A Standardised Core Outcome Set for Measurement and Reporting Sedentary Behaviour Interventional Research: The CROSBI Consensus Study
Abstract
:1. Introduction
2. Materials and Methods
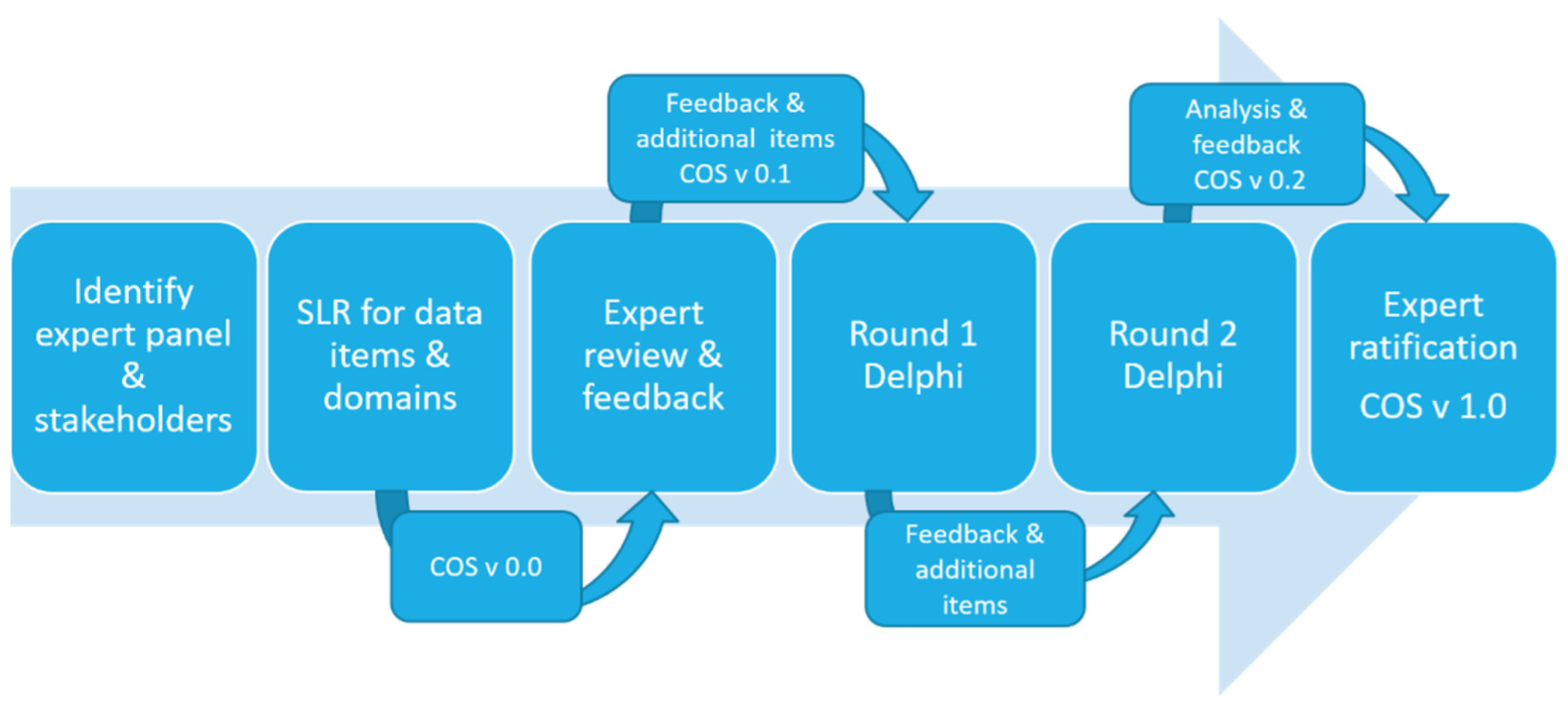
2.1. Study Design
2.2. Outcome Identification
2.3. Stakeholder Identification and Expert Review
2.4. Consensus Process Delphi Survey
2.5. Delphi Survey Round One: Expert Panel Snowball and SBRN
2.6. Delphi Survey Round Two
2.7. Retaining or Dropping Items between Rounds
2.8. Consensus for Inclusion, Definition
3. Results
3.1. Delphi Survey Participants’ Characteristics
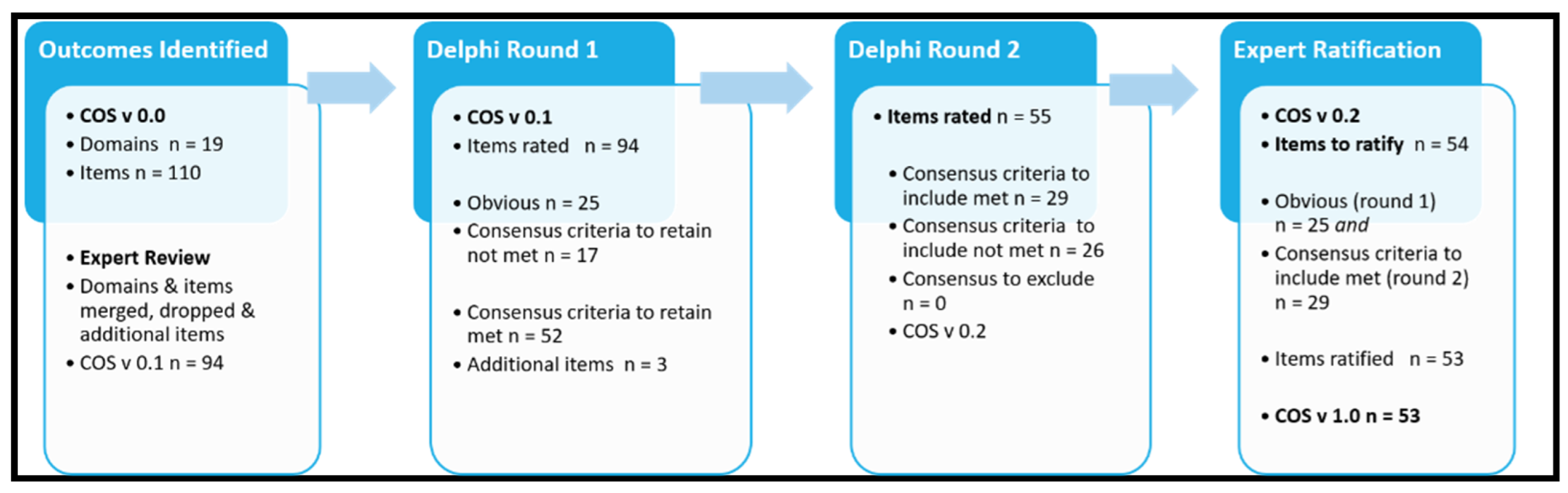
3.2. Outcomes Identified (Cos V.0 to Cos V.1)
3.3. Delphi Survey Analysis Round 1
3.4. Delphi Survey Analysis Round 2
3.5. Expert Panel Ratification (COS V 0.2 to COS V 1.0)
4. Discussion
5. Strengths and Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tremblay, M.S.; Aubert, S.; Barnes, J.D.; Saunders, T.J.; Carson, V.; Latimer-Cheung, A.E.; Chastin, S.F.M.; Altenburg, T.M.; Chinapaw, M.J.M.; on behalf of SBRN Terminology Consensus Project Participants. Sedentary Behavior Research Network (SBRN)—Terminology Consensus Project process and outcome. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biswas, A.; Oh, P.I.; Faulkner, G.E.; Bajaj, R.R.; Silver, M.A.; Mitchell, M.S.; Alter, D.A. Sedentary Time and Its Association With Risk for Disease Incidence, Mortality, and Hospitalization in Adults: A systematic review and meta-analysis. Ann. Intern. Med. 2015, 162, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Chau, J.Y.; Grunseit, A.C.; Chey, T.; Stamatakis, E.; Brown, W.J.; Matthews, C.E.; Bauman, A.E.; van der Ploeg, H.P. Daily Sitting Time and All-Cause Mortality: A Meta-Analysis. PLoS ONE 2013, 8, e80000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katzmarzyk, P.T.; Church, T.S.; Craig, C.L.; Bouchard, C. Sitting Time and Mortality from All Causes, Cardiovascular Disease, and Cancer. Med. Sci. Sports Exerc. 2009, 41, 998–1005. [Google Scholar] [CrossRef]
- Owen, N.; Sugiyama, T.; Eakin, E.E.; Gardiner, P.A.; Tremblay, M.S.; Sallis, J.F. Adults’ Sedentary Behavior: Determinants and Interventions. Am. J. Prev. Med. 2011, 41, 189–196. [Google Scholar] [CrossRef]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.-P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef]
- Young, D.R.; Hivert, M.-F.; Alhassan, S.; Camhi, S.M.; Ferguson, J.F.; Katzmarzyk, P.; Lewis, C.E.; Owen, N.; Perry, C.; Siddique, J.; et al. Sedentary Behavior and Cardiovascular Morbidity and Mortality: A Science Advisory From the American Heart Association. Circulation 2016, 134, e262–e279. [Google Scholar] [CrossRef]
- British Heart Foundation National Centre. Sedentary Behaviour Evidence Briefing; Health Service Executive: Dublin, Ireland, 2012. [Google Scholar]
- Ross, R.; Chaput, J.-P.; Giangregorio, L.M.; Janssen, I.; Saunders, Y.J.; Kho, M.E.; Poitras, V.J.; Tomasone, J.R.; El-Kotob, R.; McLaughlin, E.C.; et al. Canadian 24-hour movement guidelines: An Integration of Physical Activity, Sed-entary Behaviour, and Sleep. Appl. Phys. Nutr. Metab. 2020, 45 (Suppl. S2), S57–S102. [Google Scholar] [CrossRef]
- Austrailian Government. Australia’s Physical Activity and Sedentary Behaviour Guidelines and the Australian 24-Hour Movement Guidelines Commonwealth of Australia. 2019. Available online: https://www.health.gov.au/health-topics/physical-activity-and-exercise/physical-activity-and-exercise-guidelines-for-all-australians#about-the-guidelines (accessed on 3 July 2022).
- Prince, S.A.; Saunders, T.J.; Gresty, K.; Reid, R.D. A comparison of the effectiveness of physical activity and sedentary behaviour interventions in reducing sedentary time in adults: A systematic review and meta-analysis of controlled trials. Obes. Rev. 2014, 15, 905–919. [Google Scholar] [CrossRef]
- Martin, A.; Fitzsimons, C.; Jepson, R.; Saunders, D.H.; van der Ploeg, H.; Teixeira, P.; Gray, C.M.; Mutrie, N. Interventions with potential to reduce sedentary time in adults: Systematic review and meta-analysis. Br. J. Sports Med. 2015, 49, 1056–1063. [Google Scholar] [CrossRef] [Green Version]
- De Craemer, M.; Chastin, S.; Ahrens, W.; Bernaards, C.; Brug, J.; Buck, C.; Cardon, G.; Capranica, L.; Dargent-Molina, P.; De Lepeleere, S.; et al. Data on Determinants Are Needed to Curb the Sedentary Epidemic in Europe. Lessons Learnt from the DEDIPAC European Knowledge Hub. Int. J. Environ. Res. Public Health 2018, 15, 1406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chastin, S.F.M.; De Craemer, M.; Lien, N.; Bernaards, C.; Buck, C.; Oppert, J.-M.; Nazare, J.-A.; Lakerveld, J.; O’Donoghue, G.; Holdsworth, M.; et al. The SOS-framework (Systems of Sedentary behaviours): An international transdisciplinary consensus framework for the study of determinants, research priorities and policy on sedentary behaviour across the life course: A DEDIPAC-study. Int. J. Behav. Nutr. Phys. Act. 2016, 13, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curran, F.; Blake, C.; Cunningham, C.; Perrotta, C.; van der Ploeg, H.; Matthews, J.; O’Donoghue, G. Efficacy, characteristics, behavioural models and behaviour change strategies, of non-workplace interventions specifically targeting sedentary behaviour; a systematic review and meta-analysis of randomised control trials in healthy ambulatory adults. PLoS ONE 2021, 16, e0256828. [Google Scholar] [CrossRef] [PubMed]
- Edwardson, C.L.; Henson, J.; Biddle, S.J.H.; Davies, M.J.; Khunti, K.; Maylor, B.; Yates, T. ActivPAL and ActiGraph Assessed Sedentary Behavior and Cardiometabolic Health Markers. Med. Sci. Sports Exerc. 2020, 52, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.Y.; Bassett, D.R., Jr. The Technology of Accelerometry-Based Activity Monitors: Current and Future. Med. Sci. Sports Exerc. 2005, 37 (Suppl. S11), S490–S500. [Google Scholar] [CrossRef] [Green Version]
- Carpenter, C.; Yang, C.-H.; West, D. A Comparison of Sedentary Behavior as Measured by the Fitbit and ActivPAL in College Students. Int. J. Environ. Res. Public Health 2021, 18, 3914. [Google Scholar] [CrossRef]
- Bai, Y.; Tompkins, C.; Gell, N.; Dione, D.; Zhang, T.; Byun, W. Comprehensive comparison of Apple Watch and Fitbit monitors in a free-living setting. PLoS ONE 2021, 16, e0251975. [Google Scholar]
- Aadland, E.; Kvalheim, O.M.; Anderssen, S.A.; Resaland, G.K.; Andersen, L.B. The multivariate physical activity signature associated with metabolic health in children. Int. J. Behav. Nutr. Phys. Act. 2018, 15, 77. [Google Scholar] [CrossRef]
- Atkin, A.J.; Gorely, T.; Clemes, S.A.; Yates, T.; Edwardson, C.; Brage, S.; Salmon, J.; Marshall, S.J.; Biddle, S.J. Methods of Measurement in epidemiology: Sedentary Behaviour. Int. J. Epidemiol. 2012, 41, 1460–1471. [Google Scholar] [CrossRef] [Green Version]
- Aunger, J.; Wagnild, J. Objective and subjective measurement of sedentary behavior in human adults: A toolkit. Am. J. Hum. Biol. 2020, 34, e23546. [Google Scholar] [CrossRef]
- Bellettiere, J.; Tuz-Zahra, F.; Carlson, J.A.; Ridgers, N.D.; Liles, S.; Greenwood-Hickman, M.A.; Walker, R.L.; LaCroix, A.Z.; Jankowska, M.M.; Rosenberg, D.E.; et al. Agreement of Sedentary Behavior Metrics Derived From Hip- and Thigh-Worn Accelerometers Among Older Adults: With Implications for Studying Physical and Cognitive Health. J. Meas. Phys. Behav. 2021, 4, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Kerr, J.; Carlson, J.; Godbole, S.; Cadmus-Bertram, L.; Bellettiere, J.; Hartman, S. Improving Hip-Worn Accelerometer Estimates of Sitting Using Machine Learning Methods. Med. Sci. Sports Exerc. 2018, 50, 1518–1524. [Google Scholar] [CrossRef] [PubMed]
- Greenwood-Hickman, M.A.; Nakandala, S.; Jankowska, M.M.; Rosenberg, D.E.; Tuz-Zahra, F.; Bellettiere, J.; Carlson, J.; Hibbing, P.R.; Zou, J.; Lacroix, A.Z.; et al. The CNN Hip Accelerometer Posture (CHAP) Method for Classifying Sitting Patterns from Hip Accelerometers: A Validation Study. Med. Sci. Sports Exerc. 2021, 53, 2445–2454. [Google Scholar] [CrossRef] [PubMed]
- Boerema, S.T.; Van Velsen, L.; Vollenbroek, M.; Hermens, H.J. Pattern measures of sedentary behaviour in adults: A literature review. Digit. Health 2020, 6, 2055207620905418. [Google Scholar] [CrossRef]
- Edwardson, C.L.; Winkler, E.A.H.; Bodicoat, D.H.; Yates, T.; Davies, M.J.; Dunstan, D.W.; Healy, G.N. Considerations when using the activPAL monitor in field-based research with adult populations. J. Sport Health Sci. 2017, 6, 162–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rowlands, A.V.; Edwardson, C.L.; Davies, M.J.; Khunti, K.; Harrington, D.M.; Yates, T. Beyond Cut Points: Accelerometer Metrics that Capture the Physical Activity Profile. Med. Sci. Sports Exerc. 2018, 50, 1323–1332. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, S. Data rich, information poor (DRIP) syndrome: Is there a treatment? Radiol. Manag. 1996, 18, 45–49. [Google Scholar]
- Goossen, W.T.F.; Epping, P.J.M.M.; Feuth, T.; Dassen, T.W.N.; Hasman, A.; Heuvel, W.J.A.V.D. A Comparison of Nursing Minimal Data Sets. J. Am. Med Inform. Assoc. 1998, 5, 152–163. [Google Scholar] [CrossRef] [Green Version]
- Williamson, P.R.; Altman, D.G.; Bagley, H.; Barnes, K.L.; Blazeby, J.; Brookes, S.T.; Clarke, M.; Gargon, E.; Gorst, S.; Harman, N.; et al. The COMET Handbook: Version 1.0. Trials 2017, 18 (Suppl. S3), 280. [Google Scholar] [CrossRef] [Green Version]
- Wilkinson, M.D.; Dumontier, M.; Aalbersberg, I.J.; Appleton, G.; Axton, M.; Baak, A.; Blomberg, N.; Boiten, J.W.; da Silva Santos, L.B.; Bourne, P.E.; et al. The FAIR Guiding Principles for scientific data management and stewardship. Sci. Data 2016, 3, 160018. [Google Scholar] [CrossRef] [Green Version]
- Lakerveld, J.; Van Der Ploeg, H.P.; Kroeze, W.; Ahrens, W.; Allais, O.; Andersen, L.F.; Cardon, G.; Capranica, L.; Chastin, S.; Donnelly, A.; et al. Towards the integration and development of a cross-European research network and infrastructure: The DEterminants of DIet and Physical ACtivity (DEDIPAC) Knowledge Hub. Int. J. Behav. Nutr. Phys. Act. 2014, 11 (Suppl. S3), 143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacDonald, B.; Janssen, X.; Kirk, A.; Patience, M.; Gibson, A.-M. An Integrative, Systematic Review Exploring the Research, Effectiveness, Adoption, Implementation, and Maintenance of Interventions to Reduce Sedentary Behaviour in Office Workers. Int. J. Environ. Res. Public Health 2018, 15, 2876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirkham, J.; Gorst, S.; Altman, D.G.; Blazeby, J.; Clarke, M.; Devane, D.; Gargon, E.; Moher, D.; Schmitt, J.; Tugwell, P.; et al. Core Outcome Set–STAndards for Reporting: The COS-STAR Statement. PLoS Med. 2016, 13, e1002148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choquet, R.; Maaroufi, M.; de Carrara, A.; Messiaen, C.; Luigi, E.; Landais, P. A methodology for a minimum data set for rare diseases to support national centers of excellence for healthcare and research. J. Am. Med. Inform. Assoc. 2015, 22, 76–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Svensson-Ranallo, P.A.; Adam, T.J.; Sainfort, F. A Framework and Standardized Methodology for Developing Minimum Clinical Datasets. AMIA Jt. Summits Transl. Sci. Proc. 2011, 2011, 54–58. [Google Scholar]
- Jones, J.; Hunter, D. Consensus methods for medical and health services research. BMJ 1995, 311, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Identifying Effective Characteristics, Behavioural Models and Behavioural Change Strategies Underpinning Sedentary Behaviour Interventions in Adults: A Systematic Review. PROSPERO 2020. Available online: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=172457 (accessed on 3 July 2022).
- Guyatt, G.H.; Oxman, A.D.; Kunz, R.; Atkins, D.; Brozek, J.; Vist, G.; Alderson, P.; Glasziou, P.; Falck-Ytter, Y.; Schünemann, H.J. GRADE guidelines: 2. Framing the question and deciding on important outcomes. J. Clin. Epidemiol. 2011, 64, 395–400. [Google Scholar] [CrossRef]
- Naderifar, M.; Goli, H.; Ghaljaei, F. Snowball Sampling: A Purposeful Method of Sampling in Qualitative Research. Strides Dev. Med. Educ. 2017, 14, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Gargon, E.; Crew, R.; Burnside, G.; Williamson, P.R. Higher number of items associated with significantly lower response rates in COS Delphi surveys. J. Clin. Epidemiol. 2019, 108, 110–120. [Google Scholar] [CrossRef] [Green Version]
- Beaton, D.M.L.; Grosskleg, S.; Shea, B.; Tugwell, B. The OMERACT Handbook Version 2.1 2021. Available online: https://omeract.org/handbook/ (accessed on 4 March 2022).
- MacKenzie, R.M.; Ells, L.J.; Simpson, S.A.; Logue, J. Core outcome set for behavioural weight management interventions for adults with overweight and obesity: Standardised reporting of lifestyle weight management interventions to aid evaluation (STAR-LITE). Obes. Rev. 2020, 21, e12961. [Google Scholar] [CrossRef] [Green Version]
- Smith, S.M.; Wallace, E.; Salisbury, C.; Sasseville, M.; Bayliss, E.; Fortin, M. A Core Outcome Set for Multimorbidity Research (COSmm). Ann. Fam. Med. 2018, 16, 132–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerbens, L.; Apfelbacher, C.; Irvine, A.; Barbarot, S.; De Booij, R.; Boyce, A.; Deleuran, M.; Eichenfield, L.; Hof, M.; Middelkamp-Hup, M.; et al. TRE atment of AT opic eczema ( TREAT ) Registry Taskforce: An international Delphi exercise to identify a core set of domains and domain items for national atopic eczema photo- and systemic therapy registries. Br. J. Dermatol. 2019, 180, 790–801. [Google Scholar] [CrossRef] [PubMed]
- Prince, S.A.; Leblanc, A.G.; Colley, R.C.; Saunders, T.J. Measurement of sedentary behaviour in population health surveys: A review and recommendations. PeerJ 2017, 5, e4130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marconcin, P.; Júdice, P.B.; Ferrari, G.; Werneck, A.; Marques, A. Methods of Assessing Sedentary Behaviour. In Sedentary Behaviour—A Contemporary View [Internet]; Marques, A., Gouveia, É.R., Eds.; IntechOpen: London, UK, 2021. [Google Scholar]
- Dogra, S.; Ashe, M.C.; Biddle, S.J.H.; Brown, W.J.; Buman, M.P.; Chastin, S.; Gardiner, P.A.; Inoue, S.; Jefferis, B.J.; Oka, K.; et al. Sedentary time in older men and women: An international consensus statement and research priorities. Br. J. Sports Med. 2017, 51, 1526–1532. [Google Scholar] [CrossRef]
- Katzmarzyk, P.T.; Powell, K.E.; Jakicic, J.M.; Troiano, R.; Piercy, K.; Tennant, B.; for the 2018 Physical Activity Guidelines Advisory Committee. Sedentary Behavior and Health: Update from the 2018 Physical Activity Guidelines Advisory Committee. Med. Sci. Sports Exerc. 2019, 51, 1227–1241. [Google Scholar] [CrossRef]
- Kuster, R.P.; Grooten, W.J.A.; Baumgartner, D.; Blom, V.; Hagströmer, M.; Ekblom, Ö. Detecting prolonged sitting bouts with the ActiGraph GT3X. Scand. J. Med. Sci. Sports 2020, 30, 572–582. [Google Scholar] [CrossRef]
- Kuster, R.P.; Grooten, W.J.; Blom, V.; Baumgartner, D.; Hagströmer, M.; Ekblom, Ö. How Accurate and Precise Can We Measure the Posture and the Energy Expenditure Component of Sedentary Behaviour with One Sensor? Int. J. Environ. Res. Public Health 2021, 18, 5782. [Google Scholar] [CrossRef]
- Ellis, K.; Kerr, J.; Godbole, S.; Staudenmayer, J.; Lanckriet, G. Hip and Wrist Accelerometer Algorithms for Free-Living Behavior Classification. Med. Sci. Sports Exerc. 2016, 48, 933–940. [Google Scholar] [CrossRef] [Green Version]
- Lyden, K.; Keadle, S.K.; Staudenmayer, J.; Freedson, P.S. The activPALTM Accurately Classifies Activity Intensity Categories in Healthy Adults. Med. Sci. Sports Exerc. 2017, 49, 1022–1028. [Google Scholar] [CrossRef] [Green Version]
- MacEwen, B.T.; MacDonald, D.J.; Burr, J.F. A systematic review of standing and treadmill desks in the workplace. Prev. Med. 2015, 70, 50–58. [Google Scholar] [CrossRef]
- Dunstan, D.W.; Kingwell, B.A.; Larsen, R.; Healy, G.N.; Cerin, E.; Hamilton, M.T.; Shaw, J.E.; Bertovic, D.A.; Zimmet, P.Z.; Salmon, J.; et al. Breaking Up Prolonged Sitting Reduces Postprandial Glucose and Insulin Responses. Diabetes Care 2012, 35, 976–983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Healy, G.N.; Winkler, E.A.H.; Owen, N.; Anuradha, S.; Dunstan, D. Replacing sitting time with standing or stepping: Associations with cardio-metabolic risk biomarkers. Eur. Heart J. 2015, 36, 2643–2649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chastin, S.F.; Egerton, T.; Leask, C.; Stamatakis, E. Meta-analysis of the relationship between breaks in sedentary behavior and cardiometabolic health. Obesity 2015, 23, 1800–1810. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, P.C.; Biddle, S.J.H.; Buman, M.P.; Chastin, S.; Ekelund, U.; Friedenreich, C.M.; Katzmarzyk, P.T.; Leitzmann, M.F.; Stamatakis, E.; Van Der Ploeg, H.P.; et al. New global guidelines on sedentary behaviour and health for adults: Broadening the behavioural targets. Int. J. Behav. Nutr. Phys. Act. 2020, 17, 151. [Google Scholar] [CrossRef] [PubMed]
- Farrahi, V.; Kangas, M.; Kiviniemi, A.; Puukka, K.; Korpelainen, R.; Jämsä, T. Accumulation patterns of sedentary time and breaks and their association with cardiometabolic health markers in adults. Scand. J. Med. Sci. Sports 2021, 31, 1489–1507. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
| Round 1 Participants (n) | Round 2 Participants (n) | |
|---|---|---|
| COUNTRY | ||
| Australia | 10 | 8 |
| Canada | 7 | 7 |
| United Kingdom (UK) | 7 | 7 |
| Ireland | 5 | 4 |
| Brazil | 4 | 2 |
| United States of America (USA) | 3 | 3 |
| Netherlands | 3 | 2 |
| Belgium | 2 | 2 |
| Portugal | 2 | 1 |
| Unknown | 2 | 1 |
| France | 1 | 1 |
| Czech Republic | 1 | 1 |
| Argentina | 1 | 1 |
| Slovenia | 1 | 1 |
| Italy | 1 | 0 |
| GENDER | ||
| Female | 29 | 26 |
| Male | 19 | 13 |
| Unspecified | 2 | 2 |
| EXPERIENCE SB RESEARCH | ||
| <5 years | 21 | 13 |
| 5–10 years | 18 | 18 |
| >10 years | 11 | 10 |
| CURRENT ROLE | ||
| Academic | 24 | 22 |
| Researcher | 11 | 9 |
| Clinical | 4 | 1 |
| Academic; Researcher | 6 | 5 |
| Clinical; Academic; Researcher | 3 | 3 |
| Clinical; Academic | 1 | 1 |
| Other | 1 | 0 |
| Domain & Reporting Statistic | Outcome Name | Measure/Report | When to Measure | |
|---|---|---|---|---|
| Demographics 1 or 2 | Age | years | baseline | |
| Gender | male/female/other | baseline | ||
| Population type | healthy sedentary; healthy active; clinical cohort | baseline | ||
| BMI (body mass index) | baseline | follow-up | ||
| Device Details and Wear Time Criteria | Device type | accelerometer with inclinometry function | ||
| Device sensor position | placement of sensors on body | |||
| Device minimum wear time | hours/day | |||
| Device minimum wear time | days/week | |||
| Device Wear Time Measured 1 | Total daily wear-time | minutes or hours per day | ||
| Waking/non-sleep wear-time | minutes or hours per day, while awake | |||
| Sleep wear-time | minutes or hours per day, while sleeping | |||
| Posture Related Outcomes 1 & 3 | Sedentary time | minutes or hours per day | baseline | follow-up |
| % daily waking hours | baseline | follow-up | ||
| % of total daily hours | baseline | follow-up | ||
| Sitting time | minutes or hours per day | baseline | follow-up | |
| % daily waking hours | baseline | follow-up | ||
| % of total daily hours | baseline | follow-up | ||
| Standing time | minutes or hours per day | baseline | follow-up | |
| % daily waking hours | baseline | follow-up | ||
| % of total daily hours | baseline | follow-up | ||
| Stepping time | minutes or hours per day | baseline | follow-up | |
| % daily waking hours | baseline | follow-up | ||
| % of total daily hours | baseline | follow-up | ||
| Sedentary Breaks 1&4 | Sedentary breaks; Sit to stand or upright transitions | n/day; number of sedentary breaks daily | baseline | follow-up |
| Sedentary breaks; Movement breaks | n/day; number of sedentary breaks daily | baseline | follow-up | |
| Sedentary Bouts 1&3 | Prolonged sedentary bouts > 30 min | average duration of bout, mins | baseline | follow-up |
| total duration of bouts, minutes or hours per day | baseline | follow-up | ||
| Prolonged sedentary bouts > 60 min | average duration of bout, minutes | baseline | follow-up | |
| total duration of bouts, minutes or hours per day | baseline | follow-up | ||
| Physical Activity 1&3 | Light intensity physical activity, time | minutes/day | baseline | follow-up |
| % of daily waking hours | baseline | follow-up | ||
| Moderate intensity physical activity, time | minutes/day | baseline | follow-up | |
| % of daily waking hours | baseline | follow-up | ||
| Vigorous intensity physical activity, time | minutes/day | baseline | follow-up | |
| % of daily waking hours | baseline | follow-up | ||
| Moderate–vigorous intensity physical activity, time | minutes/day | baseline | follow-up | |
| % of daily waking hours | baseline | follow-up | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Curran, F.; Dowd, K.P.; Peiris, C.L.; van der Ploeg, H.P.; Tremblay, M.S.; O’Donoghue, G. A Standardised Core Outcome Set for Measurement and Reporting Sedentary Behaviour Interventional Research: The CROSBI Consensus Study. Int. J. Environ. Res. Public Health 2022, 19, 9666. https://doi.org/10.3390/ijerph19159666
Curran F, Dowd KP, Peiris CL, van der Ploeg HP, Tremblay MS, O’Donoghue G. A Standardised Core Outcome Set for Measurement and Reporting Sedentary Behaviour Interventional Research: The CROSBI Consensus Study. International Journal of Environmental Research and Public Health. 2022; 19(15):9666. https://doi.org/10.3390/ijerph19159666
Chicago/Turabian StyleCurran, Fiona, Kieran P. Dowd, Casey L. Peiris, Hidde P. van der Ploeg, Mark S. Tremblay, and Grainne O’Donoghue. 2022. "A Standardised Core Outcome Set for Measurement and Reporting Sedentary Behaviour Interventional Research: The CROSBI Consensus Study" International Journal of Environmental Research and Public Health 19, no. 15: 9666. https://doi.org/10.3390/ijerph19159666
APA StyleCurran, F., Dowd, K. P., Peiris, C. L., van der Ploeg, H. P., Tremblay, M. S., & O’Donoghue, G. (2022). A Standardised Core Outcome Set for Measurement and Reporting Sedentary Behaviour Interventional Research: The CROSBI Consensus Study. International Journal of Environmental Research and Public Health, 19(15), 9666. https://doi.org/10.3390/ijerph19159666






