ludwig, C.; Panton, U.H. Prevalence of cardiovascular disease in type 2 diabetes: A systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc. Diabetol. 2018, 17, 83. [Google Scholar] [CrossRef] [PubMed]
- Frank, U.; Nikol, S.; Belch, J.; Boc, V.; Brodmann, M.; Carpentier, P.H.; Chraim, A.; Canning, C.; Dimakakos, E.; Gottsäter, A.; et al. ESVM Guideline on peripheral arterial disease. Vasa 2019, 48 (Suppl. 102), 1–79. [Google Scholar] [CrossRef] [PubMed]
- Olinic, D.M.; Spinu, M.; Olinic, M.; Homorodean, C.; Tataru, D.A.; Liew, A.; Schernthaner, G.H.; Stanek, A.; Fowkes, G.; Catalano, M. Epidemiology of peripheral artery disease in Europe: VAS Educational Paper. Int. Angiol. 2018, 37, 327–334. [Google Scholar] [CrossRef]
- Constans, J.; Bura-Rivière, A.; Visona, A.; Brodmann, M.; Abraham, P.; Olinic, D.M.; Madaric, J.; Steiner, S.; Quéré, I.; Mazzolai, L.; et al. Urgent need to clarify the definition of chronic critical limb ischemia—A position paper from the European Society for Vascular Medicine. Vasa 2019, 48, 223–227. [Google Scholar] [CrossRef]
- Heiss, C.; Olinic, D.M.; Belch, J.J.F.; Brodmann, M.; Mazzolai, L.; Stanek, A.; Madaric, J.; Krentz, A.; Schlager, O.; European Society of Vascular Medicine; et al. Management of chronic peripheral artery disease patients with indication for endovascular revascularization. Vasa 2022, 51, 121–137. [Google Scholar] [CrossRef]
- Toth, G.; Johnson, N.P.; Wijns, W.; Toth, B.; Achim, A.; Fournier, S.; Barbato, E. Revascularization decisions in patients with chronic coronary syndromes: Results of the second International Survey on Interventional Strategy (ISIS-2). Int. J. Cardiol. 2021, 336, 38–44. [Google Scholar] [CrossRef]
- Takumi, T.; Miyata, M.; Ohishi, M. Physiological Assessment in Peripheral Artery Disease: Going Beyond Angiography. J. Atheroscler. Thromb. 2016, 23, 44–45. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.C.; Levin, D.C.; Parker, L.; Rao, V.M. Have CT and MR Angiography Replaced Catheter Angiography in Diagnosing Peripheral Arterial Disease? J. Am. Coll. Radiol. 2015, 12, 909–914. [Google Scholar] [CrossRef]
- Veit-Haibach, P.; Huellner, M.W.; Banyai, M.; Mafeld, S.; Heverhagen, J.; Strobel, K.; Sah, B.R. CT perfusion in peripheral arterial disease-hemodynamic differences before and after revascularisation. Eur. Radiol. 2021, 31, 5507–5513. [Google Scholar] [CrossRef]
- Sah, B.R.; Veit-Haibach, P.; Strobel, K.; Banyai, M.; Huellner, M.W. CT-perfusion in peripheral arterial disease–Correlation with angiographic and hemodynamic parameters. PLoS ONE 2019, 14, e0223066. [Google Scholar] [CrossRef] [PubMed]
- Törngren, K.; Eriksson, S.; Arvidsson, J.; Falkenberg, M.; Johnsson, Å.A.; Sjöberg, C.; Lagerstrand, K.; Nordanstig, J. A Reperfusion BOLD-MRI Tissue Perfusion Protocol Reliably Differentiate Patients with Peripheral Arterial Occlusive Disease from Healthy Controls. J. Clin. Med. 2021, 10, 3643. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T.; Takabe, S.; Yanagawa, Y.; Ohshima, Y.; Kagawa, Y.; Shibata, A.; Oyama, K. Laser Doppler blood flowmeter as a useful instrument for the early detection of lower extremity peripheral arterial disease in hemodialysis patients: An observational study. BMC Nephrol. 2019, 20, 470. [Google Scholar] [CrossRef] [PubMed]
- Gerhard-Herman, M.D.; Gornik, H.L.; Barrett, C.; Barshes, N.R.; Corriere, M.A.; Drachman, D.E.; Fleisher, L.A.; Fowkes, F.G.R.; Hamburg, N.M.; Kinlay, S.; et al. 2016 AHA/ACC Guideline on the Management of Patients with Lower Extremity Peripheral Artery Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2017, 135, e686–e725. [Google Scholar] [CrossRef]
- Aboyans, V.; Ricco, J.B.; Bartelink, M.E.L.; Björck, M.; Brodmann, M.; Cohnert, T.; Collet, J.-P.; Czerny, M.; De Carlo, M.; ESC Scientific Document Group; et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteriesEndorsed by: The European Stroke Organization (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur. Heart J. 2018, 39, 763–816. [Google Scholar] [CrossRef]
- Balletshofer, B.; Ito, W.; Lawall, H.; Malyar, N.; Oberländer, Y.; Reimer, P.; Rittig, K.; Zähringer, M. Position Paper on the Diagnosis and Treatment of Peripheral Arterial Disease (PAD) in People with Diabetes Mellitus. Exp. Clin. Endocrinol. Diabetes 2019, 127 (Suppl. 1), S105–S113. [Google Scholar] [CrossRef]
- Jakubiak, G.K.; Pawlas, N.; Cieślar, G.; Stanek, A. Chronic Lower Extremity Ischemia and Its Association with the Frailty Syndrome in Patients with Diabetes. Int. J. Environ. Res. Public Health 2020, 17, 9339. [Google Scholar] [CrossRef]
- Olinic, D.M.; Stanek, A.; Tătaru, D.A.; Homorodean, C.; Olinic, M. Acute Limb Ischemia: An Update on Diagnosis and Management. J. Clin. Med. 2019, 8, 1215. [Google Scholar] [CrossRef]
- Eikelboom, J.W.; Connolly, S.J.; Bosch, J.; Dagenais, G.R.; Hart, R.G.; Shestakovska, O.; Diaz, R.; Alings, M.; Lonn, E.M.; COMPASS Investigators; et al. Rivaroxaban with or without Aspirin in Stable Cardiovascular Disease. N. Engl. J. Med. 2017, 377, 1319–1330. [Google Scholar] [CrossRef]
- Bonaca, M.P.; Bauersachs, R.M.; Anand, S.S.; Debus, E.S.; Nehler, M.R.; Patel, M.R.; Fanelli, F.; Capell, W.H.; Diao, L.; Jaeger, N.; et al. Rivaroxaban in Peripheral Artery Disease after Revascularization. N. Engl. J. Med. 2020, 382, 1994–2004. [Google Scholar] [CrossRef]
- Wimmer, R.A.; Leopoldi, A.; Aichinger, M.; Wick, N.; Hantusch, B.; Novatchkova, M.; Taubenschmid, J.; Hämmerle, M.; Esk, C.; Bagley, J.A.; et al. Human blood vessel organoids as a model of diabetic vasculopathy. Nature 2019, 565, 505–510. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Achim, A.; Stanek, A.; Homorodean, C.; Spinu, M.; Onea, H.L.; Lazăr, L.; Marc, M.; Ruzsa, Z.; Olinic, D.M. Approaches to Peripheral Artery Disease in Diabetes: Are There Any Differences? Int. J. Environ. Res. Public Health 2022, 19, 9801. https://doi.org/10.3390/ijerph19169801
Achim A, Stanek A, Homorodean C, Spinu M, Onea HL, Lazăr L, Marc M, Ruzsa Z, Olinic DM. Approaches to Peripheral Artery Disease in Diabetes: Are There Any Differences? International Journal of Environmental Research and Public Health. 2022; 19(16):9801. https://doi.org/10.3390/ijerph19169801
Chicago/Turabian StyleAchim, Alexandru, Agata Stanek, Călin Homorodean, Mihail Spinu, Horea Laurenţiu Onea, Leontin Lazăr, Mădălin Marc, Zoltán Ruzsa, and Dan Mircea Olinic. 2022. "Approaches to Peripheral Artery Disease in Diabetes: Are There Any Differences?" International Journal of Environmental Research and Public Health 19, no. 16: 9801. https://doi.org/10.3390/ijerph19169801
APA StyleAchim, A., Stanek, A., Homorodean, C., Spinu, M., Onea, H. L., Lazăr, L., Marc, M., Ruzsa, Z., & Olinic, D. M. (2022). Approaches to Peripheral Artery Disease in Diabetes: Are There Any Differences? International Journal of Environmental Research and Public Health, 19(16), 9801. https://doi.org/10.3390/ijerph19169801










