Impact of Body Mass Index on Muscle Strength, Thicknesses, and Fiber Composition in Young Women
Abstract
:1. Introduction
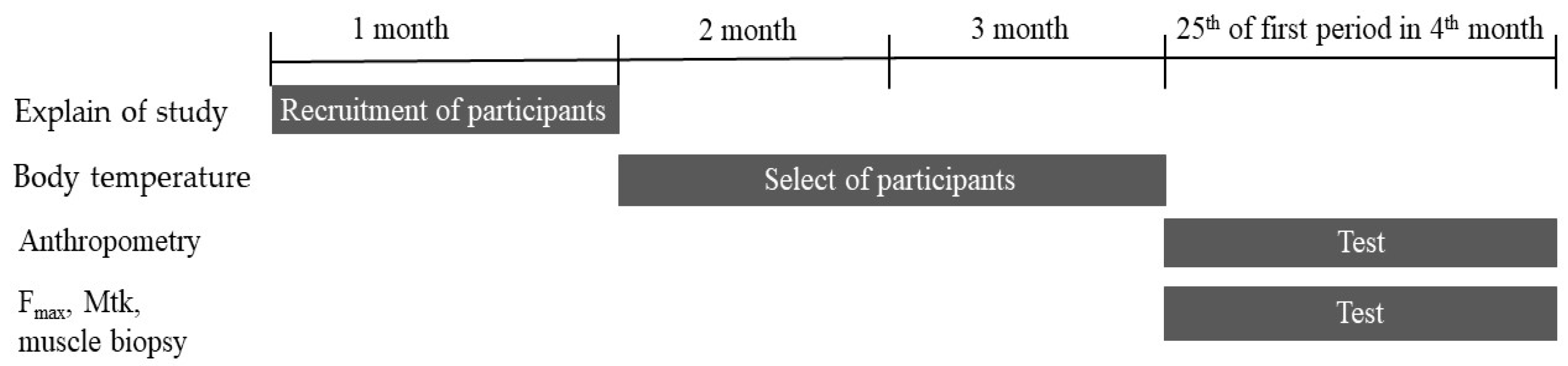
2. Materials and Methods
2.1. Menstrual Cycle Monitoring
2.2. Maximum Isometric Force (Fmax) Measurement
2.3. Muscle Thicknesses (Mtk) Measurements
2.4. Analysis of Muscle Fiber Composition
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lebrun, C.M. Effect of the different phases of the menstrual cycle and oral contraceptives on athletic performance. Sports Med. 1993, 16, 400–430. [Google Scholar] [CrossRef] [PubMed]
- Reilly, T. The Menstrual Cycle and Human Performance: An Overview. Biol. Rhythm. Res. 2000, 31, 29–40. [Google Scholar] [CrossRef]
- Oosthuyse, T.; Bosch, A.N. The effect of the menstrual cycle on exercise metabolism: Implications for exercise performance in eumenorrhoeic women. Sports Med. 2010, 40, 207–227. [Google Scholar] [CrossRef] [PubMed]
- Reis, E.; Frick, U.; Schmidtbleicher, D. Frequency variations of strength training sessions triggered by the phases of the menstrual cycle. Int. J. Sports Med. 1995, 16, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Constantini, N.W.; Dubnov, G.; Lebrun, C.M. The menstrual cycle and sport performance. Clin. Sports Med. 2005, 24, e51–e82, xiii-xiv. [Google Scholar] [CrossRef]
- Janse de Jonge, X.A. Effects of the menstrual cycle on exercise performance. Sports Med. 2003, 33, 833–851. [Google Scholar] [CrossRef]
- Lebrun, C.M. The effect of the phase of the menstrual cycle and the birth control pill on athletic performance. Clin. Sports Med. 1994, 13, 419–441. [Google Scholar] [CrossRef]
- Elliott, K.J.; Cable, N.T.; Reilly, T.; Diver, M.J. Effect of menstrual cycle phase on the concentration of bioavailable 17-beta oestradiol and testosterone and muscle strength. Clin. Sci. 2003, 105, 663–669. [Google Scholar] [CrossRef] [Green Version]
- Redman, L.M.; Weatherby, R.P. Measuring performance during the menstrual cycle: A model using oral contraceptives. Med. Sci. Sports Exerc. 2004, 36, 130–136. [Google Scholar] [CrossRef]
- Rechichi, C.; Dawson, B.; Goodman, C. Athletic performance and the oral contraceptive. Int. J. Sports Physiol. Perform 2009, 4, 151–162. [Google Scholar] [CrossRef]
- McNulty, K.L.; Elliott-Sale, K.J.; Dolan, E.; Swinton, P.A.; Ansdell, P.; Goodall, S.; Thomas, K.; Hicks, K.M. The effects of menstrual cycle phase on exercise performance in eumenorrheic women: A systematic review and meta-analysis. Sports Med. 2020, 50, 1813–1827. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.K.; Sanderson, A.G.; Birch, K.; Bruce, S.A.; Woledge, R.C. Changes in maximal voluntary force of human adductor pollicis muscle during the menstrual cycle. J. Physiol. 1996, 496 Pt 2, 551–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarwar, R.; Niclos, B.B.; Rutherford, O.M. Changes in muscle strength, relaxation rate and fatiguability during the human menstrual cycle. J. Physiol. 1996, 493 Pt 1, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Petrofsky, J.S.; LeDonne, D.M.; Rinehart, J.S.; Lind, A.R. Isometric strength and endurance during the menstrual cycle. Eur. J. Appl. Physiol. Occup. Physiol. 1976, 35, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Elliott-Sale, K.J.; McNulty, K.L.; Ansdell, P.; Goodall, S.; Hicks, K.M.; Thomas, K.; Swinton, P.A.; Dolan, E. The effects of oral contraceptives on exercise performance in women: A systematic review and meta-analysis. Sports Med. 2020, 50, 1785–1812. [Google Scholar] [CrossRef] [PubMed]
- Janse, D.E.J.X.; Thompson, B.; Han, A. Methodological Recommendations for Menstrual Cycle Research in Sports and Exercise. Med. Sci. Sports Exerc. 2019, 51, 2610–2617. [Google Scholar] [CrossRef] [PubMed]
- Frisch, R.E.; Revelle, R. Height and weight at menarche and a hypothesis of menarche. Arch. Dis. Child. 1971, 46, 695–701. [Google Scholar] [CrossRef] [Green Version]
- Frisch, R.E.; Revelle, R. Height and weight at menarche and a hypothesis of critical body weights and adolescent events. Science 1970, 169, 397–399. [Google Scholar] [CrossRef]
- Dars, S.; Sayed, K.; Yousufzai, Z. Relationship of menstrual irregularities to BMI and nutritional status in adolescent girls. Pak. J. Med. Sci. 2014, 30, 141. [Google Scholar]
- Yeung, E.H.; Zhang, C.; Albert, P.S.; Mumford, S.L.; Ye, A.; Perkins, N.J.; Wactawski-Wende, J.; Schisterman, E.F. Adiposity and sex hormones across the menstrual cycle: The BioCycle Study. Int. J. Obes. 2012, 37, 237–243. [Google Scholar] [CrossRef] [Green Version]
- Santoro, N.; Lasley, B.; McConnell, D.; Allsworth, J.; Crawford, S.; Gold, E.B.; Finkelstein, J.S.; Greendale, G.A.; Kelsey, J.; Korenman, S.; et al. Body size and ethnicity are associated with menstrual cycle alterations in women in the early menopausal transition: The Study of Women’s Health across the Nation (SWAN) Daily Hormone Study. J. Clin. Endocrinol. Metab. 2004, 89, 2622–2631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Randolph, J.F., Jr.; Sowers, M.; Gold, E.B.; Mohr, B.A.; Luborsky, J.; Santoro, N.; McConnell, D.S.; Finkelstein, J.S.; Korenman, S.G.; Matthews, K.A.; et al. Reproductive hormones in the early menopausal transition: Relationship to ethnicity, body size, and menopausal status. J. Clin. Endocrinol. Metab. 2003, 88, 1516–1522. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Polotsky, A.J.; Rochester, D.; Berga, S.L.; Loucks, T.; Zeitlian, G.; Gibbs, K.; Polotsky, H.N.; Feng, S.; Isaac, B.; et al. Pulsatile luteinizing hormone amplitude and progesterone metabolite excretion are reduced in obese women. J. Clin. Endocrinol. Metab. 2007, 92, 2468–2473. [Google Scholar] [CrossRef] [PubMed]
- Cheymol, G. Effects of obesity on pharmacokinetics implications for drug therapy. Clin. Pharm. 2000, 39, 215–231. [Google Scholar] [CrossRef] [PubMed]
- Castro-Fernandez, C.; Olivares, A.; Soderlund, D.; Lopez-Alvarenga, J.C.; Zambrano, E.; Veldhuis, J.D.; Ulloa-Aguirre, A.; Mendez, J.P. A preponderance of circulating basic isoforms is associated with decreased plasma half-life and biological to immunological ratio of gonadotropin-releasing hormone-releasable luteinizing hormone in obese men. J. Clin. Endocrinol. Metab. 2000, 85, 4603–4610. [Google Scholar] [PubMed] [Green Version]
- Azziz, R. Reproductive endocrinologic alterations in female asymptomatic obesity. Fertil Steril 1989, 52, 703–725. [Google Scholar]
- Pescatello, L.S.; Kelsey, B.K.; Price, T.B.; Seip, R.L.; Angelopoulos, T.J.; Clarkson, P.M.; Gordon, P.M.; Moyna, N.M.; Visich, P.S.; Zoeller, R.F.; et al. The muscle strength and size response to upper arm, unilateral resistance training among adults who are overweight and obese. J. Strength Cond. Res. 2007, 21, 307–313. [Google Scholar]
- Zoico, E.; Di Francesco, V.; Guralnik, J.M.; Mazzali, G.; Bortolani, A.; Guariento, S.; Sergi, G.; Bosello, O.; Zamboni, M. Physical disability and muscular strength in relation to obesity and different body composition indexes in a sample of healthy elderly women. Int J. Obes. Relat. Metab. Disord. 2004, 28, 234–241. [Google Scholar] [CrossRef] [Green Version]
- Rolland, Y.; Lauwers-Cances, V.; Pahor, M.; Fillaux, J.; Grandjean, H.; Vellas, B. Muscle strength in obese elderly women: Effect of recreational physical activity in a cross-sectional study. Am. J. Clin. Nutr. 2004, 79, 552–557. [Google Scholar] [CrossRef] [Green Version]
- Albertson, B.D.; Zinaman, M.J. The prediction of ovulation and monitoring of the fertile period. Adv. Contracept. 1987, 3, 263–290. [Google Scholar] [CrossRef]
- Kelly, G. Body temperature variability (Part 1): A review of the history of body temperature and its variability due to site selection, biological rhythms, fitness, and aging. Altern. Med. Rev. 2006, 11, 278–293. [Google Scholar] [PubMed]
- Owen, J.A., Jr. Physiology of the menstrual cycle. Am. J. Clin. Nutr. 1975, 28, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Su, H.W.; Yi, Y.C.; Wei, T.Y.; Chang, T.C.; Cheng, C.M. Detection of ovulation, a review of currently available methods. Bioeng. Transl. Med. 2017, 2, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Angelozzi, M.; Madama, M.; Corsica, C.; Calvisi, V.; Properzi, G.; McCaw, S.T.; Cacchio, A. Rate of force development as an adjunctive outcome measure for return-to-sport decisions after anterior cruciate ligament reconstruction. J. Orthop. Sports Phys. Ther. 2012, 42, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Martinson, H.; Stokes, M.J. Measurement of anterior tibial muscle size using real-time ultrasound imaging. Eur. J. Appl. Physiol. Occup. Physiol. 1991, 63, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Reimer, C.D.; Gaulrapp, H.; Kelle, H. Sonographie der Muskeln, Sehnen und Nerven; Deutscher Ärzte-Verlag: Köln, Germany, 2004. [Google Scholar]
- Evans, W.J.; Coggan, A.R. Muscle biopsy as a tool in the study of aging. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 1995, 50, 30–34. [Google Scholar] [CrossRef]
- Patel, H.P.; Jameson, K.A.; Syddall, H.E.; Martin, H.J.; Stewart, C.E.; Cooper, C.; Sayer, A.A. Developmental influences, muscle morphology, and sarcopenia in community-dwelling older men. J. Gerontol. A Biol. Sci. Med. Sci. 2012, 67, 82–87. [Google Scholar] [CrossRef] [Green Version]
- Bergström, J. Muscle electolytes in man. Scand. J. Clin. Lab. Investig. 1962, 14, 1–110. [Google Scholar]
- Goebel, H.H. Muscle Biopsy. In Diagnostic Neuropathology; Springer: Berlin/Heidelberg, Germany, 1990; pp. 203–290. [Google Scholar]
- Brooke, M.H.; Kaiser, K.K. Three “myosin adenosine triphosphatase” systems: The nature of their pH lability and sulfhydryl dependence. J. Histochem. Cytochem. 1970, 18, 670–672. [Google Scholar] [CrossRef]
- Yan, Z. Skeletal muscle adaptation and cell cycle regulation. Exerc. Sport Sci. Rev. 2000, 28, 24–26. [Google Scholar]
- Hulens, M.; Vansant, G.; Lysens, R.; Claessens, A.L.; Muls, E.; Brumagne, S. Study of differences in peripheral muscle strength of lean versus obese women: An allometric approach. Int. J. Obes. Relat. Metab. Disord. 2001, 25, 676–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lafortuna, C.L.; Maffiuletti, N.A.; Agosti, F.; Sartorio, A. Gender variations of body composition, muscle strength and power output in morbid obesity. Int. J. Obes 2005, 29, 833–841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maffiuletti, N.A.; Jubeau, M.; Munzinger, U.; Bizzini, M.; Agosti, F.; De Col, A.; Lafortuna, C.L.; Sartorio, A. Differences in quadriceps muscle strength and fatigue between lean and obese subjects. Eur. J. Appl. Physiol. 2007, 101, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Hickey, M.S.; Carey, J.O.; Azevedo, J.L.; Houmard, J.A.; Pories, W.J.; Israel, R.G.; Dohm, G.L. Skeletal muscle fiber composition is related to adiposity and in vitro glucose transport rate in humans. Am. J. Physiol. 1995, 268 Pt 1, E453–E457. [Google Scholar] [CrossRef] [PubMed]
- Wade, A.J.; Marbut, M.M.; Round, J.M. Muscle fibre type and aetiology of obesity. Lancet 1990, 335, 805–808. [Google Scholar] [CrossRef]
- Sung, E.; Han, A.; Hinrichs, T.; Vorgerd, M.; Manchado, C.; Platen, P. Effects of follicular versus luteal phase-based strength training in young women. Springerplus 2014, 3, 668. [Google Scholar] [CrossRef] [Green Version]
- Rickenlund, A.; Carlstrom, K.; Ekblom, B.; Brismar, T.B.; Von Schoultz, B.; Hirschberg, A.L. Effects of oral contraceptives on body composition and physical performance in female athletes. J. Clin. Endocrinol. Metab. 2004, 89, 4364–4370. [Google Scholar] [CrossRef] [Green Version]
- Wirth, J.C.; Lohman, T.G. The relationship of static muscle function to use of oral contraceptives. Med. Sci. Sports Exerc. 1982, 14, 16–20. [Google Scholar] [CrossRef]
- Peters, C.; Burrows, M. Androgenicity of the progestin in oral contraceptives does not affect maximal leg strength. Contraception 2006, 74, 487–491. [Google Scholar] [CrossRef]
- Higginbotham, S. Contraceptive considerations in obese women: Release date 1 September 2009, SFP Guideline 20091. Contraception 2009, 80, 583–590. [Google Scholar]
- Lexell, J.; Taylor, C.; Sjostrom, M. Analysis of sampling errors in biopsy techniques using data from whole muscle cross sections. J. Appl. Physiol. 1985, 59, 1228–1235. [Google Scholar] [CrossRef] [PubMed]
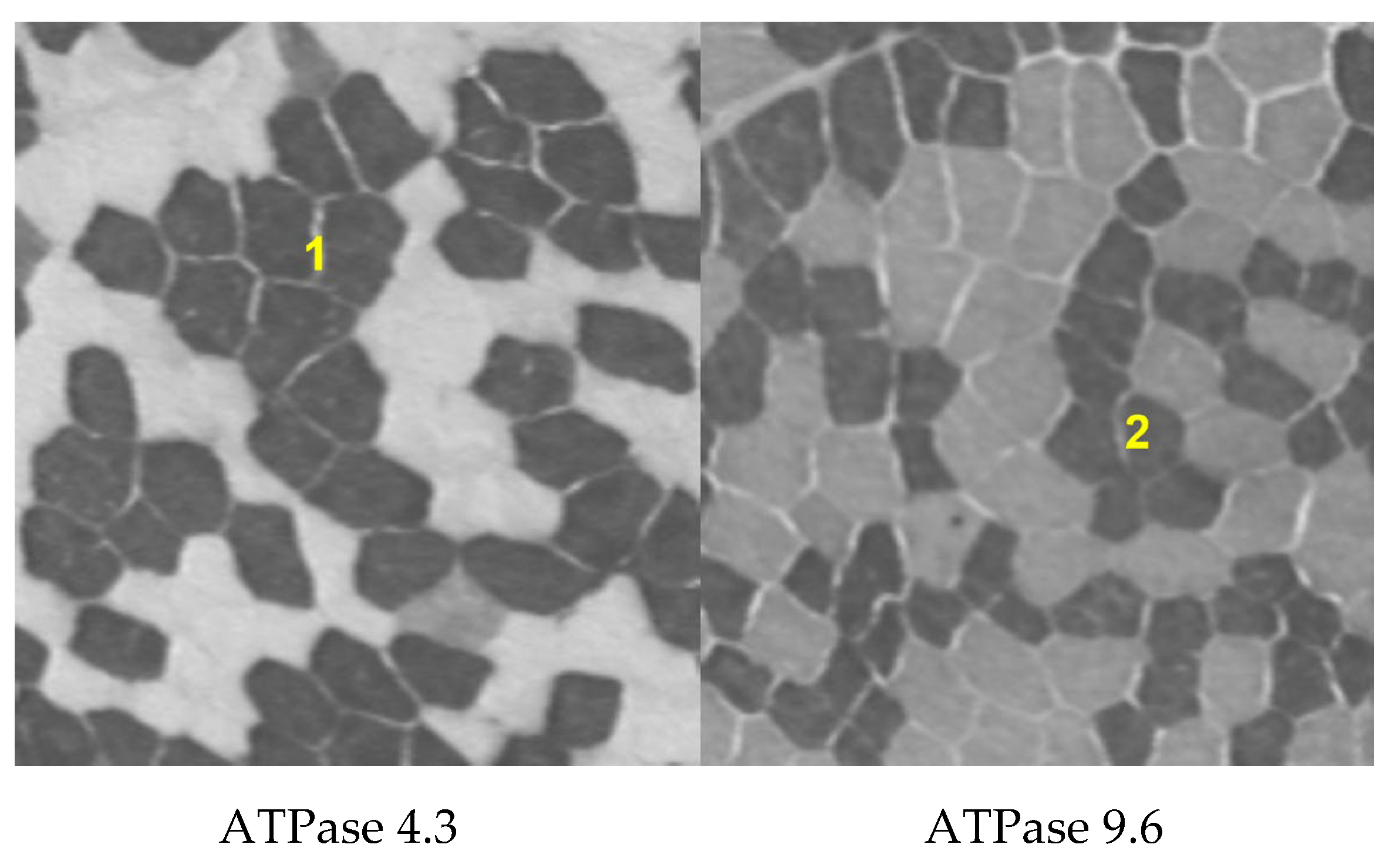
| Characteristics | non-OC (n = 31) | OC (n = 27) | ||||
|---|---|---|---|---|---|---|
| BMIlow (n = 10) | BMInorm (n = 10) | BMIhigh (n = 11) | BMIlow (n = 9) | BMInorm (n = 9) | BMIhigh (n = 9) | |
| Age (y) | 25.10 ± 4.61 | 26.18 ± 4.62 | 25.00 ± 4.69 | 24.25 ± 4.33 | 24.90 ± 4.09 | 25.20 ± 5.31 |
| Height (m) | 1.66 ± 0.04 | 1.62 ± 0.06 | 1.64 ± 0.04 | 1.65 ± 0.08 | 1.66 ± 0.06 | 1.67 ± 0.06 |
| Weight (kg) | 49.70 ± 3.59 | 58.91 ± 5.03 | 75.00 ± 5.13 | 48.13 ± 6.13 | 60.80 ± 4.32 | 77.20 ± 4.85 |
| BMI (kg/m2) | 17.94 ± 1.14 | 22.43 ± 1.19 | 27.84 ± 1.91 | 17.67 ± 0.95 | 22.13 ± 0.99 | 27.28 ± 1.65 |
| Variables | non-OC (n = 31) | OC (n = 27) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BMIlow (n = 10) | BMInorm (n = 10) | BMIhigh (n = 11) | F | p | BMIlow (n = 9) | BMInorm (n = 9) | BMIhigh (n = 9) | F | p | |
| Fmax (N) | 619.26 ± 68.50 | 657.09 ± 128.18 | 782.44 ± 157.00 | 1.65 | 0.23 | 655.42 ± 114.76 | 842.02 ± 136.32 | 1087.42 ± 311.05 | 5.77 | 0.01 * |
| Mtk (cm2) | 4.05 ± 0.51 | 4.34 ± 0.75 | 5.12 ± 0.63 | 2.19 | 0.15 | 3.88 ± 1.96 | 4.15 ± 0.37 | 4.71 ± 0.37 | 4.52 | 0.03 * |
| Fmax/Mtk (N) | 155.62 ± 31.36 | 156.31 ± 44.79 | 152.00 ± 18.72 | 0.14 | 0.99 | 168.66 ± 36.26 | 203.06 ± 27.93 | 232.46 ± 71.54 | 1.94 | 0.18 |
| Group | non-OC (n = 5) | OC (n = 5) | ||
|---|---|---|---|---|
| Muscle fiber type | Type 1 | Type 2 | Type 1 | Type 2 |
| BMIlow (%) | 49.57 | 50.43 | 59.23 | 40.77 |
| BMInorm(%) | 47.92 | 52.08 | 49.83 | 50.17 |
| BMIhigh(%) | 59.38 | 40.62 | 32.96 | 67.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sung, E.-S.; Han, A.; Hinrichs, T.; Vorgerd, M.; Platen, P. Impact of Body Mass Index on Muscle Strength, Thicknesses, and Fiber Composition in Young Women. Int. J. Environ. Res. Public Health 2022, 19, 9789. https://doi.org/10.3390/ijerph19169789
Sung E-S, Han A, Hinrichs T, Vorgerd M, Platen P. Impact of Body Mass Index on Muscle Strength, Thicknesses, and Fiber Composition in Young Women. International Journal of Environmental Research and Public Health. 2022; 19(16):9789. https://doi.org/10.3390/ijerph19169789
Chicago/Turabian StyleSung, Eun-Sook, Ahreum Han, Timo Hinrichs, Matthias Vorgerd, and Petra Platen. 2022. "Impact of Body Mass Index on Muscle Strength, Thicknesses, and Fiber Composition in Young Women" International Journal of Environmental Research and Public Health 19, no. 16: 9789. https://doi.org/10.3390/ijerph19169789
APA StyleSung, E.-S., Han, A., Hinrichs, T., Vorgerd, M., & Platen, P. (2022). Impact of Body Mass Index on Muscle Strength, Thicknesses, and Fiber Composition in Young Women. International Journal of Environmental Research and Public Health, 19(16), 9789. https://doi.org/10.3390/ijerph19169789







