Effectiveness of Microcurrent Therapy for Treating Pressure Ulcers in Older People: A Double-Blind, Controlled, Randomized Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
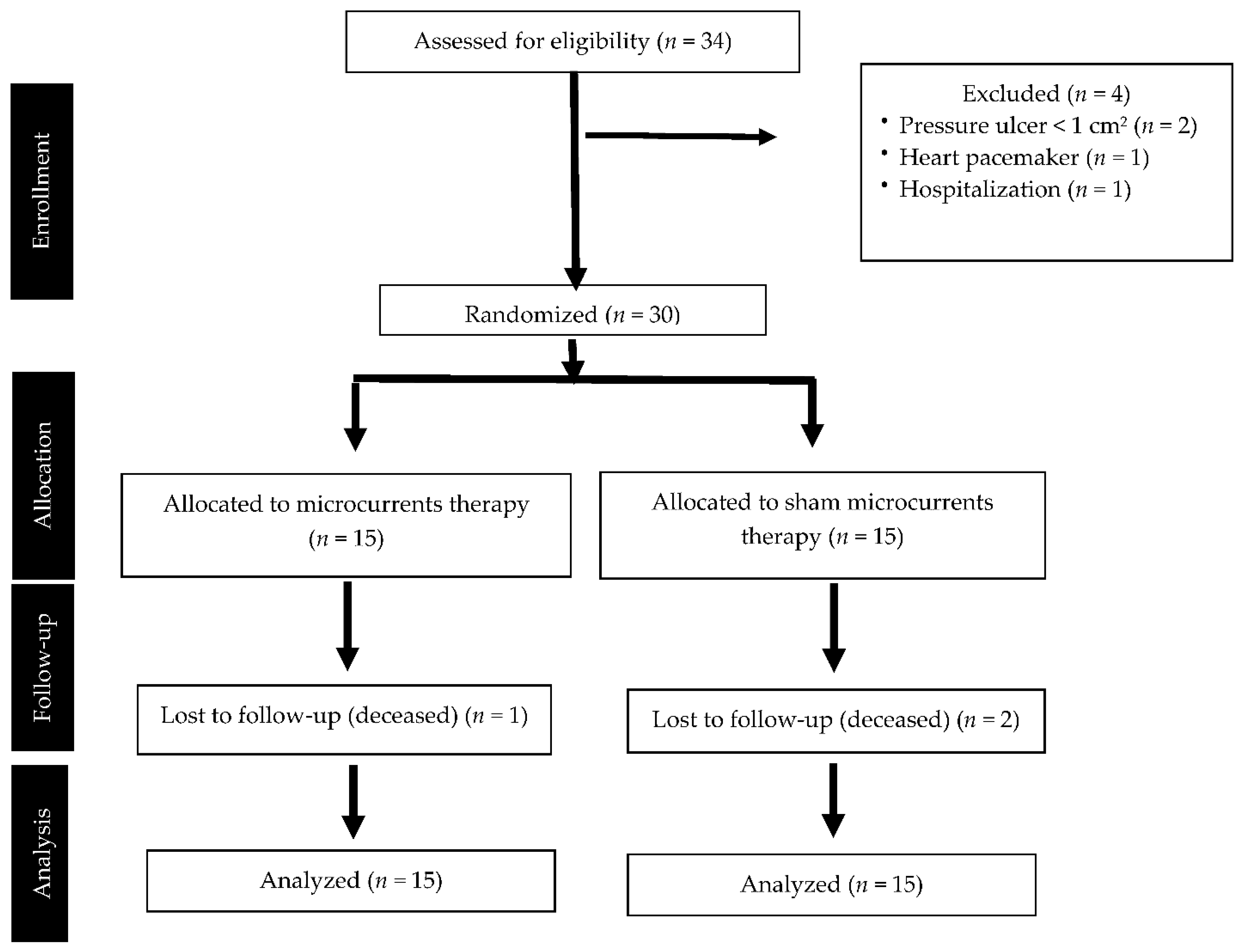
2.2. Participants and Setting
2.3. Intervention
2.4. Variables
2.5. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics of Participants at Baseline
3.2. Effect on Pressure Ulcer Healing
3.3. Effect on Secondary Variables
3.4. Blinding Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Menzel, J. Pressure Ulcers in the Elderly, as a Public Health Problem. J. Gen. Pract. 2014, 2, 174. [Google Scholar] [CrossRef]
- McGinnis, E.; Briggs, M.; Collinson, M.; Wilson, L.; Dealey, C.; Brown, J.; Nixon, J. Pressure ulcer related pain in community populations: A prevalence survey. BMC Nurs. 2014, 13, 16. [Google Scholar] [CrossRef] [PubMed]
- Gorecki, C.; Brown, J.M.; Nelson, E.A.; Briggs, M.; Schoonhoven, L.; Dealey, C.; Defloor, T.; Nixon, J.; on behalf of the European Quality of Life Pressure Ulcer Project group. Impact of Pressure Ulcers on Quality of Life in Older Patients: A Systematic Review. J. Am. Geriatr. Soc. 2009, 57, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Barry, M.; Nugent, L. Pressure ulcer prevention in frail older people. Nurs. Stand. 2015, 30, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.; Cadarette, S.; Wodchis, W.; Wong, J.; Mittmann, N.; Krahn, M. Cost-of-illness studies in chronic ulcers: A systematic review. J. Wound Care 2017, 26, S4–S14. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, L.; Mushahwar, V.K.; Ho, C.; Dukelow, S.P.; Chan, L.L.; Chan, K.M. The mechanisms and evidence of efficacy of electrical stimulation for healing of pressure ulcer: A systematic review. Wound Repair Regen. 2014, 22, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Qaseem, A.; Humphrey, L.L.; Forciea, M.A.; Starkey, M.; Denberg, T.D. Treatment of pressure ulcers: A clinical practice guideline from the American College of Physicians. Ann. Intern. Med. 2015, 162, 370–379. [Google Scholar] [CrossRef]
- Houghton, P.E. Clinical Trials Involving Biphasic Pulsed Current, MicroCurrent, and/or Low-Intensity Direct Current. Adv. Wound Care 2014, 3, 166–183. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M. Electrical fields in wound healing—An overriding signal that directs cell migration. Semin. Cell Dev. Biol. 2009, 20, 674–682. [Google Scholar] [CrossRef]
- Todd, I.; Clothier, R.H.; Huggins, M.L.; Patel, N.; Searle, K.C.; Jeyarajah, S.; Pradel, L.; Lacey, K.L. Electrical Stimulation of Transforming Growth Factor-β1 Secretion by Human Dermal Fibroblasts and the U937 Human Monocytic Cell Line. Altern. Lab. Anim. 2001, 29, 693–701. [Google Scholar] [CrossRef]
- Lee, B.Y.; Wendell, K.; Al-Waili, N.; Butler, G. Ultra-low microcurrent therapy: A novel approach for treatment of chronic resistant wounds. Adv. Ther. 2007, 24, 1202–1209. [Google Scholar] [CrossRef] [PubMed]
- Ramadhinara, A.; Poulas, K. Use of wireless microcurrent stimulation for the treatment of diabetes-related wounds: 2 case reports. Adv. Skin Wound Care. 2013, 26, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.Y.; Al-Waili, N.; Stubbs, D.; Wendell, K.; Butler, G.; Al-Waili, T.; Al-Waili, A. Ultra-low microcurrent in the management of diabetes mellitus, hypertension and chronic wounds: Report of twelve cases and discussion of mechanism of action. Int. J. Med Sci. 2009, 7, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Wirsing, P.G.; Habrom, A.D.; Zehnder, T.M.; Friedli, S.; Blatti, M. Wireless micro current stimulation–an innovative electrical stimulation method for the treatment of patients with leg and diabetic foot ulcers. Int. Wound J. 2015, 12, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.O. A study to detect the efficacy of Micro-current Electrical Therapy on decubitus wound. J. Med. Sci. 2007, 7, 1320–1324. [Google Scholar] [CrossRef]
- Lessiani, G.; Galati, V.; Iodice, P. Efficacy of Modulated Microcurrent Stimulation in Pressure Ulcers Treatment: A Monocentric, Prospective, Double-Blind, Randomized Study. J. Nov. Physiother. 2014, 4, 224. [Google Scholar]
- National Pressure Ulcer Advisory Panel; European Pressure Ulcer Advisory Panel. Prevention and Treatment of Pressure Ulcers: Clinical Practice Guideline; National Pressure Ulcer Advisory Panel: Washington, DC, USA, 2009. [Google Scholar]
- de Souza, D.M.S.T.; de Gouveia Santos, V.L.C.; Iri, H.K.; Sadasue Oguri, M.Y. Predictive Validity of the Braden Scale for Pressure Ulcer Risk in Elderly Residents of Long-Term Care Facilities. Geriatr. Nurs. 2010, 31, 95–104. [Google Scholar] [CrossRef]
- World Medical Association. Declaration of Helsinki. Ethical principles for medical research involving human subjects. In Proceedings of the 64th WMA General Assembly, Fortaleza, Brazil, 16–19 October 2013; p. 28. [Google Scholar]
- Schultz, G.S.; Sibbald, R.G.; Falanga, V.; Ayello, E.A.; Dowsett, C.; Harding, K.; Romanelli, M.; Stacey, M.C.S.; Teot, L.; Vanscheidt, W. Wound bed preparation: A systematic approach to wound management. Wound Repair Regen. 2003, 11, S1–S28. [Google Scholar] [CrossRef] [PubMed]
- Blanco Zapata, R.M.; López García, E.; Quesada Ramos, C.; García Rodríguez, M.R. Guide to Recommendations Based on Evidence in Prevention and Treatment of Pressure Ulcers in Adults; San Sebastián: Osakidetza, Yemen, 2015. [Google Scholar]
- Vertesi, A.; Lever, J.A.; Molloy, D.W.; Sanderson, B.; Tuttle, I.; Pokoradi, L.; Principi, E. Standardized Mini-Mental State Examination. Use and interpretation. Can. Fam. Physician 2001, 47, 2018–2023. [Google Scholar]
- Stotts, N.A.; Rodeheaver, G.T.; Thomas, D.R.; Frantz, R.A.; Bartolucci, A.A.; Sussman, C.; Maklebust, J. An Instrument to Measure Healing in Pressure UlcersDevelopment and Validation of the Pressure Ulcer Scale for Healing (PUSH). J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, M795–M799. [Google Scholar] [CrossRef] [PubMed]
- Timar-Banu, O.; Beauregard, H.; Tousignant, J.; Lassonde, M.; Harris, P.; Viau, G.; Vachon, L.; Levy, E.; Abribat, T. Development of noninvasive and quantitative methodologies for the assessment of chronic ulcers and scars in humans. Wound Repair Regen. 2001, 9, 123–132. [Google Scholar] [CrossRef]
- Bang, H.; Flaherty, S.P.; Kolahi, J.; Park, J. Blinding assessment in clinical trials: A review of statistical methods and a proposal of blinding assessment protocol. Clin. Res. Regul. Aff. 2010, 27, 42–51. [Google Scholar] [CrossRef]
- Kolahi, J.; Bang, H.; Park, J. Towards a proposal for assessment of blinding success in clinical trials: Up-to-date review. Community Dent. Oral Epidemiol. 2009, 37, 477–484. [Google Scholar] [CrossRef]
- James, K.E.; Bloch, D.A.; Lee, K.K.; Kraemer, H.C.; Fuller, R.K. An index for assessing blindness in a multi-centre clinical trial: Disulfiram for alcohol cessation—A VA cooperative study. Stat. Med. 1996, 15, 1421–1434. [Google Scholar] [CrossRef]
- Avendaño-Coy, J.; López-Muñoz, P.; Serrano-Muñoz, D.; Comino-Suárez, N.; Avendaño-López, C.; Martin-Espinosa, N. Electrical microcurrent stimulation therapy for wound healing: A meta-analysis of randomized clinical trials. J. Tissue Viability 2021, 31, 268–277. [Google Scholar] [CrossRef]
- Guest, J.F.; Singh, H.; Rana, K.; Vowden, P. Cost-effectiveness of an electroceutical device in treating non-healing venous leg ulcers: Results of an RCT. J. Wound Care 2018, 27, 230–243. [Google Scholar] [CrossRef]
- Unal, A.; Gulsen, C.; Altug, F. Effectiveness of microcurrent therapy on sacral pressure ulcer: Our first experience. Niger. J. Clin. Pract. 2017, 20, 397–398. [Google Scholar]
- Angeloti, P.F.; Santos, G.M.T.; Mendonça, F.A.S. Antimicrobial effect of the microcurrent application after burns of 2° degree induced in the skin of the back in Wistar mice. Discov. Sci. 2013, 5, 37–39. [Google Scholar]
- Ibrahim, Z.M.; Waked, I.S.; Ibrahim, O. Negative pressure wound therapy versus microcurrent electrical stimulation in wound healing in burns. J. Wound Care 2019, 28, 214–219. [Google Scholar] [CrossRef]
- Polak, A.; Kucio, C.; Kloth, L.; Paczula, M.; Hordynska, E.; Ickowicz, T.; Blaszczak, E.; Kucio, E.; Oleszczyk, K.; Ficek, K.; et al. A Randomized, Controlled Clinical Study to Assess the Effect of Anodal and Cathodal Electrical Stimulation on Periwound Skin Blood Flow and Pressure Ulcer Size Reduction in Persons with Neurological Injuries. Ostomy Wound Manag. 2018, 64, 10–29. [Google Scholar] [CrossRef]
- Brem, H.; Tomic-Canic, M. Cellular and molecular basis of wound healing in diabetes. J. Clin. Investig. 2007, 117, 1219–1222. [Google Scholar] [CrossRef]
| Outcomes | Participants (n = 30) | Microcurrents Group (n = 15) | Sham Group (n = 15) | Intergroup Differences (p Value) |
|---|---|---|---|---|
| Age (years) Mean (SD) | 87.6 (5.7) | 88.8 (5.0) | 86.3 (6.2) | (p = 0.24) a |
| Gender (Men/Women) n (%) | 7 (23.3%)/23 (76.7%) | 3 (20.0%)/12 (80.0%) | 4 (26.7%)/11 (73.3%) | (p = 1.0) c |
| Weight (kg) Mean (SD) | 63.5 (14.1) | 64.6 (15.3) | 62.5 (13.2) | (p = 0.69) a |
| PU duration (days) Mean (SD) | 62.8 (63.7) | 60.3 (38.6) | 65.3 (83.2) | (p = 0.84) a |
| PU grade (II–IV) Median/Mode | 3/3 | 3/3 | 3/3 | (p = 0.26) d |
| PU area (cm2) Mean (SD) | 7.4 (6.7) | 5.2 (4.6) | 9.5 (8.0) | (p = 0.09) a |
| PUSH scale Mean (SD) | 11.2 (2.5) | 10.7 (2.6) | 11.7 (2.4) | (p = 0.25) a |
| PU infection Yes/No n (%) | 3 (10.0%)/27 (90.0%) | 1 (6.7%)/14 (93.3%) | 2 (13.3%)/13 (86.7%) | (p = 1.0) c |
| Braden scale Mean (SD) | 11.6 (2.2) | 12.5 (1.6) | 10.8 (2.5) | (p = 0.04) *a |
| Diabetes Yes/No n (%) | 10 (33.3%)/20 (66.7%) | 8 (53.3%)/7 (46.7%) | 2 (13.3%)/13 (86.7%) | (p = 0.02) *b |
| Anti-decubitus mattress Yes/No n (%) | 15 (50.0%)/15 (50.0%) | 7 (53.3%)/8 (46.7%) | 8 (53.3%)/7 (46.7%) | (p = 0.72) b |
| Protein supplements Yes/No n (%) | 10 (33.3%)/20 (66.7%) | 3 (10.0%)/12 (90.0%) | 7 (46.7%)/8 (53.3%) | (p =0.12) b |
| Comorbidity (number of diseases) Mean (SD) | 3.4 (0.9) | 3.5 (0.8) | 3.3 (1.0) | (p = 0.55) a |
| Mini-mental test Mean (SD) | 12.5 (9.4) | 12.5 (9.5) | 12.4 (9.7) | (p = 0.97) a |
| Systolic blood pressure (mmHg) Mean (SD) | 124.8 (15.1) | 124.0 (17.4) | 125.6 (13.0) | (p = 0.78) a |
| Diastolic blood pressure (mmHg) Mean (SD) | 70.4 (11.0) | 69.3 (9.9) | 71.6 (12.1) | (p = 0.57) a |
| Radial pulse (beats/min) Mean (SD) | 79.2 (10.4) | 76.1 (11.5) | 82.2 (8.5) | (p = 0.11) a |
| Blood glucose (mg/dL) Mean (SD) | 99.8 (22.5) | 103.8 (21.7) | 95.8 (23.3) | (p = 0.34) a |
| Periulcer flowmetry Mean (SD) | 76.0 (50.5) | 68.8 (36.3) | 85.6 (67.7) | (p = 0.60) a |
| Outcomes | Intragroup Comparison Versus Baseline | Intergroup Comparison of Changes versus Baseline | ||||
|---|---|---|---|---|---|---|
| Active Group | Sham Group | Mean Change Active Minus Sham at T2 | Mean Change Active Minus Sham at T3 | |||
| T1 Minus T2 | T1 Minus T3 | T1 Minus T2 | T1 Minus T3 | |||
| PUSH scale % Mean (CI95%) | 22.5% ** (8.5–36.5) | 34.4% ** (19.0–49.7) | 5.7% (−8.3–19.6) | 9.1% (−6.3–24.5) | 16.8% * (0.5–33.1) | 25.3% ** (7.6–43.0) |
| Pressure ulcer area % Mean (CI95%) | 22.0% ** (8.9–35.1) | 30.2% ** (15.5–44.1) | 1.9% (−11.2–15.0) | 1.6% (−13.1–16.2) | 20.1% * (5.2–35.0) | 28.6% ** (11.9–45.3) |
| Pressure ulcer depth % Mean (CI95%) | 12.7% (−1.2–26.6) | 19.5% (−8.1–47.1) | 6.4% (−7.5–20.3) | 15.0% (−12.6–42.6) | 6.3% (−9.5–22.2) | 4.5% (−26.9–35.8) |
| Outcomes | Intragroup Comparison versus Baseline | Intergroup Comparison of Changes versus Baseline | ||||
|---|---|---|---|---|---|---|
| Active Group | Sham Group | Mean Change Active Minus Sham at T2 | Mean Change Active Minus Sham at T3 | |||
| T1 Minus T2 | T1 Minus T3 | T1 Minus T2 | T1 Minus T3 | |||
| Periulcer flowmetry (%) Mean (CI95%) | −11.4% (−64.1–41.3) | 24.8% (−20.2–69.8) | 4.4% (−56.4–65.3) | 29.3% (−22.7–81.2) | −15.8% (−78.9–47.3) | −4.5% (−58.3–49.4) |
| Systolic blood pressure (mmHg) Mean (CI95%) | 1.2 (−6.9–9.3) | 1.3 (−8.0–10.6) | 1.7 (−6.4–9.8) | 5.4 (−3.8–14.7) | −0.5 (−9.8–8.8) | −4.1 (−14.7–6.4) |
| Diastolic blood pressure (mmHg) Mean (CI95%) | −3.9 (−9.5–1.7) | −0.8 (−7.7–6.1) | −1.3 (−6.9–4.3) | 2.8 (−4.1–9.8) | −2.6 (−9.0–3.8) | −3.6 (−11.5–4.3) |
| Radial pulse (beats/min) Mean (CI95%) | 1.7 (−4.8–8.2) | 5.0 (−0.1–10.1) | 4.1 (−2.4–10.6) | 7.2 ** (2.1–12.3) | −2.4 (−9.8–5.0) | −2.2 (−8.0–3.6) |
| Blood glucose (mg/dL) Mean (CI95%) | −4.6 (−15.7–6.6) | −12.3 (−34.1–9.4) | 0.7 (−10:4–11.9) | 5.3 (−16.5–27.1) | −5.3 (−18.0–7.4) | −17.6 (−42.4–7.1) |
| Allocation | Therapist Guess, n (%) | |||
|---|---|---|---|---|
| Active MCT | Sham MCT | Do Not Know | Total | |
| Active MCT | 7 (23.3) | 3 (10.0) | 5 (16.7) | 15 (50.0) |
| Sham MCT | 8 (26.7) | 2 (6.7) | 5 (16.7) | 15 (50.0) |
| Total | 15 (50.0) | 5 (16.7) | 10 (33.3) | 30 (100.0) |
| Allocation | Assessor Guess, n (%) | |||
| Active MCT | Sham MCT | Do Not Know | Total | |
| Active MCT | 5 (16.7) | 2 (6.7) | 8 (26.7) | 15 (50.0) |
| Sham MCT | 5 (16.7) | 3 (10.0) | 7 (23.3) | 15 (50.0) |
| Total | 10 (33.3) | 5 (16.7) | 15 (50.0) | 30 (100.0) |
| Methods | Index | p-Value | 95% Confidence Interval | Conclusion |
|---|---|---|---|---|
| James’s BI | 0.70 | 1.00 | 0.58 to 0.82 | Blinded |
| Bang’s BI—Active/2 × 3 | 0.27 | 0.09 | −0.06 to 0.59 | Blinded |
| Bang’s BI—Sham/2 × 3 | −0.40 | 0.99 | −0.70 to −0.09 | Opposite guess |
| James’s BI | 0.73 | 1.00 | 0.60 to 0.85 | Blinded |
| Bang’s BI—Active/2 × 3 | 0.20 | 0.12 | −0.08 to 0.48 | Blinded |
| Ban’s BI g—Sham/2 × 3 | −0.13 | 0.76 | −0.43 to 0.17 | Blinded |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avendaño-Coy, J.; Martín-Espinosa, N.M.; Ladriñán-Maestro, A.; Gómez-Soriano, J.; Suárez-Miranda, M.I.; López-Muñoz, P. Effectiveness of Microcurrent Therapy for Treating Pressure Ulcers in Older People: A Double-Blind, Controlled, Randomized Clinical Trial. Int. J. Environ. Res. Public Health 2022, 19, 10045. https://doi.org/10.3390/ijerph191610045
Avendaño-Coy J, Martín-Espinosa NM, Ladriñán-Maestro A, Gómez-Soriano J, Suárez-Miranda MI, López-Muñoz P. Effectiveness of Microcurrent Therapy for Treating Pressure Ulcers in Older People: A Double-Blind, Controlled, Randomized Clinical Trial. International Journal of Environmental Research and Public Health. 2022; 19(16):10045. https://doi.org/10.3390/ijerph191610045
Chicago/Turabian StyleAvendaño-Coy, Juan, Noelia M. Martín-Espinosa, Arturo Ladriñán-Maestro, Julio Gómez-Soriano, María Isabel Suárez-Miranda, and Purificación López-Muñoz. 2022. "Effectiveness of Microcurrent Therapy for Treating Pressure Ulcers in Older People: A Double-Blind, Controlled, Randomized Clinical Trial" International Journal of Environmental Research and Public Health 19, no. 16: 10045. https://doi.org/10.3390/ijerph191610045
APA StyleAvendaño-Coy, J., Martín-Espinosa, N. M., Ladriñán-Maestro, A., Gómez-Soriano, J., Suárez-Miranda, M. I., & López-Muñoz, P. (2022). Effectiveness of Microcurrent Therapy for Treating Pressure Ulcers in Older People: A Double-Blind, Controlled, Randomized Clinical Trial. International Journal of Environmental Research and Public Health, 19(16), 10045. https://doi.org/10.3390/ijerph191610045






