Effects of Tribulus terrestris L. on Sport and Health Biomarkers in Physically Active Adult Males: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Selection Criteria
2.3. Quality Assessment
2.4. Data Extraction
3. Results
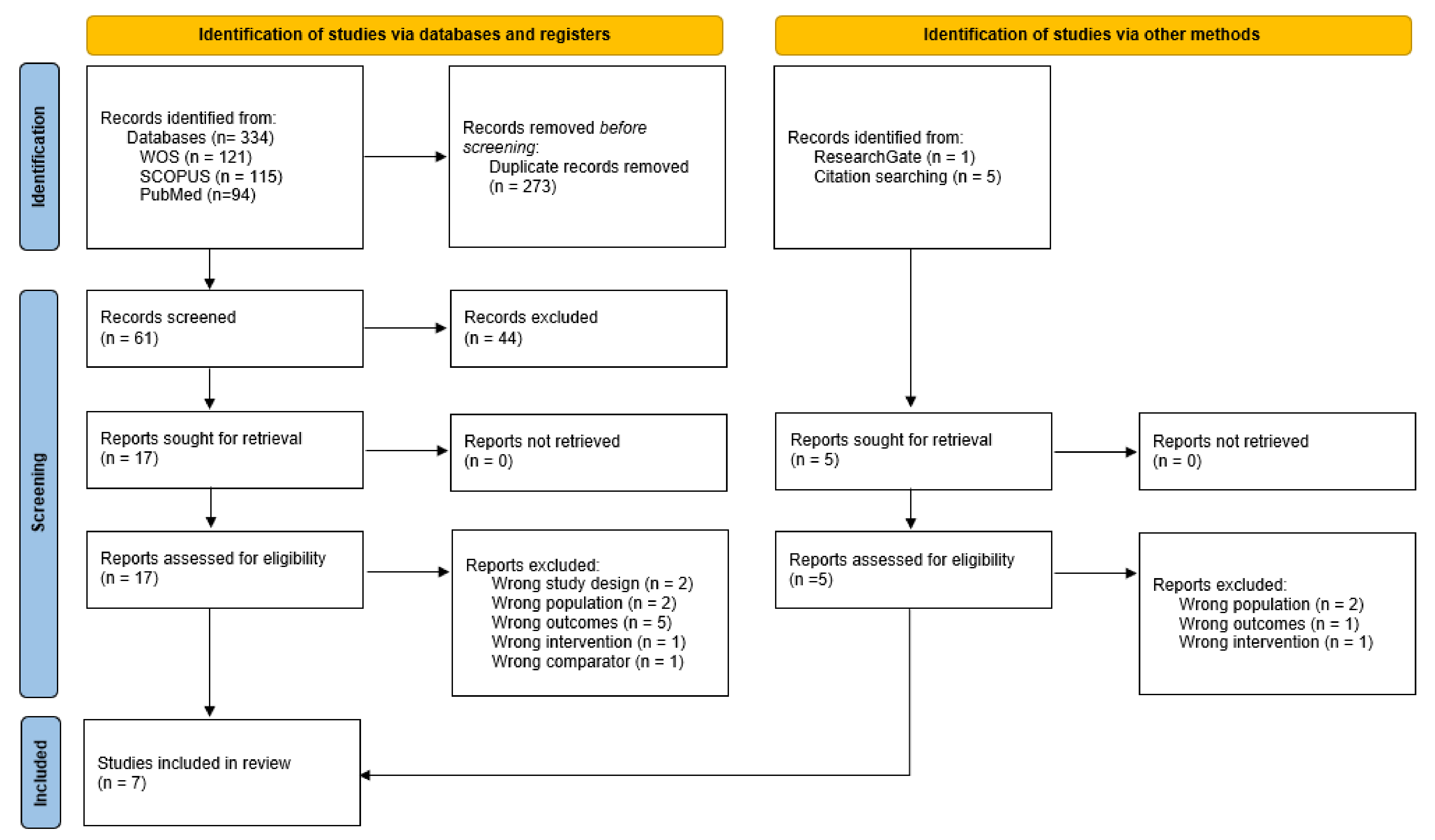
3.1. Study Selection
3.2. Quality Assessment
3.3. Characteristics of the Participants and Interventions
3.4. Outcome Evaluation
3.4.1. Immunological Biomarkers
3.4.2. Hematological Biomarkers
3.4.3. Biochemical Biomarkers
3.4.4. Renal Biomarkers
3.4.5. Lipid Biomarkers
3.4.6. Inflammatory Biomarkers
3.4.7. Hormonal Biomarkers
4. Discussion
4.1. Tribulus terrestris L. Supplementation
4.2. Hematological Biomarkers
4.3. Immunological Biomarkers
4.4. Biochemical Biomarkers
4.5. Inflammatory Biomarkers
4.6. Renal Biomarkers
4.7. Lipid Biomarkers
4.8. Hormonal Biomarkers
4.9. Limitations and Stregths
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fernández-Lázaro, D. Ergogenic Strategies for Optimizing Performance and Health in Regular Physical Activity Participants: Evaluation of the Efficacy of Compressive Cryotherapy, Exposure to Intermittent Hypoxia at Rest and Sectorized Training of the Inspiratory Muscles. Ph.D. Thesis, University of León, León, Spain, 2020. Available online: https://dialnet.unirioja.es/servlet/tesis?codigo=286163&info=resumen&idioma=SPA (accessed on 7 May 2021).
- Fernández-Lázaro, D.; Mielgo-Ayuso, J.; Calvo, J.S.; Martínez, A.C.; García, A.C.; Fernandez-Lazaro, C.I. Modulation of exercise-induced muscle damage, inflammation, and oxidative markers by curcumin supplementation in a physically active population: A systematic review. Nutrients 2020, 12, 501. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Lázaro, D.; Mielgo-Ayuso, J.; Martínez, A.C.; Seco-Calvo, J. Iron and physical activity: Bioavailability enhancers, properties of black pepper (bioperine®) and potential applications. Nutrients 2020, 12, 1886. [Google Scholar] [CrossRef] [PubMed]
- Silano, V.; Coppens, P.; Larrañaga-Guetaria, A.; Minghetti, P.; Roth-Ehrang, R. Regulations applicable to plant food supplements and related products in the European Union. Food Funct. 2011, 2, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Lázaro, D.; Fernandez-Lazaro, C.I.; Mielgo-Ayuso, J.; Navascués, L.J.; Martínez, A.C.; Seco-Calvo, J. The role of selenium mineral trace element in exercise: Antioxidant defense system, muscle performance, hormone response, and athletic performance. A systematic review. Nutrients 2020, 12, 1790. [Google Scholar] [CrossRef]
- Dietary Supplements Market Size & Trends Report, 2021–2028. 2021, pp. 1–8. Available online: https://www.grandviewresearch.com/industry-analysis/dietary-supplements-market (accessed on 14 July 2022).
- Williams, M. Dietary Supplements and Sports Performance: Herbals. J. Int. Soc. Sports Nutr. 2006, 3, 1. [Google Scholar] [CrossRef]
- Chhatre, S.; Nesari, T.; Somani, G.; Kanchan, D.; Sathaye, S. Phytopharmacological overview of Tribulus terrestris. Pharmacogn. Rev. 2014, 8, 45–51. [Google Scholar] [CrossRef]
- Sellami, M.; Slimeni, O.; Pokrywka, A.; Kuvačić, G.; Hayes, L.D.; Milic, M.; Padulo, J. Herbal medicine for sports: A review. J. Int. Soc. Sports Nutr. 2018, 15, 14. [Google Scholar] [CrossRef]
- Qureshi, A.; Naughton, D.P.; Petroczi, A. A systematic review on the herbal extract Tribulus terrestris and the roots of its putative aphrodisiac and performance enhancing effect. J. Diet. Suppl. 2014, 11, 64–79. [Google Scholar] [CrossRef]
- Pokrywka, A.; Obmiński, Z.; Malczewska-Lenczowska, J.; Fijałek, Z.; Turek-Lepa, E.; Grucza, R. Insights into supplements with Tribulus terrestris used by athletes. J. Hum. Kinet. 2014, 41, 99–105. [Google Scholar] [CrossRef]
- Ma, Y.; Guo, Z.; Wang, X. Tribulus terrestris extracts alleviate muscle damage and promote anaerobic performance of trained male boxers and its mechanisms: Roles of androgen, IGF-1, and IGF binding protein-3. J. Sport Health Sci. 2017, 6, 474–481. [Google Scholar] [CrossRef]
- Yin, L.; Wang, Q.; Wang, X.; Song, L.-N. Effects of Tribulus terrestris saponins on exercise performance in overtraining rats and the underlying mechanisms. Can. J. Physiol. Pharmacol. 2016, 94, 1193–1201. [Google Scholar] [CrossRef]
- Rogerson, S.; Riches, C.J.; Jennings, C.; Weatherby, R.P.; Meir, R.A.; Marshall-Gradisnik, S.M. The effect of five weeks of Tribulus terrestris supplementation on muscle strength and body composition during preseason training in elite rugby league players. J. Strength Cond. Res. 2007, 21, 348–353. [Google Scholar]
- Fernández-Lázaro, D.; Mielgo-Ayuso, J.; Soto, M.D.V.; Adams, D.P.; González-Bernal, J.J.; Seco-Calvo, J. The Effects of 6 Weeks of Tribulus terrestris L. Supplementation on Body Composition, Hormonal Response, Perceived Exertion, and CrossFit® Performance: A Randomized, Single-Blind, Placebo-Controlled Study. Nutrients 2021, 13, 3696. [Google Scholar] [CrossRef]
- Heidari, M.R.; Mehrabani, M.; Pardakhty, A.; Khazaeli, P.; Zahedi, M.J.; Yakhchali, M.; Vahedian, M. The analgesic effect of Tribulus terrestris extract and comparison of gastric ulcerogenicity of the extract with indomethacine in animal experiments. Ann. N. Y. Acad. Sci. 2007, 1095, 418–427. [Google Scholar] [CrossRef]
- Talemi, M.N.P.E.; Ardakani, S.M.P.; Roozbeh, B. Tribulus terrestris may decrease muscle damage markers following a high-intensity resistance exercise: A pilot study. Int. J. Vitam. Nutr. Res. 2021, 91, 500–506. [Google Scholar] [CrossRef]
- Alzahrani, S.; Ezzat, W.; Elshaer, R.E.; Abd El-Lateef, A.S.; Mohammad, H.M.F.; Elkazaz, A.Y.; Toraih, E.; Zaitone, S.A. Standardized Tribulus terrestris extract protects against rotenone-induced oxidative damage and nigral dopamine neuronal loss in mice. J. Physiol. Pharmacol. 2018, 69, 979–994. [Google Scholar]
- Fernández-Lázaro, D.; Mielgo-Ayuso, J.; del Valle Soto, M.; Adams, D.P.; Gutiérrez-Abejón, E.; Seco-Calvo, J. Impact of Optimal Timing of Intake of Multi-Ingredient Performance Supplements on Sports Performance, Muscular Damage, and Hormonal Behavior across a Ten-Week Training Camp in Elite Cyclists: A Randomized Clinical Trial. Nutrients 2021, 13, 3746. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Law, M.; Stewart, C.; Pollock, N.; Letts, L.; Bosch, J.; Westmorland, M. Guidelines for Critical Review of Qualitative Studies; McMaster University Occupational Therapy Evidence-Based Practice Research Group: Hamilton, ON, Canada, 1998; pp. 1–9. [Google Scholar]
- Milasius, K.; Dadeliene, R.; Skernevicius, J. The influence of the Tribulus terrestris extract on the parameters of the functional preparedness and athletes’ organism homeostasis. Fiziol. Zh. 2009, 55, 89–96. [Google Scholar]
- Poprzecki, S.; Zebrowska, A.; Cholewa, J. Ergogenic effects of Tribulus terrestris supplementation in men by. J. Hum. Kinet. 2005, 13, 41–50. [Google Scholar]
- Wilk, M.; Michalczyk, M.; Chycki, J.; Maszczyk, A.; Czuba, M.; Roczniok, R.; Wilk, M.; Zając, A. Endocrine Responses to Physical Training and Tribulus terrestris Supplementation in Middle-Age Men. Cent. Eur. J. Sport Sci. Med. 2016, 13, 65–71. [Google Scholar]
- Akhtari, E.; Raisi, F.; Keshavarz, M.; Hosseini, H.; Sohrabvand, F.; Bioos, S.; Kamalinejad, M.; Ghobadi, A. Tribulus terrestris for treatment of sexual dysfunction in women: Randomized double-blind placebo-controlled study. Daru 2014, 22, 40. [Google Scholar] [CrossRef]
- Kamenov, Z.; Fileva, S.; Kalinov, K.; Jannini, E.A. Evaluation of the efficacy and safety of Tribulus terrestris in male sexual dysfunction—A prospective, randomized, double-blind, placebo-controlled clinical trial. Maturitas 2017, 99, 20–26. [Google Scholar] [CrossRef]
- Jameel, J.K.A.; Kneeshaw, P.J.; Rao, V.S.R.; Drew, P.J. Gynaecomastia and the plant product “Tribulis terrestris”. Breast 2004, 13, 428–430. [Google Scholar] [CrossRef]
- Campanelli, M.; De Thomasis, R.; Tenaglia, R.L. Priapism caused by ‘Tribulus terrestris’. Int. J. Impot. Res. 2016, 28, 39–40. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.; Lazar, I.; Nadasdy, G.M.; Nadasdy, T.; Satoskar, A.A. Acute kidney injury and hyperbilirubinemia in a young male after ingestion of Tribulus terrestris. Clin. Nephrol. 2015, 83, 177–183. [Google Scholar] [CrossRef]
- Talasaz, A.H.; Abbasi, M.R.; Abkhiz, S.; Dashti-Khavidaki, S. Tribulus terrestris-induced severe nephrotoxicity in a young healthy male. Nephrol. Dial. Transplant. 2010, 25, 3792–3793. [Google Scholar] [CrossRef]
- Alonso Lebrero, E.; Manuel Barat Baviera, J.; Pilar Conchello Moreno, M.; Estruch Riba, R.; Antonia Ferrús Pérez, M.; Font Pérez, G. Report of the Scientific Committee of the Spanish Agency for Consumer Affairs, Food Safety and Nutrition (AECOSAN) on the conditions of use of certain substances to be used in food supplements. J. Sci. Comm. AESAN 2015, 22, 79–131. [Google Scholar]
- Gandhi, S.; Srinivasan, B.P.; Akarte, A.S. Potential nephrotoxic effects produced by steroidal saponins from hydro alcoholic extract of Tribulus terrestris in STZ-induced diabetic rats. Toxicol. Mech. Methods 2013, 23, 548–557. [Google Scholar] [CrossRef] [PubMed]
- World Anti-Doping Agency (WADA). The Prohibited List. Available online: https://www.wada-ama.org/en/prohibited-list (accessed on 17 May 2021).
- Coviello, A.D.; Kaplan, B.; Lakshman, K.M.; Chen, T.; Singh, A.B.; Bhasin, S. Effects of Graded Doses of Testosterone on Erythropoiesis in Healthy Young and Older Men. J. Clin. Endocrinol. Metab. 2008, 93, 914–919. [Google Scholar] [CrossRef] [PubMed]
- Tilwari, A.; Devi, P.U.; Prabha, B.; Shukla, N.P. Immunomodulatory effect of fractions of saponins from Tribulus terrestris on non-specific immunity using in vitro phagocytosis. Int. J. Drug Discov. Herb. Res. 2011, 1, 202–207. [Google Scholar]
- Tilwari, A.; Shukla, N.; Devi, P.U. Effect of five medicinal plants used in Indian system of medicines on immune function in Wistar rats. Afr. J. Biotechnol. 2013, 10, 16637–16645. [Google Scholar]
- Fernández-Lázaro, D.; Fernandez-Lazaro, C.I.; Mielgo-Ayuso, J.; Adams, D.P.; García Hernández, J.L.; González-Bernal, J.; González-Gross, M. Glycophosphopeptical AM3 Food Supplement: A Potential Adjuvant in the Treatment and Vaccination of SARS-CoV-2. Front. Immunol. 2021, 12, 698672. [Google Scholar] [CrossRef]
- Fernández-Lázaro, D.; González-Bernal, J.J.; Sánchez-Serrano, N.; Navascués, L.J.; Del Río, A.A.; Mielgo-Ayuso, J. Physical exercise as a multimodal tool for COVID-19: Could it be used as a preventive strategy? Int. J. Environ. Res. Public Health 2020, 17, 8496. [Google Scholar] [CrossRef]
- Zhang, S.; Li, H.; Yang, S.J. Tribulosin protects rat hearts from ischemia/reperfusion injury. Acta Pharmacol. Sin. 2010, 31, 671–678. [Google Scholar] [CrossRef]
- Rajendar, B.; Bharavi, K.; Rao, G.S.; Kishore, P.V.S.; Ravi Kumar, P.; Satish Kumar, C.S.V.; Patel, T.P. Protective effect of an aphrodisiac herb Tribulus terrestris Linn on cadmium-induced testicular damage. Indian J. Pharmacol. 2011, 43, 568–573. [Google Scholar]
- Li, M.; Qu, W.; Wang, Y.; Wan, H.; Tian, C. Hypoglycemic effect of saponin from Tribulus terrestris. Zhong Yao Cai = Zhongyaocai = J. Chin. Med. Mater. 2002, 25, 420–422. [Google Scholar]
- Oh, J.S.; Baik, S.H.; Ahn, E.-K.; Jeong, W.; Hong, S.S. Anti-inflammatory activity of Tribulus terrestris in RAW264.7 Cells (54.2). J. Immunol. 2012, 188, 54–62. [Google Scholar]
- Lombardo, B.; Izzo, V.; Terracciano, D.; Ranieri, A.; Mazzaccara, C.; Fimiani, F.; Cesaro, A.; Gentile, L.; Leggiero, E.; Pero, R.; et al. Laboratory medicine: Health evaluation in elite athletes. Clin. Chem. Lab. Med. 2019, 57, 1450–1473. [Google Scholar] [CrossRef]
- Almasi, F.; Khazaei, M.; Chehrei, S.; Ghanbari, A. Hepatoprotective Effects of Tribulus terrestris Hydro-Alcholic Extract on Non-Alcoholic Fatty Liver-Induced Rats. Int. J. Morphol. 2017, 35, 345–350. [Google Scholar] [CrossRef]
- Kavitha, P.; Ramesh, R.; Bupesh, G.; Stalin, A.; Subramanian, P. Hepatoprotective activity of Tribulus terrestris extract against acetaminophen-induced toxicity in a freshwater fish (Oreochromis mossambicus). Vitr. Cell. Dev. Biol. Anim. 2011, 47, 698–706. [Google Scholar] [CrossRef]
- Ștefănescu, R.; Tero-Vescan, A.; Negroiu, A.; Aurică, E.; Vari, C.E. A Comprehensive Review of the Phytochemical, Pharmacological, and Toxicological Properties of Tribulus terrestris L. Biomolecules 2020, 10, 752. [Google Scholar] [CrossRef]
- Coutinho, A.E.; Chapman, K.E. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol. Cell. Endocrinol. 2011, 335, 2–13. [Google Scholar] [CrossRef]
- Ranjithkumar, R.; Alhadidi, Q.; Shah, Z.A.; Ramanathan, M. Tribulusterine Containing Tribulus terrestris Extract Exhibited Neuroprotection Through Attenuating Stress Kinases Mediated Inflammatory Mechanism: In Vitro and In Vivo Studies. Neurochem. Res. 2019, 44, 1228–1242. [Google Scholar] [CrossRef]
- Gunarathne, R.; Nadeeshani, H.; Lu, A.; Li, J.; Zhang, B.; Ying, T.; Lu, J. Potential Nutraceutical Use of Tribulus terrestris L. in Human Health. Food Rev. Int. 2022, 1, 1–30. [Google Scholar] [CrossRef]
- Anand, R.; Patnaik, G.K.; Kulshreshtha, D.K.; Dhawan, B.N. Activity of certain fractions of Tribulus terrestris fruits against experimentally induced urolithiasis in rats. Indian J. Exp. Biol. 1994, 32, 548–552. [Google Scholar]
- Aggarwal, A.; Tandon, S.; Kumar Singla, S.; Tandon, C. A novel antilithiatic protein from Tribulus terrestris having cytoprotective potency. Protein Pept. Lett. 2012, 19, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Rubio Pérez, F.J.; Franco Bonafonte, L.; Ibarretxe Guerediaga, D.; Oyon Belaza, M.P.; Ugarte Peyron, P. Effect of an individualized physical exercise program on lipid profile in sedentary patients with cardiovascular risk factors. Clin. Investig. Arterioscler. 2017, 29, 201–208. [Google Scholar]
- Kraus, W.E.; Houmard, J.A.; Duscha, B.D.; Knetzger, K.J.; Wharton, M.B.; McCartney, J.S.; Bales, C.W.; Henes, S.; Samsa, G.P.; Otvos, J.D.; et al. Effects of the amount and intensity of exercise on plasma lipoproteins. N. Engl. J. Med. 2002, 347, 1483–1492. [Google Scholar] [CrossRef]
- Leon, A.S.; Sanchez, O.A. Response of blood lipids to exercise training alone or combined with dietary intervention. Med. Sci. Sports Exerc. 2001, 33, S502–S515. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, J.R.; Mesa, J.L.M.; Mingorance, I.; Rodríguez-Cuartero, A.; Castillo, M.J. Sports with a high degree of physical stress negatively affect plasma lipid profile. Rev. Española Cardiol. 2004, 1, 57, 499–506. [Google Scholar]
- Santos-Silva, A.; Rebelo, M.I.; Castro, E.M.B.; Belo, L.; Guerra, A.; Rego, C.; Quintanilha, A. Leukocyte activation, erythrocyte damage, lipid profile and oxidative stress imposed by high competition physical exercise in adolescents. Clin. Chim. Acta 2001, 306, 119–126. [Google Scholar] [CrossRef]
- Khan, S.; Kabir, H.; Jalees, F.; Asif, M.; Naquvi, K.J. Antihyperlipidemic potential of fruits of Tribulus terrestris Linn. Int. J. Biomed. Res. 2011, 2, 98–101. [Google Scholar] [CrossRef][Green Version]
- El-Tantawy, W.H.; Hassanin, L.A. Hypoglycemic and hypolipidemic effects of alcoholic extract of Tribulus alatus in streptozotocin-induced diabetic rats: A comparative study with T. terrestris (Caltrop). Indian J. Exp. Biol. 2007, 45, 785–790. [Google Scholar]
- Chu, S.; Qu, W.; Pang, X.; Sun, B.; Huang, X. Effect of saponin from Tribulus terrestris on hyperlipidemia. Zhong Yao Cai 2003, 26, 341–344. [Google Scholar]
- Wang, X.; Wang, R.; Yin, L.; Liu, G. Effects of Tribulus terrestris on immune function in over-trained rats and its mechanism: The role of glucocorticoid and glucocorticoid receptor. Endocr. Abstr. 2013, 1, 32. [Google Scholar]
- Gauthaman, K.; Ganesan, A.P. The hormonal effects of Tribulus terrestris and its role in the management of male erectile dysfunction—An evaluation using primates, rabbit and rat. Phytomedicine 2008, 15, 44–54. [Google Scholar] [CrossRef]
- Singh, S.; Nair, V.; Gupta, Y.K. Evaluation of the aphrodisiac activity of Tribulus terrestris Linn. in sexually sluggish male albino rats. J. Pharmacol. Pharmacother. 2012, 3, 43–47. [Google Scholar] [CrossRef]
- Iacono, F.; Prezioso, D.; Illiano, E.; Romeo, G.; Ruffo, A.; Amato, B. Sexual asthenia: Tradamixina versus Tadalafil 5 mg daily. BMC Surg. 2012, 12, S23. [Google Scholar] [CrossRef]
- Zhu, W.; Du, Y.; Meng, H.; Dong, Y.; Li, L. A review of traditional pharmacological uses, phytochemistry, and pharmacological activities of Tribulus terrestris. Chem. Cent. J. 2017, 11, 60. [Google Scholar] [CrossRef]
- Neychev, V.K.; Mitev, V.I. The aphrodisiac herb Tribulus terrestris does not influence the androgen production in young men. J. Ethnopharmacol. 2005, 101, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Frystyk, J. Exercise and the growth hormone-insulin-like growth factor axis. Med. Sci. Sports Exerc. 2010, 42, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, I.H. Sarcopenia: Origins and clinical relevance. Clin. Geriatr. Med. 2011, 27, 337–339. [Google Scholar] [CrossRef] [PubMed]
- Giannoulis, M.G.; Martin, F.C.; Nair, K.S.; Umpleby, A.M.; Sonksen, P. Hormone replacement therapy and physical function in healthy older men. Time to talk hormones? Endocr. Rev. 2012, 33, 314–377. [Google Scholar] [CrossRef]
- Díaz Martínez, A.E.; Alcaide Martín, M.J.; González-Gross, M. Basal Values of Biochemical and Hematological Parameters in Elite Athletes. Int. J. Environ. Res. Public Health 2022, 19, 3059. [Google Scholar] [CrossRef]
| Study | Item | Total | % | Quality Score | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | ||||
| Ma et al., 2017 [12] | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 15 | 93.8 | E |
| Rogerson et al., 2007 [14] | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 15 | 93.8 | E |
| Fernández-Lázaro et al., 2021 [15] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 16 | 100 | E |
| Talemi et al., 2021 [17] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 16 | 100 | E |
| Milasius, et al., 2019 [22] | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 13 | 86.7 | VG |
| Poprzecki et al., 2005 [23] | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 13 | 86.7 | VG |
| Wilk et al., 2012 [24] | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 13 | 86.7 | VG |
| Characteristics | Types | Study |
|---|---|---|
| Participants | Elite athletes | [12,14,23] |
| Well-trained athletes | [15,22] | |
| No regular training before the study | [17,24] | |
| Supplementation product | Manufactured | [17,24] |
| Registered product® | [12,14,15,22,23] | |
| % Saponins of the supplementation product | 100% | [22,23,24] |
| 60% | [14] | |
| >40% | [15,17] | |
| 40% | [12] | |
| Total dose (mg) × day−1 | 1875 | [22] |
| 1800–2700 | [23] | |
| 1250 | [12] | |
| 900–1800 | [24] | |
| 770 | [15] | |
| 500 | [17] | |
| 450 | [14] | |
| Duration | 12 weeks | [24] |
| 6 weeks | [15] | |
| 5 weeks | [14] | |
| 4 weeks | [23] | |
| 3 weeks–4 weeks (rest)–3 weeks | [12] | |
| 2 weeks | [17] | |
| 20 days | [22] | |
| Dose schedule | After exercise and before going to sleep | [23] |
| a.m. | [12] | |
| a.m. and before going to sleep | [24] | |
| a.m. and p.m. | [17,22] | |
| No reported | [14,15] |
| First Author, Year of Publication, and Country | Study Design | Participants (Baseline Sample Size and Characteristics, Withdrawals, and Final Group Sample Size) | Intervention | Outcomes | Results | |
|---|---|---|---|---|---|---|
| Ma et al., 2017, China [12] | Random-ized, double-blind, placebo-controlled trial | 15  boxers (national second-level athletes, 2–3 y of training) boxers (national second-level athletes, 2–3 y of training) Age (mean ± SD): CG: 16.6 ± 1.9 y IG: 16.1 ± 1.8 y Weight (mean ± SD): CG: 62.8 ± 15.2 kg IG: 64.1 ± 6.6 kg Body fat (mean ± SD): CG: 9.6 ± 3.2% IG: 9.8 ± 2.4% 2 withdrawals/lost to follow-up 13 participants completed the study 7 participants CG 6 participants IG | 2 × 625 mg TT (Pronova Biocare, Sweden) (>40% steroidal saponins) “Placebo” (starch) 2 cap every morning 3 wk supplementation For high-volume training 4 wk rest 3 wk supplementation For high-intensity training | BUN CK DHT T IGF-1 IGFBP-3 | IG vs. CG | |
| high-volume training | high-intensity training | |||||
| ↓BUN ↑CK ↓DHT ↓Testosterone ↓IGF-1 ↓*IGFBP-3 | ↓BUN ↓*CK ↓DHT ↓Testosterone ↑IGF-1 ↓*IGFBP-3 | |||||
| IG: change from baseline | ||||||
| high-volume training | high-intensity training | |||||
| ↑BUN ↑CK ↓DHT ↑Testosterone ↓IGF-1 ↓*IGFBP-3 |  BUN BUN↓*CK ↓DHT ↑Testosterone ↓IGF-1 ↓*IGFBP-3 | |||||
| Rogerson et al., 2007, Australia [14] | Random-ized, double-blind, placebo-controlled trial | 22  male elite rugby players male elite rugby playersAge (mean ± SD) CG: 19 ± 1.3 y IG: 20.5 ± 3.8 y Weight (mean ± SD) CG: 87.6 ± 9.0 kg IG: 88.5 ± 10.5 kg No withdrawals reported 11 participants CG 11 participants IG | 450 mg/cap of TT extract (Body Science, Sydney, Australia) (60% steroidal saponins; 40% flavonoids, alkaloids, phenols). “Placebo” inert herbs identical TT 1 cap × day 5 wk | Testosterone/Epites-tosterone | IG vs. CG ↓Testosterone/Epitestosterone IG: Change from baseline ↓Testosterone/Epitestosterone | |
| Fernández-Lázaro et al., 2021, Spain [15] | Random-ized, single-blind, placebo-controlled trial | 30  CrossFit®-trained athletes CrossFit®-trained athletesAge (mean ± SD): CG: 33.1 ± 5.7 y IG: 32.9 ± 6.3 y Body mass (mean ± SD) CG: 80.1 ± 10.7 kg IG: 81.2 ± 11.5 kg No withdrawals reported 15 participants CG 15 participants IG | 2 caps × 385 mg TT (Quamtrax Europe, Spain) (40% steroidal saponins) “Placebo” (maltodextrin) 2 caps empty stomach 6 wk | Testosterone Cortisol Testosterone/Cortisol | IG vs. CG ↑*Testosterone ↓Cortisol ↑Testosterone/Cortisol IG: Change from baseline  Testosterone Testosterone↓Cortisol ↑Testosterone/Cortisol | |
| Talemi et al., 2021, Iran [17] | Random-ized, double-blind, placebo-controlled trial | 18  healthy physically active through resistance exercise training healthy physically active through resistance exercise trainingAge (mean ± SD) 22.44 ± 2.54 y BMI (mean ± SD) 26.15 ± 1.62 kg/m2 No withdrawals reported 9 participants CG 9 participants IG | 2 × 250 mg TT TT powder and extract (27 mg total phenolic content, 100 mg furostanol saponins, and 34 mg total flavonoids) “Placebo” (maltodextrin) 2 wk daily: morning and evening intakes after meal | IL-6 Hs-CRP CK LDH | IG vs. CG ↓IL-6 ↓Hs-CRP ↓CK ↓*LDH IG: Change from baseline ↑*IL-6 ↑Hs-CRP ↓CK ↑LDH | |
| Milasius, et al., 2019, Lithuania [22] | Placebo-controlled study | 32  athletes’ endurance sport athletes’ endurance sportAge (range): 20-22 y Weight (mean ± SD): CG 76.0 ± 8.2 kg IG 75.3 ± 7.7 kg BMI (mean ± SD) CG: 22.9 ± 1.7 kg/m2 IG: 23.1 ± 1.9 kg/m2 No withdrawals reported 12 participants CG 20 participants IG | 3 × 625 mg TT powder (Optimum Nutrition, EE.UU.) (100% furostanol saponins) a.m. (1 × cap) and p.m. (2 × caps) intakes 20 days | RCB Hb HCT MVC ERS WBC LYM MON GRAN CK Cr Ua Urea Chol Tg Bilirubin Testosterone Cortisol | IG vs. CG ↓RCB ↑HB ↑HCT ↓MVC ↑ERS ↓WBC ↓LYM ↑MON ↑GRAN ↑*CK ↓Cr ↑Ua ↓Urea ↓Chol ↑Tg ↓Bilirubin xTestosterone xCortisol | IG: Change from baseline ↓RCB ↓HB ↑HCT  MVC MVC↓ERS  WBC WBC↓*LYM ↓MON ↑*GRAN ↑*CK ↓Cr ↓Ua ↓Urea ↓Chol ↑Tg ↓Bilirubin ↑Testosterone ↑Cortisol |
| Poprzecki et al., 2005, Poland [23] | Placebo-controlled study | 24  competitive basketball players competitive basketball playersAge (mean ± SD): 26 ± 3.4 y Weight (mean ± SD): 91.5 ± 9.0 kg No withdrawals reported 8 participants CG 8 participants IG | “Tribusteron 90” 450 mg/cap (100% Steroidal saponins) 1st 4 caps × day, 2 wk (1800 mg saponins) 2st 6 caps × day, 2 wk (2700 mg saponins) “Placebo” 450 mg/caps gelatin. Twice daily: 30 min before training and 20 min before going to bed 4 wk | Testosterone Luteinizing Hormone Estradiol | IG vs. CG ↓Testosterone ↑Luteinizing Hormone ↑Estradiol IG: Change from baseline ↓*Testosterone ↑Luteinizing Hormone ↑*Estradiol | |
| Wilk et al., 2012, Poland [24] | Random-ized, placebo-controlled trial | 14  with 4 training sessions * wk, (2 sessions anaerobic power, 2 of aerobic endurance exercise) with 4 training sessions * wk, (2 sessions anaerobic power, 2 of aerobic endurance exercise)Age (range): 45–60 y BMI (range): 25–30 kg/m2 Body fat (range): 23–30% No withdrawals/lost to follow-up reported | 1st 6 wk: 900 mg TT (100% steroidal saponins) 2 caps × 300 mg morning on an empty stomach 1 cap × 300 mg bedtime 2nd 6 wk: 1800 mg TT (100% steroidal saponins) 4 caps × 300 mg morning on an empty stomach 2 caps × 300 mg bedtime “Placebo” caps gelatin 12 wk | Chol LDH-Chol HDL-Chol GH IGF-1 Testosterone | IG vs. CG ↓*Chol ↑*HDL-Chol ↓*LDH-Chol ↑*GH ↑*IGF-1 ↑*Testosterone IG: Change from baseline ↓*Chol ↑*HDL-Chol ↓*LDH-Chol ↑*GH ↑*IGF-1 ↑*Testosterone | |
 = no significant change. ↑* = significant increase; ↓* = significant decrease; CG = control group; IG = intervention group; wk = weeks; caps = capsules; TT = Tribulus Terrestris; DHT = dihydrotestosterone; IGF-1 = Insulin-like growth factor 1; IGFBP-3 = Insulin-like growth factor binding protein 3; GH = growth hormone; CK = creatine kinase; LDH = lactate dehydrogenase; Cr = creatinine; Ua = uric acid; BUN = blood urine nitrogen; RCB = red blood cells; HB = Hemoglobin; HCT = Hematocrit; ESR = erythrocyte sedimentation rate; MCV = mean corpuscular volume; WBC = white blood cells; LYM = lymphocytes MON = monocytes; GRAN = granulocytes; Chol = cholesterol; Tg = triglycerides; LDH-Chol = low-density lipoprotein;
= no significant change. ↑* = significant increase; ↓* = significant decrease; CG = control group; IG = intervention group; wk = weeks; caps = capsules; TT = Tribulus Terrestris; DHT = dihydrotestosterone; IGF-1 = Insulin-like growth factor 1; IGFBP-3 = Insulin-like growth factor binding protein 3; GH = growth hormone; CK = creatine kinase; LDH = lactate dehydrogenase; Cr = creatinine; Ua = uric acid; BUN = blood urine nitrogen; RCB = red blood cells; HB = Hemoglobin; HCT = Hematocrit; ESR = erythrocyte sedimentation rate; MCV = mean corpuscular volume; WBC = white blood cells; LYM = lymphocytes MON = monocytes; GRAN = granulocytes; Chol = cholesterol; Tg = triglycerides; LDH-Chol = low-density lipoprotein;  = males; HDL-Chol = high-density lipoprotein; IL-6 = interleukin 6; Hs-CRP = high-sensitivity C-reactive protein; y = years; kg = kilograms; m2 = square meters; mg = milligrams.
= males; HDL-Chol = high-density lipoprotein; IL-6 = interleukin 6; Hs-CRP = high-sensitivity C-reactive protein; y = years; kg = kilograms; m2 = square meters; mg = milligrams.Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Lázaro, D.; Fernandez-Lazaro, C.I.; Seco-Calvo, J.; Garrosa, E.; Adams, D.P.; Mielgo-Ayuso, J. Effects of Tribulus terrestris L. on Sport and Health Biomarkers in Physically Active Adult Males: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 9533. https://doi.org/10.3390/ijerph19159533
Fernández-Lázaro D, Fernandez-Lazaro CI, Seco-Calvo J, Garrosa E, Adams DP, Mielgo-Ayuso J. Effects of Tribulus terrestris L. on Sport and Health Biomarkers in Physically Active Adult Males: A Systematic Review. International Journal of Environmental Research and Public Health. 2022; 19(15):9533. https://doi.org/10.3390/ijerph19159533
Chicago/Turabian StyleFernández-Lázaro, Diego, Cesar I. Fernandez-Lazaro, Jesús Seco-Calvo, Evelina Garrosa, David P. Adams, and Juan Mielgo-Ayuso. 2022. "Effects of Tribulus terrestris L. on Sport and Health Biomarkers in Physically Active Adult Males: A Systematic Review" International Journal of Environmental Research and Public Health 19, no. 15: 9533. https://doi.org/10.3390/ijerph19159533
APA StyleFernández-Lázaro, D., Fernandez-Lazaro, C. I., Seco-Calvo, J., Garrosa, E., Adams, D. P., & Mielgo-Ayuso, J. (2022). Effects of Tribulus terrestris L. on Sport and Health Biomarkers in Physically Active Adult Males: A Systematic Review. International Journal of Environmental Research and Public Health, 19(15), 9533. https://doi.org/10.3390/ijerph19159533











