Effects of a Home-Based Exercise Program on Health-Related Quality of Life and Physical Fitness in Dementia Caregivers: A Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Trail Design and Participants
2.2. Intervention
2.3. Measures
2.4. Statistical Analysis
3. Results
3.1. Sociodemographic and Caregiving-Related Characteristics of Participants
3.2. Intervention Adherence
3.3. Effects of Intervention
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mebane-Sims, I. Alzheimer’s Association, 2018 Alzheimer’s disease facts and figures. Alzheimers Dement 2018, 14, 367–429. [Google Scholar]
- World Health Organization Dementia Fact Sheet. Available online: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed on 20 September 2020).
- Alzheimer’s Association. Alzheimer’s disease facts and figures. Alzheimers Dement 2017, 13, 325–373. [Google Scholar]
- Graff, M.J.L.; Adang, E.M.M.; Vernooij-Dassen, M.J.M.; Dekker, J.; Jönsson, L.; Thijssen, M.; Hoefnagels, W.H.L.; Olde Rikkert, M.G.M. Community Occupational Therapy for Older Patients with Dementia and Their Care Givers: Cost Effectiveness Study. BMJ 2008, 336, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Shultz, R.; Newsom, J.; Mittelmark, M.; Burton, L.; Hirsch, C.; Jackson, S. Health Effects of Caregiving. The Caregiver Health Effects Study. Ann. Behav. Med. 1997, 19, 110–116. [Google Scholar] [CrossRef]
- Chan, S.W.C. Family Caregiving in Dementia: The Asian Perspective of a Global Problem. Dement. Geriatr. Cogn. Disord. 2011, 30, 469–478. [Google Scholar] [CrossRef]
- National Research Council. The Role of Human Factors in Home Health Care: Workshop Summary; National Research Council: Rockville, MD, USA, 2010. [Google Scholar]
- Chiao, C.Y.; Wu, H.S.; Hsiao, C.Y. Caregiver Burden for Informal Caregivers of Patients with Dementia: A Systematic Review. Int. Nurs. Rev. 2015, 62, 340–350. [Google Scholar] [CrossRef]
- Collins, L.G.; Swartz, K. Caregiver Care. Am. Fam. Physician 2011, 83, 1309–1317. [Google Scholar]
- Corvin, J.; Chan, I.; Tezak, A.; Carpenter, K.; Aguado Loi, C.; Gonzales, J.; Hoare, I. Caring for Individuals with Chronic Illness and Minor Depression: Latino Perceptions of Caregiver Burden. J. Gerontol. Soc. Work 2017, 60, 79–95. [Google Scholar] [CrossRef]
- Erol, R.; Brooker, D.; Peel, E. Women and Dementia: A Global Research Review; Alzheimer’s Disease International: London, UK, 2015. [Google Scholar]
- Pinquart, M.; Sörensen, S. Differences between Caregivers and Noncaregivers in Psychological Health and Physical Health: A Meta-Analysis. Psychol. Aging 2003, 18, 250–267. [Google Scholar] [CrossRef]
- Bertrand, R.M.; Fredman, L.; Saczynski, J. Are All Caregivers Created Equal? Stress in Caregivers to Adults with and without Dementia. J. Aging Health 2006, 18, 534–551. [Google Scholar] [CrossRef]
- Ory, M.G.; Hoffman, R.R.; Yee, J.L.; Tennstedt, S.; Schulz, R. Prevalence and Impact of Caregiving: A Detailed Comparison Between Dementia and Nondementia Caregivers. Gerontologist 1999, 39, 177–186. [Google Scholar] [CrossRef]
- Wilcox, S.; King, A.C. Sleep Complaints in Older Women Who Are Family Caregivers. J. Gerontol. Ser. B 1999, 54B, P189–P198. [Google Scholar] [CrossRef]
- Chang, H.Y.; Chiou, C.J.; Chen, N. Sen Impact of Mental Health and Caregiver Burden on Family Caregivers’ Physical Health. Arch. Gerontol. Geriatr. 2010, 50, 267–271. [Google Scholar] [CrossRef]
- Cuijpers, P. Depressive Disorders in Caregivers of Dementia Patients: A Systematic Review. Aging Ment. Heal. 2005, 9, 325–330. [Google Scholar] [CrossRef]
- Tremont, G. Family Caregiving in Dementia. Med. Health. R. I 2011, 94, 36–38. [Google Scholar]
- Valles, M.N.; Gutierrez, V.; Luquin, A.M.; Martin, M.A.; de Castro, F. Health and Social Problems of Caregivers of Patients with Dementia. Atención Primaria 1998, 22, 481–485. [Google Scholar]
- Verbakel, E.; Metzelthin, S.F.; Kempen, G.I.J.M. Caregiving to Older Adults: Determinants of Informal Caregivers’ Subjectivewell-Being and Formal and Informal Support as Alleviating Conditions. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 2018, 73, 1099–1111. [Google Scholar] [CrossRef]
- Wright, L.K.; Hickey, J.V.; Buckwalter, K.C.; Hendrix, S.A.; Kelechi, T. Emotional and Physical Health of Spouse Caregivers of Persons with Alzheimer’s Disease and Stroke. J. Adv. Nurs. 1999, 30, 552–563. [Google Scholar] [CrossRef]
- Schulz, R.; Sherwood, P.R. Physical and Mental Health Effects of Family Caregiving. Am. J. Nurs. 2008, 44, 105–113. [Google Scholar] [CrossRef]
- Cochrane, J.J.; Goering, P.N.; Rogers, J.M. The Mental Health of Informal Caregivers in Ontario: An Epidemiological Survey. Am. J. Public Health 1997, 87, 2002–2007. [Google Scholar] [CrossRef]
- Schulz, R.; Beach, S. Caregiving as a Risk Factor for Mortality: The Caregiver Health Effects Study. JAMA 1999, 282, 2215–2219. [Google Scholar] [CrossRef]
- Shaw, W.S.; Patterson, T.L.; Ziegler, M.G.; Dimsdale, J.E.; Semple, S.J.; Grant, I. Accelerated Risk of Hypertensive Blood Pressure Recordings among Alzheimer Caregivers. J. Psychosom. Res. 1999, 46, 215–227. [Google Scholar] [CrossRef]
- Capistrant, B.D.; Moon, J.R.; Glymour, M.M. Spousal Caregiving and Incident Hypertension. Am. J. Hypertens. 2012, 25, 437–443. [Google Scholar] [CrossRef]
- King, A.C.; Oka, R.K.; Young, D.R. Ambulatory Blood Pressure and Heart Rate Responses to the Stress of Work and Caregiving in Older Women. J. Gerontol. 1994, 49, M239–M245. [Google Scholar] [CrossRef]
- Bauer, M.E.; Vedhara, K.; Perks, P.; Wilcock, G.K.; Lightman, S.L.; Shanks, N. Chronic Stress in Caregivers of Dementia Patients Is Associated with Reduced Lymphocyte Sensitivity to Glucocorticoids. J. Neuroimmunol. 2000, 103, 84–92. [Google Scholar] [CrossRef]
- Lee, S.; Colditz, G.A.; Berkman, L.F.; Kawachi, I. Caregiving and Risk of Coronary Heart Disease in U.S. Women: A Prospective Study. Am. J. Prev. Med. 2003, 24, 113–119. [Google Scholar] [CrossRef]
- Mausbach, B.T.; Patterson, T.L.; Rabinowitz, Y.G.; Grant, I.; Schulz, R. Depression and Distress Predict Time to Cardiovascular Disease in Dementia Caregivers. Heal. Psychol. 2007, 26, 539–544. [Google Scholar] [CrossRef]
- Von Känel, R.; Mausbach, B.T.; Patterson, T.L.; Dimsdale, J.E.; Aschbacher, K.; Mills, P.J.; Ziegler, M.G.; Ancoli-Israel, S.; Grant, I. Increased Framingham Coronary Heart Disease Risk Score in Dementia Caregivers Relative to Non-Caregiving Controls. Gerontology 2008, 54, 131–137. [Google Scholar] [CrossRef]
- Vitaliano, P.P.; Zhang, J.; Scanlan, J.M. Is Caregiving Hazardous to One’s Physical Health? A Meta-Analysis. Psychol. Bull. 2003, 129, 946–972. [Google Scholar] [CrossRef]
- Badia, X.; Lara, N.; Roset, M. Quality of Life, Time Commitment and Burden Perceived by the Principal Informal Caregiver of Alzheimer’s Patients. Atención Primaria 2004, 34, 170–177. [Google Scholar] [CrossRef]
- Meyer, C.; Dow, B.; Bilney, B.E.; Moore, K.J.; Bingham, A.L.; Hill, K.D. Falls in Older People Receiving In-Home Informal Care across Victoria: Influence on Care Recipients and Caregivers. Australas. J. Ageing 2010, 31, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Gusi, N.; Prieto, J.; Madruga, M.; Garcia, J.M.; Gonzalez-Guerrero, J.L. Health-Related Quality of Life and Fitness of the Caregiver of Patient with Dementia. Med. Sci. Sports Exerc. 2009, 41, 1182–1187. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, C.A.; Miller, M.C.; Sui, X.; West, D.S. Weight Status and Sedentary Behavior of Alzheimer’s Disease Caregivers. Am. J. Health Behav. 2020, 44, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Cuthbert, C.A.; King-Shier, K.; Ruether, D.; Tapp, D.M.; Culos-Reed, S.N. What Is the State of the Science on Physical Activity Interventions for Family Caregivers? A Systematic Review and RE-AIM Evaluation. J. Phys. Act. Heal. 2017, 14, 578–595. [Google Scholar] [CrossRef]
- Epps, F.; To, H.; Liu, T.T.; Karanjit, A.; Warren, G. Effect of Exercise Training on the Mental and Physical Well-Being of Caregivers for Persons Living with Chronic Illnesses: A Systematic Review and Meta-Analysis. J. Appl. Gerontol. 2019, 40, 18–27. [Google Scholar] [CrossRef]
- King, A.C.; Baumann, K.; O’Sullivan, P.; Wilcox, S.; Castro, C. Effects of Moderate-Intensity Exercise on Physiological, Behavioral, and Emotional Responses to Family Caregiving: A Randomized Controlled Trial. J. Gerontol. Ser. A 2002, 57, M26–M36. [Google Scholar] [CrossRef]
- Connell, C.M.; Janevic, M.R. Effects of a Telephone-Based Exercise Intervention for Dementia Caregiving Wives: A Randomized Controlled Trial. J. Appl. Gerontol. 2009, 28, 171–194. [Google Scholar] [CrossRef]
- Danucalov, M.A.D.; Kozasa, E.H.; Ribas, K.T.; Galduróz, J.C.F.; Garcia, M.C.; Verreschi, I.T.N.; Oliveira, K.C.; Romani De Oliveira, L.; Leite, J.R. A Yoga and Compassion Meditation Program Reduces Stress in Familial Caregivers of Alzheimer’s Disease Patients. Evid. Based Complement. Altern. Med. 2013, 2016, 513149. [Google Scholar] [CrossRef]
- Castro, C.M.; Wilcox, S.; O’Sullivan, P.; Baumann, K.; King, A.C. An Exercise Program for Women Who Are Caring for Relatives with Dementia. Psychosom. Med. 2002, 64, 458–468. [Google Scholar] [CrossRef]
- Hill, K.; Smith, R.; Fearn, M.; Rydberg, M.; Oliphant, R. Physical and Psychological Outcomes of a Supported Physical Activity Program for Older Carers. J. Aging Phys. Act. 2007, 15, 257–271. [Google Scholar] [CrossRef][Green Version]
- Hirano, A.; Suzuki, Y.; Kuzuya, M.; Onishi, J.; Ban, N.; Umegaki, H. Influence of Regular Exercise on Subjective Sense of Burden and Physical Symptoms in Community-Dwelling Caregivers of Dementia Patients: A Randomized Controlled Trial. Arch. Gerontol. Geriatr. 2011, 53, e158–e163. [Google Scholar] [CrossRef]
- Madruga, M.; Gozalo, M.; Prieto, J.; Rohlfs Domínguez, P.; Gusi, N. Effects of a Home-Based Exercise Program on Mental Health for Caregivers of Relatives with Dementia: A Randomized Controlled Trial. Int. Psychogeriatrics 2020, 33, 359–372. [Google Scholar] [CrossRef]
- Orgeta, V.; Miranda-Castillo, C. Does Physical Activity Reduce Burden in Carers of People with Dementia? A Literature Review. Int. J. Geriatr. Psychiatry 2014, 29, 771–783. [Google Scholar] [CrossRef]
- Waelde, L.C.; Thompson, L.; Gallagher-Thompson, D. A Pilot Study of a Yoga and Meditation Intervention for Dementia Caregiver Stress. J. Clin. Psychol. 2004, 60, 677–687. [Google Scholar] [CrossRef]
- Stella, F.; Canonici, A.P.; Gobbi, S.; Santos-Galduroz, R.F.; de Castilho Cação, J.; Gobbi, L.T.B. Attenuation of Neuropsychiatric Symptoms and Caregiver Burden in Alzheimer’s Disease by Motor Intervention: A Controlled Trial. Clinics 2011, 66, 1553–1560. [Google Scholar] [CrossRef]
- Teri, L.; Gibbons, L.E.; McCurry, S.M.; Logsdon, R.G.; Buchner, D.M.; Barlow, W.E.; Kukull, W.A.; LaCroix, A.Z.; McCormick, W.; Larson, E.B. Exercise Plus Behavioral Management in Patients with Alzheimer Disease: A Randomized Controlled Trial. J. Am. Med. Assoc. 2003, 290, 2015–2022. [Google Scholar] [CrossRef]
- Prick, A.E.; De Lange, J.; Twisk, J.; Pot, A.M. The Effects of a Multi-Component Dyadic Intervention on the Psychological Distress of Family Caregivers Providing Care to People with Dementia: A Randomized Controlled Trial. Int. Psychogeriatr. 2015, 27, 2031–2044. [Google Scholar] [CrossRef]
- Farran, C.J.; Etkin, C.D. Effect of Moderate to Vigorous Physical Activity Intervention on Improving Dementia Family Caregiver Physical Function: A Randomized Controlled Trial. J. Alzheimers Dis. Park. 2016, 6, 253. [Google Scholar] [CrossRef]
- Farran, C.J.; Paun, O.; Cothran, F.; Etkin, C.D.; Rajan, K.B.; Eisenstein, A.; Maryam, N. Impact of an Individualized Physical Activity Intervention on Improving Mental Health Outcomes in Family Caregivers of Persons with Dementia: A Randomized Controlled Trial. AIMS Med. Sci. 2015, 3, 15. [Google Scholar] [CrossRef]
- Jackson, G.A.; Browne, D. Supporting Carers of People with Dementia: What Is Effective? BJPsych Adv. 2017, 23, 179–186. [Google Scholar] [CrossRef]
- Etkin, C.D.; Farran, C.J.; Barnes, L.L.; Shah, R.C. Recruitment and Enrollment of Caregivers for a Lifestyle Physical Activity Clinical Trial. Res. Nurs. Heal. 2012, 35, 70–81. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, B.; Bailey, S.; Walker, A. Promoting the Health and Well Being of Older Carers: A Proactive Strategy. Aust. Heal. Rev. 2003, 26, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Farina, N.; Williams, A.; Clarke, K.; Hughes, L.J.; Thomas, S.; Lowry, R.G.; Banerjee, S. Barriers, Motivators and Facilitators of Physical Activity in People with Dementia and Their Family Carers in England: Dyadic Interviews. Aging Ment. Heal. 2020, 18, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Farran, C.J.; Staffileno, B.A.; Gilley, D.W.; McCann, J.J.; Li, Y.; Castro, C.M.; King, A.C. A Lifestyle Physical Activity Intervention for Caregivers of Persons with Alzheimer’s Disease. Am. J. Alzheimers Dis. Other Demen. 2008, 23, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Marquez, D.X.; Bustamante, E.E.; Kozey-Keadle, S.; Kraemer, J.; Carrion, I. Physical Activity and Psychosocial and Mental Health of Older Caregivers and Non-Caregivers. Geriatr. Nurs. 2012, 33, 358–365. [Google Scholar] [CrossRef]
- Chan, S.W.C.; Lautenschlager, N.; Dow, B.; Ma, S.L.; Wong, C.S.M.; Lam, L.C.W. A Home-Based Exercise Intervention for Caregivers of Persons with Dementia: Study Protocol for a Randomised Controlled Trial. Trials 2016, 17, 460. [Google Scholar] [CrossRef]
- Yan, T.; Wilber, K.H.; Simmons, W.J. Motivating High-Risk Older Adults to Exercise: Does Coaching Matter? Home Health Care Serv. Q. 2011, 30, 84–95. [Google Scholar] [CrossRef]
- Ishii, S.; Weintraub, N.; Mervis, J.R. Apathy: A Common Psychiatric Syndrome in the Elderly. J. Am. Med. Dir. Assoc. 2009, 10, 381–393. [Google Scholar] [CrossRef]
- Steinberg, M.; Sheppard Leoutsakos, J.M.; Podewills, L.J.; Lyketsos, C.G. Evaluation of a Home-Based Exercise Program in the Treatment of Alzheimer’s Disease: The Maximizing Independence in Dementia (MIND) Study. Int. J. Geriatr. Psychiatry 2009, 24, 680–685. [Google Scholar] [CrossRef]
- Madruga, M.; Prieto, J.; Rohlfs, P.; Gusi, N. Cost-Effectiveness and Effects of a Home-Based Exercise Intervention for Female Caregivers of Relatives with Dementia: Study Protocol for a Randomized Controlled Trial. Healthcare 2020, 8, 54. [Google Scholar] [CrossRef]
- Zijlstra, T.R.; Braakman-Jansen, L.M.A.; Taal, E.; Rasker, J.J.; van de Laar, M.A.F.J. Cost-Effectiveness of Spa Treatment for Fibromyalgia: General Health Improvement Is Not for Free. Rheumatology 2007, 46, 1454–1459. [Google Scholar] [CrossRef][Green Version]
- Thomas, S.; Reading, J.; Shephard, R.J. Revision of the Physical Activity Readiness Questionnaire (PAR-Q). Canandial J. Sport Sci. 1992, 17, 338–345. [Google Scholar]
- Zarit, S.H.; Reever, K.E.; Bach-Peterson, J. Relatives of the Impaired Elderly: Correlates of Feelings of Burden. Gerontologist 1980, 20, 649–655. [Google Scholar] [CrossRef]
- Martín, M.; Salvadó, I.; Nadal, S.; Miji, C.L.; Rico, J.M.; Lanz, P.; Taussig, M.I. Adaptación Para Nuestro Medio de La Escala de Sobrecarga Del Cuidador (Caragiver Burden Interview) de Zarit. Rev. Gerontol. 1996, 6, 338–346. [Google Scholar]
- Mahoney, F.I.; Barthel, D.W. Functional Evaluation: The Barthel Index. Md. State Med. J. 1965, 14, 61–65. [Google Scholar]
- Alonso, J.; Prieto, L.; Anto, J.M. The Spanish Version of the SF-36 Health Survey (the SF-36 Health Questionnaire): An Instrument for Measuring Clinical Results [in Spanish]. Med. Clin. 1995, 104, 771–776. [Google Scholar]
- Ware, J.E.; Sherbourne, C.D. The MOS 36-Item Short-Form Health Survey (SF-36). Conceptual Framework and Item Selection. Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef]
- Oja, P.; Tuxworth, B. Eurofit for Adults, Assessment of Health-Related Fitness; UKK Institute for Health Promotion Research: Tampere, Finland, 1995. [Google Scholar]
- Nordenskiold, U.M.; Grimby, G. Grip Force in Patients with Rheumatoid Arthritis and Fibromyalgia and in Healthy Subjects. A Study with the Grippit Instrument. Scand. J. Rheumatol. 1993, 22, 14–19. [Google Scholar] [CrossRef]
- Ito, T.; Shirado, O.; Suzuki, H.; Takahashi, M.; Kaneda, K.; Strax, T.E. Lumbar Trunk Muscle Endurance Testing: An Inexpensive Alternative to a Machine for Evaluation. Arch. Phys. Med. Rehabil. 1996, 77, 75–79. [Google Scholar] [CrossRef]
- Wells, K.E.; Dillon, E.K. The Sit and Reach—A Test of Back and Leg Flexibility. Res. Q. Exerc. Sport 1952, 23, 115–118. [Google Scholar] [CrossRef]
- Csuka, M.; McCarty, D.J. Simple Method for Measurement of Lower Extremity Muscle Strength. Am. J. Med. 1985, 78, 77–81. [Google Scholar] [CrossRef]
- Podsiadlo, D.; Richardson, S. The Timed “up & Go”: A Test of Basic Functional Mobility for Frail Elderly Persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [CrossRef]
- Duncan, P.W.; Weiner, D.K.; Chandler, J.; Studenski, S. Functional Reach: A New Clinical Measure of Balance. J. Gerontol. 1990, 45, M192–M197. [Google Scholar] [CrossRef]
- Weller, I.M.; Thomas, S.G.; Cox, M.H.; Corey, P.N. A Study to Validate the Canadian Aerobic Fitness Test. Can. J. Public Heal. 1992, 83, 120–124. [Google Scholar] [CrossRef]
- Cohen, J. A Power Primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef]
- Hunter, J.; Schmidt, F. Method of Meta-Analysis: Correcting Error and Bias in Research Findings, 2nd ed.; Sage: Newcastle-upon-Tyne, UK, 2004. [Google Scholar]
- World Health Organization. Waist Circumference and Waist-Hip Ratio: Report of a WHO Expert Consultation, Geneva, 8–11 December 2008; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Mingjun, J.; Yongqiu, Z.; Qinghua, X.; Yuanfa, C.; Ying, W.; Xueying, Q.; Defu, M. Dose–Response Relationship between Body Mass Index and Risks of All-Cause Mortality and Disability among the Elderly: A Systematic Review and Meta-Analysis. Clin. Nutr. 2019, 38, 1511–1523. [Google Scholar] [CrossRef]
- Argimon, J.M.; Limon, E.; Vila, J.; Cabezas, C. Health-Related Quality of Life in Carers of Patients with Dementia. Fam. Pract. 2004, 21, 454–457. [Google Scholar] [CrossRef]
- Dawood, S. Caregiver Burden, Quality of Life and Vulnerability towards Psychopathology in Caregivers of Patients with Dementia/Alzheimer’s Disease. J. Coll. Physicians Surg. 2016, 26, 892–895. [Google Scholar]
- Garzón-Maldonado, F.J.; Gutiérrez-Bedmar, M.; García-Casares, N.; Pérez-Errázquin, F.; Gallardo-Tur, A.; Martínez-Valle Torres, M.D. Health-Related Quality of Life in Caregivers of Patients with Alzheimer Disease. Neurologia 2017, 32, 508–515. [Google Scholar] [CrossRef]
- Lee, H.S.; Lee, J. Effects of Exercise Interventions on Weight, Body Mass Index, Lean Body Mass and Accumulated Visceral Fat in Overweight and Obese Individuals: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Int. J. Environ. Res. Public Health 2021, 18, 2635. [Google Scholar] [CrossRef]
- Evans, W.J.; Campbell, W.W. Sarcopenia and Age-Related Changes in Body Composition and Functional Capacity. J. Nutr 1993, 123, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Gusi, N.; Prieto, J.; Olivares, P.R.; Delgado, S.; Quesada, F.; Cebrian, C. Normative Fitness Performance Scores of Community-Dwelling Older Adults in Spain. J. Aging Phys. Act. 2012, 20, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Rikli, R.E.; Jones, C.J. Functional Fitness Normative Scores for Community-Residing Older Adutls, Ages 60–94. J. Aging Phys. Act. 1999, 7, 60–179. [Google Scholar] [CrossRef]
- Fredman, L.; Bertrand, R.M.; Martire, L.M.; Hochberg, M.; Harris, E.L. Leisure-Time Exercise and Overall Physical Activity in Older Women Caregivers and Non-Caregivers from the Caregiver-SOF Study. Prev. Med. 2006, 43, 226–229. [Google Scholar] [CrossRef]
- Wilcox, S.; Castro, C.; King, A.C.; Housemann, R.; Brownson, R.C. Determinants of Leisure Time Physical Activity in Rural Compared with Urban Older and Ethnically Diverse Women in the United States. J. Epidemiol. Community Health 2000, 54, 667–672. [Google Scholar] [CrossRef]
- Anderson, C.S.; Linto, J.; Stewart-Wynne, E.G. A Population-Based Assessment of the Impact and Burden of Caregiving for Long-Term Stroke Survivors. Stroke 1995, 26, 843–849. [Google Scholar] [CrossRef]
- Dunn, N.J.; Strain, L.A. Caregivers at Risk?: Changes in Leisure Participation. J. Leis. Res. 2001, 33, 32–55. [Google Scholar] [CrossRef]
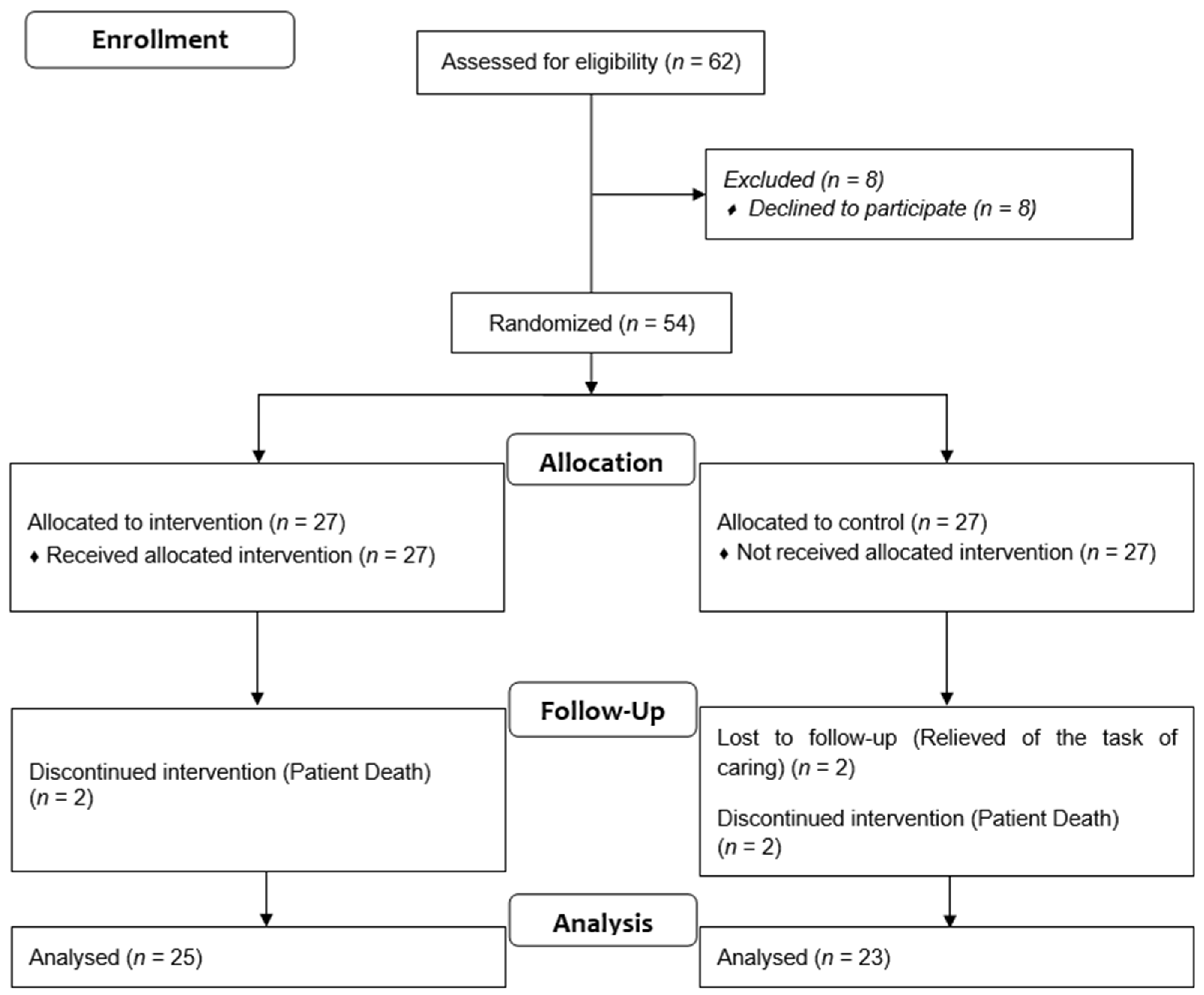
| All (n = 48) | Intervention (n = 25) | Control (n = 23) | t-Test/χ2 | p 1 | |
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | |||
| Age (years) | 60.17 (8.49) | 60.96 (8.99) | 59.30 (8.01) | −0.671 | 0.505 |
| Age of patient (years) | 79.85 (7.92) | 79.44 (10.12) | 80.30 (4.67) | −0.385 | 0.703 |
| Caregiver years | 5.48 (3.31) | 5.28 (2.94) | 5.70 (3.72) | −0.431 | 0.668 |
| Diagnostic years of the patient | 6.94 (4.17) | 7.00 (4.58) | 6.87 (3.77) | −0.107 | 0.915 |
| Zarit Subjective Burden | 55.73 (14.31) | 55.72 (14.21) | 55.74 (14.74) | −0.005 | 0.996 |
| Barthel Index patient | 31.81 (29.64) | 31.68 (26.38) | 31.96 (33.43) | −0.032 | 0.975 |
| (n, %) | (n, %) | (n, %) | |||
| Alzheimer’s disease | 39 (81.3) | 21 (84.0) | 18 (78.3) | 0.291 | 0.865 |
| Relative: daughter | 28 (58.3) | 13 (52.0) | 15 (65.2) | 0.894 | 0.639 |
| Living with patient | 37 (77.1) | 20 (80.0) | 17 (73.9) | 0.251 | 0.616 |
| Living in urban areas | 33 (68.75) | 20 (80.0) | 13 (56.5) | 3.074 | 0.080 |
| Marital status: married | 35 (72.9) | 17 (68.0) | 18 (78.3) | 2.950 | 0.566 |
| Living with husband | 36 (75.0) | 17 (68.0) | 19 (82.6) | 2.699 | 0.259 |
| Education: primary school | 29 (60.4) | 16 (64.0) | 13 (56.5) | 3.600 | 0.308 |
| Not smoker | 42 (87.5) | 24 (96.0) | 18 (78.3) | 4.782 | 0.188 |
| Not alcohol consumer | 35 (72.9) | 19 (76.0) | 16 (69.6) | 5.183 | 0.394 |
| Physical inactivity 2 | 35 (72.9) | 16 (64.0) | 19 (82.6) | 10.525 | 0.161 |
| Outcomes | Group | Baseline Mean (SD) | 9 Months Mean (SD) | Between-Group Effect Mean (95% IC) | Effect Size 1 | p 2 | p 3 | p 4 |
|---|---|---|---|---|---|---|---|---|
| Physical functioning | Intervention | 81.20 (14.59) | 84.60 (17.13) | 3.62 (−4.54 to 11.78) | 0.19 | 0.377 | 0.405 | 0.381 |
| Control | 80.43 (22.10) | 80.22 (21.50) | ||||||
| Physical role limitations | Intervention | 59.00 (40.10) | 62.00 (43.97) | 0.83 (−18.88 to 20.53) | 0.02 | 0.933 | 0.718 | 0.936 |
| Control | 68.47 (39.32) | 70.65 (38.18) | ||||||
| Bodily pain | Intervention | 66.48 (22.96) | 64.36 (25.89) | 0.31 (−14.41 to 15.04) | 0.01 | 0.966 | 0.857 | 0.966 |
| Control | 60.47 (32.29) | 58.04 (30.26) | ||||||
| General health | Intervention | 57.16 (17.49) | 64.12 (16.90) | 8.70 (0.39 to 17.00) | 0.43 * | 0.040 | 0.029 | 0.043 |
| Control | 57.39 (21.91) | 55.65 (19.19) | ||||||
| Vitality | Intervention | 54.80 (18.96) | 63.20 (18.53) | 8.18 (0.29 to 16.07) | 0.42 * | 0.042 | 0.054 | 0.035 |
| Control | 50.22 (19.27) | 50.43 (23.25) | ||||||
| Social functioning | Intervention | 73.91 (33.27) | 78.26 (28.51) | −12.85 (−25.01 to −0.69) | 0.43 * | 0.039 | 0.045 | 0.040 |
| Control | 83.00 (24.17) | 74.50 (27.36) | ||||||
| Emotional role limitations | Intervention | 74.67 (38.82) | 76.00 (36.67) | 1.33 (−16.04 to 18.71) | 0.03 | 0.878 | 0.900 | 0.875 |
| Control | 66.67 (46.05) | 66.67 (43.81) | ||||||
| Mental health | Intervention | 59.52 (16.54) | 68.16 (15.81) | 7.60 (−0.09 to 15.28) | 0.39 | 0.053 | 0.071 | 0.052 |
| Control | 56.17 (21.79) | 57.21 (20.45) | ||||||
| SF-36 PCS | Intervention | 46.44 (7.67) | 46.85 (9.67) | 0.80 (−4.08 to 5.98) | 0.01 | 0.743 | 0.846 | 0.724 |
| Control | 47.40 (8.60) | 47.02 (9.64) | ||||||
| SF-36 MCS | Intervention | 44.21 (11.82) | 46.04 (12.19) | 0.91 (−4.43 to 6.25) | 0.07 | 0.733 | 0.578 | 0.747 |
| Control | 40.29 (13.95) | 41.21 (12.86) |
| Outcomes | Group | Baseline Mean (SD) | 9 Months Mean (SD) | Between-Group Effect Mean (95% IC) | Effect Size 1 | p 2 | p 3 | p 4 |
|---|---|---|---|---|---|---|---|---|
| Weight (Kg) | Intervention | 69.19 (18.26) | 70.41 (18.31) | 2.03 (0.12 to 3.95) | 0.13 * | 0.038 | 0.042 | 0.039 |
| Control | 67.50 (12.07) | 66.68 (12.04) | ||||||
| BMI (kg·m−2) | Intervention | 27.9 (6.73) | 28.2 (6.69) | 0.69 (−0.08 to 1.45) | 0.12 | 0.077 | 0.087 | 0.080 |
| Control | 27.6 (5.12) | 27.2 (4.86) | ||||||
| Waist–Hip ratio | Intervention | 0.814 (0.064) | 0.856 (0.100) | 0.05 (0.01 to 0.09) | 0.76 * | 0.012 | 0.014 | 0.011 |
| Control | 0.843 (0.063) | 0.836 (0.062) | ||||||
| Bi-handgrip strength (kg·m2) | Intervention | 45.46 (12.16) | 47.18 (9.95) | 3.22 (0.29 to 6.17) | 0.31 * | 0.033 | 0.038 | 0.030 |
| Control | 44.89 (7.64) | 43.39 (8.46) | ||||||
| Endurance flexor trunk (s) | Intervention | 31.46 (25.09) | 65.49 (43.29) | 31.81 (12.27 to 51.35) | 0.92 ** | 0.002 | 0.002 | 0.002 |
| Control | 35.33 (41.77) | 37.56 (40.06) | ||||||
| Endurance extensor trunk (s) | Intervention | 44.19 (44.77) | 82.23 (42.85) | 39.86 (19.45 to 6.27) | 0.84 *** | <0.001 | <0.001 | <0.001 |
| Control | 45.70 (48.66) | 43.87 (48.42) | ||||||
| Flexibility (cm) | Intervention | 24.84 (9.79) | 25.04 (9.48) | 2.50 (−0.51 to 5.52) | 0.27 | 0.101 | 0.126 | 0.105 |
| Control | 23.59 (8.29) | 21.29 (9.05) | ||||||
| Leg strength (s) | Intervention | 22.69 (6.63) | 15.66 (5.77) | −7.28 (−11.56 to −3.00) | −0.89 ** | 0.001 | 0.001 | 0.001 |
| Control | 19.74 (9.26) | 19.99 (9.48) | ||||||
| Time up and go (s) | Intervention | 6.29 (1.39) | 6.05 (2.28) | −0.74 (−1.90 to 0.41) | −0.31 | 0.203 | 0.196 | 0.205 |
| Control | 7.34 (3.07) | 7.84 (4.34) | ||||||
| Functional reach (cm) | Intervention | 29.78 (4.91) | 33.16 (7.17) | 3.08 (−0.37 to 6.52) | 0.51 | 0.079 | 0.033 | 0.082 |
| Control | 28.35 (6.91) | 28.65 (7.07) | ||||||
| PAC (VO2max) | Intervention | 22.45 (3.78) | 25.01 (3.37) | 2.14 (0.62 to 3.65) | 0.63 ** | 0.007 | 0.018 | 0.006 |
| Control | 22.57 (2.79) | 23.00 (2.57) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prieto-Prieto, J.; Madruga, M.; Adsuar, J.C.; González-Guerrero, J.L.; Gusi, N. Effects of a Home-Based Exercise Program on Health-Related Quality of Life and Physical Fitness in Dementia Caregivers: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2022, 19, 9319. https://doi.org/10.3390/ijerph19159319
Prieto-Prieto J, Madruga M, Adsuar JC, González-Guerrero JL, Gusi N. Effects of a Home-Based Exercise Program on Health-Related Quality of Life and Physical Fitness in Dementia Caregivers: A Randomized Controlled Trial. International Journal of Environmental Research and Public Health. 2022; 19(15):9319. https://doi.org/10.3390/ijerph19159319
Chicago/Turabian StylePrieto-Prieto, Josué, Miguel Madruga, José Carmelo Adsuar, José Luis González-Guerrero, and Narcís Gusi. 2022. "Effects of a Home-Based Exercise Program on Health-Related Quality of Life and Physical Fitness in Dementia Caregivers: A Randomized Controlled Trial" International Journal of Environmental Research and Public Health 19, no. 15: 9319. https://doi.org/10.3390/ijerph19159319
APA StylePrieto-Prieto, J., Madruga, M., Adsuar, J. C., González-Guerrero, J. L., & Gusi, N. (2022). Effects of a Home-Based Exercise Program on Health-Related Quality of Life and Physical Fitness in Dementia Caregivers: A Randomized Controlled Trial. International Journal of Environmental Research and Public Health, 19(15), 9319. https://doi.org/10.3390/ijerph19159319








