The Impact of International Nonproprietary Names Integration on Prescribing Reimbursement Medicines for Arterial Hypertension and Analysis of Medication Errors in Latvia
Abstract
:1. Introduction
- list A, which includes medicines of equivalent effectiveness;
- list B, in which the medicines included do not have equivalent effectiveness with the medicine to be reimbursed;
2. Materials and Methods
2.1. Study Subjects and Data Collection
2.2. Sample Size and Sampling Technique
2.3. Reference Medication and Medication Error Identification
2.4. Data Analysis
3. Results
3.1. Patients’ Demographic Data
3.2. Prescription Information Filled by Physicians
3.3. Description of Prescribed Medicine Dispensing and Detected Errors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Guidelines on the Use of International Nonproprietary Names (INNs) for Pharmaceutical Substances; International Nonproprietary Names (INN) Programme; World Health Organization: Geneva, Switzerland, 2017; pp. 5–7. [Google Scholar]
- National Health Service Republic of Latvia. Medicines to Be Reimbursed. Available online: https://www.vmnvd.gov.lv/en/medicines-be-reimbursed (accessed on 30 June 2022).
- World Health Organization. Medicines Reimbursement Policies in Europe; World Health Organization Regional Office for Europe: Copenhagen, Denmark, 2018. [Google Scholar]
- Cabinet of Ministers. Procedures for the Reimbursement of Expenditures for the Acquisition of Medicinal Products and Medical Devices Intended for the Outpatient Medical Treatment; Latvijas Vēstnesis: Riga, Latvia, 2018. [Google Scholar]
- European Observatory on Health Systems and Policies. Latvia: Country Health Profile 2021, State of Health in the EU. Eur. J. Public Health 2021, 28, cky213.595. [Google Scholar] [CrossRef]
- State Agency of Medicines of the Republic of Latvia. Campaign “Know and Don’t Overpay”. Available online: https://www.zva.gov.lv/en/news-and-publications/news/campaign-know-and-dont-overpay-has-been-launched-inform-patients-how-reimbursed-medicines-will-be (accessed on 30 May 2022).
- Williams, B.; Mancia, G.; Spiering, W.; Rosei, E.A.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; De Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for themanagement of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef] [PubMed]
- Gavrilova, A.; Bandere, D.; Logviss, K.; Šmits, D.; Urtāne, I. Adherence level to arterial hypertension treatment: A cross-sectional patient survey and retrospective analysis of the nhs prescription database. Healthcare 2021, 9, 1085. [Google Scholar] [CrossRef] [PubMed]
- Gavrilova, A.; Bandere, D.; Rutkovska, I.; Šmits, D.; Mauriņa, B.; Poplavska, E.; Urtāne, A.I. Knowledge about Disease, Medication Therapy, and Related Medication Adherence Levels among Patients with Hypertension. Medicina 2019, 55, 715. [Google Scholar] [CrossRef] [PubMed]
- DiPette, D.J.; Skeete, J.; Ridley, E.; Campbell, N.R.C.; Lopez-Jaramillo, P.; Kishore, S.P.; Jaffe, M.G.; Coca, A.; Townsend, R.R.; Ordunez, P. Fixed-dose combination pharmacologic therapy to improve hypertension control worldwide: Clinical perspective and policy implications. J. Clin. Hypertens. 2019, 21, 4. [Google Scholar] [CrossRef] [PubMed]
- State Agency of Medicines Republic of Latvia. Medicinal Product Register of Latvia. Available online: https://dati.zva.gov.lv/zalu-registrs/en (accessed on 29 June 2022).
- State Agency of Medicine Republic of Latvia. Statistics on Medicines Consumption 2018; State Agency of Medicines: Riga, Latvia, 2018. [Google Scholar]
- World Health Organization. Who Launches Global Effort to Halve Medication-Related Errors in 5 Years; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Dilliard, R.; Hagemeier, N.E.; Ratliff, B.; Maloney, R. An analysis of pharmacists’ workplace patient safety perceptions across practice setting and role characteristics. Explor. Res. Clin. Soc. Pharm. 2021, 2, 100042. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Medication Errors. Available online: https://www.ema.europa.eu/en/human-regulatory/post-authorisation/pharmacovigilance/medication-errors (accessed on 12 July 2022).
- The National Health Service of Latvia. Reimbursement Drug Lists. Available online: https://www.vmnvd.gov.lv/lv/kompensejamo-zalu-saraksti (accessed on 27 June 2022).
- Mahfoud, F.; Kieble, M.; Enners, S.; Kintscher, U.; Laufs, U.; Böhm, M.; Schulz, M. Use of fixed-dose combination antihypertensives in Germany between 2016 and 2020: An example of guideline inertia. Clin. Res. Cardiol. 2022, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Salmane Kulikovska, I.; Poplavska, E.; Ceha, M.; Mezinska, S. Use of generic medicines in Latvia: Awareness, opinions and experiences of the population 11 Medical and Health Sciences 1117 Public Health and Health Services. J. Pharm. Policy Pract. 2019, 12, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Tirivangani, T.; Alpo, B.; Kibuule, D.; Gaeseb, J.; Adenuga, B.A. Impact of COVID-19 pandemic on pharmaceutical systems and supply chain—a phenomenological study. Explor. Res. Clin. Soc. Pharm. 2021, 2, 100037. [Google Scholar] [CrossRef] [PubMed]
- Guerin, P.J.; Singh-Phulgenda, S.; Strub-Wourgaft, N. The consequence of COVID-19 on the global supply of medical products: Why Indian generics matter for the world? F1000Research 2020, 9, 225. [Google Scholar] [CrossRef] [PubMed]
- Mohamed Ibrahim, O.; Ibrahim, R.M.; Al Meslamani, A.Z.; Al Mazrouei, N. Dispensing errors in community pharmacies in the United Arab Emirates: Investigating incidence, types, severity, and causes. Pharm. Pract. 2020, 18, 2111. [Google Scholar] [CrossRef] [PubMed]
- Maharaj, S.; Brahim, A.; Brown, H.; Budraj, D.; Caesar, V.; Calder, A.; Carr, D.; Castillo, D.; Cedeno, K.; Janodia, M.D. Identifying dispensing errors in pharmacies in a medical science school in Trinidad and Tobago. J. Pharm. Policy Pract. 2020, 13, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Flynn, E.A.; Barker, K.N.; Carnahan, B.J. National observational study of prescription dispensing accuracy and safety in 50 pharmacies. J. Am. Pharm. Assoc. 2003, 43, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, P.; Herborg, H.; Mortensen, A.R.; Knudsen, M.; Hellebek, A. Preventing medication errors in community pharmacy: Frequency and seriousness of medication errors. Qual. Saf. Health Care 2007, 16, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Gavrilova, A.; Latkovskis, G.; Urtāne, I. Patterns of Direct Oral Anticoagulant Prescriptions in Latvia: A Retrospective Analysis of Electronic Records. In Proceedings of the International Scientific Conference on Medicine if the 80th Scientific Conference of the University of Latvia, Riga, Latvia, 25–26 March 2022; Volume 58, p. 13. [Google Scholar]
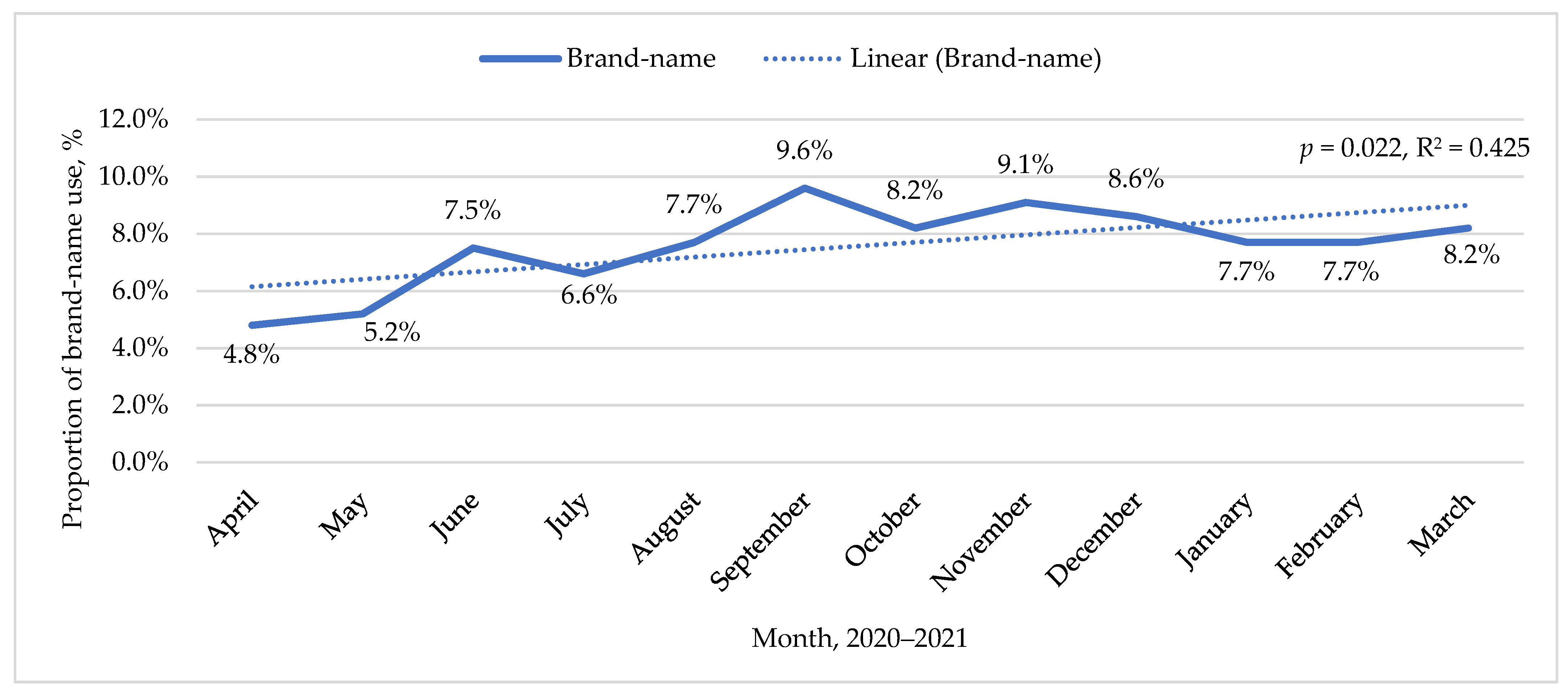
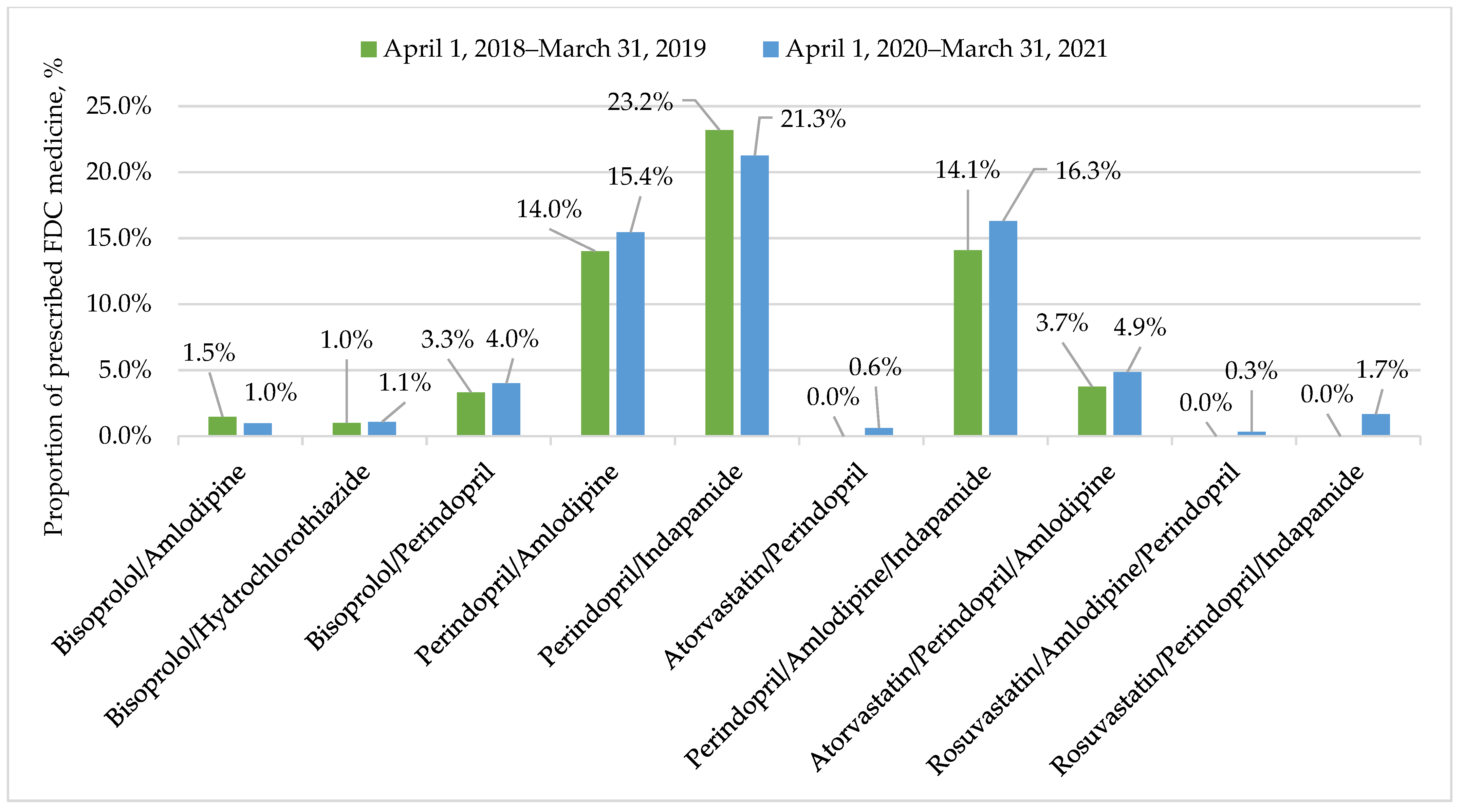
| Variable | Period | |
|---|---|---|
| 1 April 2018–31 March 2019 | 1 April 2020–31 March 2021 | |
| Total number of prescriptions, n | 8279 | 8260 |
| Number of patients, n | 7876 | 7912 |
| Age, years (mean ± SD) | 68.6 (12.4) | 68.0 (12.6) |
| Female sex, n (%) | 5102 (64.8) | 4949 (62.6) |
| Residence area *, n (%): | ||
| City | 3845 (48.8) | 4275 (54.0) |
| Countryside | 4024 (51.1) | 3626 (45.8) |
| Variables | Period | |
|---|---|---|
| 1 April 2018–31 March 2019 | 1 April 2020–31 March 2021 | |
| Total number of prescriptions, n | 8279 | 8260 |
| INN use, n (%) | 175 (2.1) | 7625 (92.3) |
| Brand-name use, n (%) | 8104 (97.9) | 635 (7.7) |
| Reference medicine or the cheapest in the reimbursement group *, n (%) | 1087 (13.4) | 134 (21.1) |
| More expensive than reference medicine or the cheapest in the reimbursement group *, n (%) | 5829 (71.9) | 355 (55.9) |
| Do not have analogues *, n (%) | 1188 (14.7) | 146 (23.0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gavrilova, A.; Zolovs, M.; Latkovskis, G.; Urtāne, I. The Impact of International Nonproprietary Names Integration on Prescribing Reimbursement Medicines for Arterial Hypertension and Analysis of Medication Errors in Latvia. Int. J. Environ. Res. Public Health 2022, 19, 10156. https://doi.org/10.3390/ijerph191610156
Gavrilova A, Zolovs M, Latkovskis G, Urtāne I. The Impact of International Nonproprietary Names Integration on Prescribing Reimbursement Medicines for Arterial Hypertension and Analysis of Medication Errors in Latvia. International Journal of Environmental Research and Public Health. 2022; 19(16):10156. https://doi.org/10.3390/ijerph191610156
Chicago/Turabian StyleGavrilova, Anna, Maksims Zolovs, Gustavs Latkovskis, and Inga Urtāne. 2022. "The Impact of International Nonproprietary Names Integration on Prescribing Reimbursement Medicines for Arterial Hypertension and Analysis of Medication Errors in Latvia" International Journal of Environmental Research and Public Health 19, no. 16: 10156. https://doi.org/10.3390/ijerph191610156
APA StyleGavrilova, A., Zolovs, M., Latkovskis, G., & Urtāne, I. (2022). The Impact of International Nonproprietary Names Integration on Prescribing Reimbursement Medicines for Arterial Hypertension and Analysis of Medication Errors in Latvia. International Journal of Environmental Research and Public Health, 19(16), 10156. https://doi.org/10.3390/ijerph191610156






