The Combination of Sleep Disorders and Depression Significantly Increases Cancer Risk: A Nationwide Large-Scale Population-Based Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Study Subjects
2.3. Variables of Interest
2.4. Statistical Analysis
3. Results
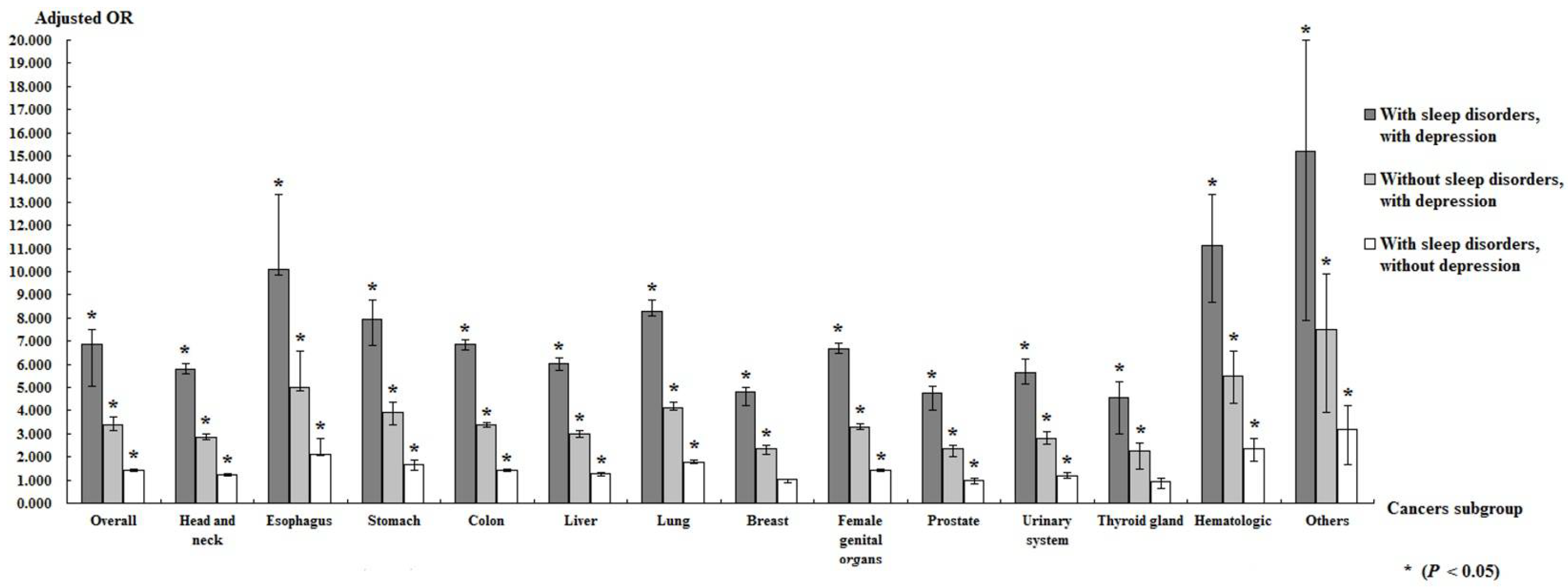
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vyazovskiy, V.V.; Delogu, A. NREM and REM Sleep: Complementary Roles in Recovery after Wakefulness. Neurosci. Rev. J. Bringing Neurobiol. Neurol. Psychiatry 2014, 20, 203–219. [Google Scholar] [CrossRef] [PubMed]
- Ohayon, M.M.; Smirne, S. Prevalence and consequences of insomnia disorders in the general population of Italy. Sleep Med. 2002, 3, 115–120. [Google Scholar] [CrossRef]
- Morin, C.M.; LeBlanc, M.; Daley, M.; Gregoire, J.P.; Mérette, C. Epidemiology of insomnia: Prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006, 7, 123–130. [Google Scholar] [CrossRef]
- Kaneita, Y.; Ohida, T.; Osaki, Y.; Tanihata, T.; Minowa, M.; Suzuki, K.; Wada, K.; Kanda, H.; Hayashi, K. Insomnia among Japanese adolescents: A nationwide representative survey. Sleep 2006, 29, 1543–1550. [Google Scholar] [CrossRef]
- Cho, Y.W.; Shin, W.C.; Yun, C.H.; Hong, S.B.; Kim, J.; Earley, C.J. Epidemiology of insomnia in korean adults: Prevalence and associated factors. J. Clin. Neurol. 2009, 5, 20–23. [Google Scholar] [CrossRef]
- Wallander, M.A.; Johansson, S.; Ruigómez, A.; García Rodríguez, L.A.; Jones, R. Morbidity associated with sleep disorders in primary care: A longitudinal cohort study. Prim. Care Companion J. Clin. Psychiatry 2007, 9, 338–345. [Google Scholar] [CrossRef]
- Mai, E.; Buysse, D.J. Insomnia: Prevalence, Impact, Pathogenesis, Differential Diagnosis, and Evaluation. Sleep Med. Clin. 2008, 3, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.J.; Mallory, L.J.; Lichstein, K.L.; Durrence, H.H.; Riedel, B.W.; Bush, A.J. Comorbidity of chronic insomnia with medical problems. Sleep 2007, 30, 213–218. [Google Scholar] [CrossRef]
- Thompson, C.L.; Li, L. Association of sleep duration and breast cancer OncotypeDX recurrence score. Breast Cancer Res. Treat. 2012, 134, 1291–1295. [Google Scholar] [CrossRef]
- Thompson, C.L.; Larkin, E.K.; Patel, S.; Berger, N.A.; Redline, S.; Li, L. Short duration of sleep increases risk of colorectal adenoma. Cancer 2011, 117, 841–847. [Google Scholar] [CrossRef]
- Wu, M.; Zeng, J.; Chen, Y.; Zeng, Z.; Zhang, J.; Cai, Y.; Ye, Y.; Fu, L.; Xian, L.; Chen, Z. Experimental chronic jet lag promotes growth and lung metastasis of Lewis lung carcinoma in C57BL/6 mice. Oncol. Rep. 2012, 27, 1417–1428. [Google Scholar] [PubMed]
- Hu, L.Y.; Chen, P.M.; Hu, Y.W.; Shen, C.C.; Perng, C.L.; Su, T.P.; Yen, S.H.; Tzeng, C.H.; Chiou, T.J.; Yeh, C.M.; et al. The risk of cancer among patients with sleep disturbance: A nationwide retrospective study in Taiwan. Ann. Epidemiol. 2013, 23, 757–761. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.T.; Huang, M.I.; Manber, R. Cognitive behavior therapy for chronic insomnia occurring within the context of medical and psychiatric disorders. Clin. Psychol. Rev. 2005, 25, 559–592. [Google Scholar] [CrossRef]
- Jia, Y.; Li, F.; Liu, Y.F.; Zhao, J.P.; Leng, M.M.; Chen, L. Depression and cancer risk: A systematic review and meta-analysis. Public Health 2017, 149, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Roest, A.M.; Martens, E.J.; de Jonge, P.; Denollet, J. Anxiety and risk of incident coronary heart disease: A meta-analysis. J. Am. Coll. Cardiol. 2010, 56, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, A.; Kuper, H.; Hemingway, H. Depression as an aetiologic and prognostic factor in coronary heart disease: A meta-analysis of 6362 events among 146 538 participants in 54 observational studies. Eur. Heart J. 2006, 27, 2763–2774. [Google Scholar] [CrossRef]
- Mitchell, A.J.; Chan, M.; Bhatti, H.; Halton, M.; Grassi, L.; Johansen, C.; Meader, N. Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological, and palliative-care settings: A meta-analysis of 94 interview-based studies. Lancet Oncol. 2011, 12, 160–174. [Google Scholar] [CrossRef]
- Oerlemans, M.E.; van den Akker, M.; Schuurman, A.G.; Kellen, E.; Buntinx, F. A meta-analysis on depression and subsequent cancer risk. Clin. Pract. Epidemiol. Ment. Health CP EMH 2007, 3, 29. [Google Scholar] [CrossRef]
- Lin, L.-Y.; Warren-Gash, C.; Smeeth, L.; Chen, P.-C. Data resource profile: The National Health Insurance Research Database (NHIRD). Epidemiol. Health 2018, 40, e2018062. [Google Scholar]
- Cheng, C.L.; Kao, Y.H.; Lin, S.J.; Lee, C.H.; Lai, M.L. Validation of the National Health Insurance Research Database with ischemic stroke cases in Taiwan. Pharmacoepidemiol. Drug Saf. 2011, 20, 236–242. [Google Scholar] [CrossRef]
- Lai, M.N.; Wang, S.M.; Chen, P.C.; Chen, Y.Y.; Wang, J.D. Population-based case-control study of Chinese herbal products containing aristolochic acid and urinary tract cancer risk. J. Natl. Cancer Inst. 2010, 102, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Martínez-García, M.Á.; Campos-Rodríguez, F.; Almendros, I. Sleep Disorders and Cancer. Curr. Sleep Med. Rep. 2016, 2, 1–11. [Google Scholar] [CrossRef][Green Version]
- Fang, H.-F.; Miao, N.-F.; Chen, C.-D.; Sithole, T.; Chung, M.-H. Risk of Cancer in Patients with Insomnia, Parasomnia, and Obstructive Sleep Apnea: A Nationwide Nested Case-Control Study. J. Cancer 2015, 6, 1140–1147. [Google Scholar] [CrossRef]
- Nelson, K. Depression may increase cancer risk. Lancet Oncol. 2003, 4, 390. [Google Scholar] [CrossRef]
- Sotelo, J.L.; Musselman, D.; Nemeroff, C. The biology of depression in cancer and the relationship between depression and cancer progression. Int. Rev. Psychiatry 2014, 26, 16–30. [Google Scholar] [CrossRef]
- Shah, P.N.; Mhatre, M.C.; Kothari, L.S. Effect of Melatonin on Mammary Carcinogenesis in Intact and Pinealectomized Rats in Varying Photoperiods. Cancer Res. 1984, 44, 3403–3407. [Google Scholar] [PubMed]
- Blask, D.E.; Brainard, G.C.; Dauchy, R.T.; Hanifin, J.P.; Davidson, L.K.; Krause, J.A.; Sauer, L.A.; Rivera-Bermudez, M.A.; Dubocovich, M.L.; Jasser, S.A.; et al. Melatonin-depleted blood from premenopausal women exposed to light at night stimulates growth of human breast cancer xenografts in nude rats. Cancer Res. 2005, 65, 11174–11184. [Google Scholar] [CrossRef]
- Li, L.; Ren, F.; Qi, C.; Xu, L.; Fang, Y.; Liang, M.; Feng, J.; Chen, B.; Ning, W.; Cao, J. Intermittent hypoxia promotes melanoma lung metastasis via oxidative stress and inflammation responses in a mouse model of obstructive sleep apnea. Respir. Res. 2018, 19, 28. [Google Scholar] [CrossRef]
- Almendros, I.; Montserrat, J.M.; Torres, M.; Bonsignore, M.R.; Chimenti, L.; Navajas, D.; Farré, R. Obesity and intermittent hypoxia increase tumor growth in a mouse model of sleep apnea. Sleep Med. 2012, 13, 1254–1260. [Google Scholar] [CrossRef]
- Kolstad, H.A. Nightshift work and risk of breast cancer and other cancers—A critical review of the epidemiologic evidence. Scand. J. Work Environ. Health 2008, 34, 5–22. [Google Scholar] [CrossRef]
- Costa, G.; Haus, E.; Stevens, R. Shift work and cancer—Considerations on rationale, mechanisms, and epidemiology. Scand. J. Work Environ. Health 2010, 36, 163–179. [Google Scholar] [CrossRef] [PubMed]
- Savvidis, C.; Koutsilieris, M. Circadian rhythm disruption in cancer biology. Mol. Med. 2012, 18, 1249–1260. [Google Scholar] [CrossRef]
- Sliwinski, T.; Rozej, W.; Morawiec-Bajda, A.; Morawiec, Z.; Reiter, R.; Blasiak, J. Protective action of melatonin against oxidative DNA damage: Chemical inactivation versus base-excision repair. Mutat. Res. 2007, 634, 220–227. [Google Scholar] [PubMed]
- Besedovsky, L.; Lange, T.; Born, J. Sleep and immune function. Pflug. Arch. Eur. J. Physiol. 2012, 463, 121–137. [Google Scholar] [CrossRef]
- Huang, T.; Poole, E.M.; Okereke, O.I.; Kubzansky, L.D.; Eliassen, A.H.; Sood, A.K.; Wang, M.; Tworoger, S.S. Depression and risk of epithelial ovarian cancer: Results from two large prospective cohort studies. Gynecol. Oncol. 2015, 139, 481–486. [Google Scholar] [PubMed]
- Archer, G.; Pikhart, H.; Head, J. Do depressive symptoms predict cancer incidence?: 17-year follow-up of the Whitehall II study. J. Psychosom. Res. 2015, 79, 595–603. [Google Scholar] [CrossRef]
- Spiegel, D.; Giese-Davis, J. Depression and cancer: Mechanisms and disease progression. Biol. Psychiatry 2003, 54, 269–282. [Google Scholar] [CrossRef]
- Burke, H.M.; Davis, M.C.; Otte, C.; Mohr, D.C. Depression and cortisol responses to psychological stress: A meta-analysis. Psychoneuroendocrinology 2005, 30, 846–856. [Google Scholar] [CrossRef]
- Goshen, I.; Kreisel, T.; Ben-Menachem-Zidon, O.; Licht, T.; Weidenfeld, J.; Ben-Hur, T.; Yirmiya, R. Brain interleukin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Mol. Psychiatry 2008, 13, 717–728. [Google Scholar]
- Howren, M.B.; Lamkin, D.M.; Suls, J. Associations of depression with C-reactive protein, IL-1, and IL-6: A meta-analysis. Psychosom. Med. 2009, 71, 171–186. [Google Scholar] [PubMed]
- Gelfo, V.; Romaniello, D.; Mazzeschi, M.; Sgarzi, M.; Grilli, G.; Morselli, A.; Manzan, B.; Rihawi, K.; Lauriola, M. Roles of IL-1 in Cancer: From Tumor Progression to Resistance to Targeted Therapies. Int. J. Mol. Sci. 2020, 21, 6009. [Google Scholar] [CrossRef] [PubMed]
- Nunes, S.O.; Reiche, E.M.; Morimoto, H.K.; Matsuo, T.; Itano, E.N.; Xavier, E.C.; Yamashita, C.M.; Vieira, V.R.; Menoli, A.V.; Silva, S.S.; et al. Immune and hormonal activity in adults suffering from depression. Braz. J. Med. Biol. Res. = Rev. Bras. De Pesqui. Med. E Biol. 2002, 35, 581–587. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Verkasalo, P.K.; Lillberg, K.; Stevens, R.G.; Hublin, C.; Partinen, M.; Koskenvuo, M.; Kaprio, J. Sleep duration and breast cancer: A prospective cohort study. Cancer Res. 2005, 65, 9595–9600. [Google Scholar] [CrossRef] [PubMed]
- Stevens, R.G. Light-at-night, circadian disruption and breast cancer: Assessment of existing evidence. Int. J. Epidemiol. 2009, 38, 963–970. [Google Scholar] [CrossRef]
- Crum, R.M.; Storr, C.L.; Chan, Y.F.; Ford, D.E. Sleep disturbance and risk for alcohol-related problems. Am. J. Psychiatry 2004, 161, 1197–1203. [Google Scholar] [CrossRef]
- Phillips, B.A.; Danner, F.J. Cigarette smoking and sleep disturbance. Arch. Intern. Med. 1995, 155, 734–737. [Google Scholar] [CrossRef]
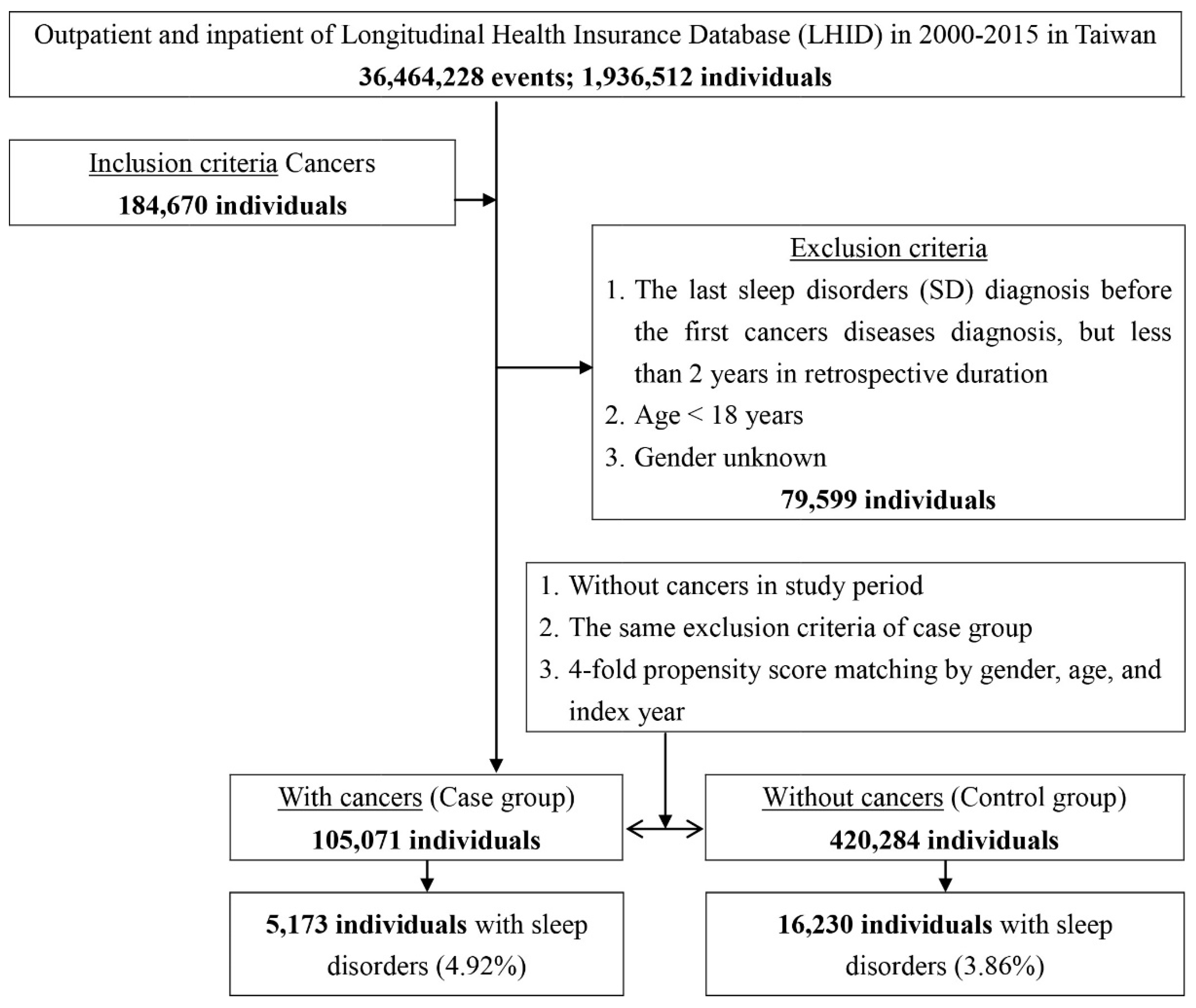

| Cancers | Total | With | Without | p | |||
|---|---|---|---|---|---|---|---|
| Variables | n | % | n | % | n | % | |
| Total | 525,355 | 105,071 | 20.00 | 420,284 | 80.00 | ||
| Gender | 0.999 | ||||||
| Male | 300,050 | 57.11 | 60,010 | 57.11 | 240,040 | 57.11 | |
| Female | 225,305 | 42.89 | 45,061 | 42.89 | 180,244 | 42.89 | |
| Age (years) | 63.42 ± 16.63 | 63.49 ± 15.13 | 63.40 ± 16.99 | 0.117 | |||
| Age group (years) | 0.999 | ||||||
| 18–44 | 68,105 | 12.96 | 13,621 | 12.96 | 54,484 | 12.96 | |
| 45–64 | 192,775 | 36.69 | 38,555 | 36.69 | 154,220 | 36.69 | |
| ≥65 | 264,475 | 50.34 | 52,895 | 50.34 | 211,580 | 50.34 | |
| Married | 0.004 | ||||||
| Without | 67,423 | 12.83 | 13,445 | 12.80 | 53,978 | 12.84 | |
| With | 276,913 | 52.71 | 55,697 | 53.01 | 221,216 | 52.63 | |
| Divorce | 61,468 | 11.70 | 12,456 | 11.85 | 49,012 | 11.66 | |
| Spouse death | 119,467 | 22.74 | 23,454 | 22.32 | 96,013 | 22.84 | |
| Unknown | 84 | 0.02 | 19 | 0.02 | 65 | 0.02 | |
| Education | <0.001 | ||||||
| Elementary/junior high school | 50,760 | 9.66 | 10,154 | 9.66 | 40,606 | 9.66 | |
| (Vocational) high school | 296,352 | 56.41 | 59,901 | 57.01 | 236,451 | 56.26 | |
| Univeristy/college/graduate | 178,211 | 33.92 | 35,010 | 33.32 | 143,201 | 34.07 | |
| Others | 32 | 0.01 | 6 | 0.01 | 26 | 0.01 | |
| Insured premium (NT$) | 0.951 | ||||||
| <18,000 | 469,293 | 89.33 | 93,831 | 89.30 | 375,462 | 89.34 | |
| 18,000–34,999 | 39,827 | 7.58 | 7982 | 7.60 | 31,845 | 7.58 | |
| ≥35,000 | 16,235 | 3.09 | 3258 | 3.10 | 12,977 | 3.09 | |
| DM | 0.359 | ||||||
| Without | 417,407 | 79.45 | 83,374 | 79.35 | 334,033 | 79.48 | |
| With | 107,948 | 20.55 | 21,697 | 20.65 | 86,251 | 20.52 | |
| HT | <0.001 | ||||||
| Without | 410,044 | 78.05 | 81,343 | 77.42 | 328,701 | 78.21 | |
| With | 115,311 | 21.95 | 23,728 | 22.58 | 91,583 | 21.79 | |
| Depression | <0.001 | ||||||
| Without | 517,915 | 98.58 | 102,600 | 97.65 | 415,315 | 98.82 | |
| With | 7440 | 1.42 | 2471 | 2.35 | 4969 | 1.18 | |
| Stroke | <0.001 | ||||||
| Without | 474,272 | 90.28 | 94,073 | 89.53 | 380,199 | 90.46 | |
| With | 51,083 | 9.72 | 10,998 | 10.47 | 40,085 | 9.54 | |
| Dementia | <0.001 | ||||||
| Without | 519,570 | 98.90 | 104,173 | 99.15 | 415,397 | 98.84 | |
| With | 5785 | 1.10 | 898 | 0.85 | 4887 | 1.16 | |
| CKD | 0.025 | ||||||
| Without | 466,555 | 88.81 | 93,106 | 88.61 | 373,449 | 88.86 | |
| With | 58,800 | 11.19 | 11,965 | 11.39 | 46,835 | 11.14 | |
| Season | <0.001 | ||||||
| Spring (March–May) | 120,264 | 22.89 | 24,086 | 22.92 | 96,178 | 22.88 | |
| Summer (June–August) | 131,700 | 25.07 | 25,691 | 24.45 | 106,009 | 25.22 | |
| Autumn (September–November) | 151,076 | 28.76 | 28,862 | 27.47 | 122,214 | 29.08 | |
| Winter (December–February) | 122,315 | 23.28 | 26,432 | 25.16 | 95,883 | 22.81 | |
| Location | <0.001 | ||||||
| Northern Taiwan | 213,017 | 40.55 | 45,624 | 43.42 | 167,393 | 39.83 | |
| Middle Taiwan | 149,055 | 28.37 | 26,980 | 25.68 | 122,075 | 29.05 | |
| Southern Taiwan | 131,872 | 25.10 | 27,744 | 26.41 | 104,128 | 24.78 | |
| Eastern Taiwan | 29,294 | 5.58 | 4402 | 4.19 | 24,892 | 5.92 | |
| Outlets islands | 2117 | 0.40 | 321 | 0.31 | 1796 | 0.43 | |
| Urbanization level | <0.001 | ||||||
| 1 (The highest) | 164,309 | 31.28 | 39,844 | 37.92 | 124,465 | 29.61 | |
| 2 | 236,316 | 44.98 | 48,746 | 46.39 | 187,570 | 44.63 | |
| 3 | 38,416 | 7.31 | 4060 | 3.86 | 34,356 | 8.17 | |
| 4 (The lowest) | 86,314 | 16.43 | 12,421 | 11.82 | 73,893 | 17.58 | |
| Level of care | <0.001 | ||||||
| Hospital center | 189,094 | 35.99 | 57,044 | 54.29 | 132,050 | 31.42 | |
| Regional hospital | 234,975 | 44.73 | 37,663 | 35.85 | 197,312 | 46.95 | |
| Local hospital | 101,286 | 19.28 | 10,364 | 9.86 | 90,922 | 21.63 | |
| Sleep disorders | <0.001 | ||||||
| Without | 503,952 | 95.93 | 99,898 | 95.08 | 404,054 | 96.14 | |
| With | 21,403 | 4.07 | 5173 | 4.92 | 16,230 | 3.86 | |
| Apnea | 0.508 | ||||||
| Without | 523,931 | 99.73 | 104,776 | 99.72 | 419,155 | 99.73 | |
| With | 1424 | 0.27 | 295 | 0.28 | 1129 | 0.27 | |
| Insomnia | <0.001 | ||||||
| Without | 512,702 | 97.59 | 101,590 | 96.69 | 411,112 | 97.82 | |
| With | 12,653 | 2.41 | 3481 | 3.31 | 9172 | 2.18 | |
| Non-apnea non-insomnia sleep disorders | 0.035 | ||||||
| Without | 517,051 | 98.42 | 103,356 | 98.37 | 413,695 | 98.43 | |
| With | 8304 | 1.58 | 1715 | 1.63 | 6589 | 1.57 | |
| Variables | Crude OR | 95% CI | 95% CI | p | Adjusted OR | 95% CI | 95% CI | p |
|---|---|---|---|---|---|---|---|---|
| Sleep disorders | ||||||||
| Without | Reference | Reference | ||||||
| With | 1.789 | 1.648 | 1.991 | <0.001 | 1.440 | 1.392 | 1.489 | <0.001 |
| Gender | ||||||||
| Male | 1.289 | 1.248 | 1.331 | <0.001 | 1.153 | 1.120 | 1.186 | <0.001 |
| Female | Reference | Reference | ||||||
| Age group | ||||||||
| 18–44 | Reference | Reference | ||||||
| 45–64 | 1.441 | 1.367 | 1.518 | <0.001 | 1.442 | 1.367 | 1.520 | <0.001 |
| ≥65 | 1.793 | 1.706 | 1.885 | <0.001 | 1.715 | 1.828 | 1.807 | <0.001 |
| Married | ||||||||
| Without | 0.816 | 0.345 | 1.672 | 0.492 | 0.842 | 0.452 | 1.726 | 0.374 |
| With | Reference | Reference | ||||||
| Divorce | 1.208 | 0.897 | 1.397 | 0.124 | 1.113 | 0.571 | 1.453 | 0.288 |
| Spouse death | 1.552 | 0.701 | 2.163 | 0.106 | 1.298 | 0.642 | 1.772 | 0.115 |
| Unknown | 0.000 | - | - | 0.931 | 0.000 | - | - | 0.989 |
| Education | ||||||||
| Elementary/junior high school | Reference | Reference | ||||||
| (Vocational) high school | 1.154 | 0.495 | 1.798 | 0.462 | 1.018 | 0.312 | 1.843 | 0.509 |
| Univeristy/college/graduate | 1.298 | 0.501 | 1.881 | 0.337 | 1.174 | 0.477 | 2.092 | 0.426 |
| Others | 0.000 | - | - | 0.872 | 0.000 | - | - | 0.911 |
| Insured premium (NT$) | ||||||||
| <18,000 | Reference | Reference | ||||||
| 18,000–34,999 | 0.935 | 0.832 | 1.052 | 0.264 | 0.952 | 0.847 | 1.071 | 0.415 |
| ≥35,000 | 0.617 | 0.468 | 0.981 | 0.041 | 0.761 | 0.577 | 1.014 | 0.073 |
| DM | ||||||||
| Without | Reference | Reference | ||||||
| With | 1.095 | 1.058 | 1.133 | <0.001 | 1.071 | 1.034 | 1.110 | <0.001 |
| HT | ||||||||
| Without | Reference | Reference | ||||||
| With | 1.948 | 1.818 | 2.177 | 0.001 | 1.914 | 1.884 | 1.945 | <0.001 |
| Depression | ||||||||
| Without | Reference | Reference | ||||||
| With | 3.072 | 2.822 | 3.344 | <0.001 | 3.408 | 3.128 | 3.714 | <0.001 |
| Stroke | ||||||||
| Without | Reference | Reference | ||||||
| With | 1.188 | 1.133 | 1.244 | <0.001 | 1.126 | 1.073 | 1.181 | <0.001 |
| Dementia | ||||||||
| Without | Reference | Reference | ||||||
| With | 1.882 | 1.693 | 2.091 | <0.001 | 1.612 | 1.449 | 1.794 | <0.001 |
| CKD | ||||||||
| Without | Reference | Reference | ||||||
| With | 1.889 | 1.741 | 2.049 | <0.001 | 1.708 | 1.574 | 1.855 | <0.001 |
| Season | ||||||||
| Spring | Reference | Reference | ||||||
| Summer | 1.113 | 1.068 | 1.160 | <0.001 | 1.108 | 1.063 | 1.155 | <0.001 |
| Autumn | 1.325 | 1.274 | 1.377 | <0.001 | 1.305 | 1.255 | 1.356 | <0.001 |
| Winter | 1.121 | 1.075 | 1.169 | <0.001 | 1.111 | 1.062 | 1.157 | <0.001 |
| Location | Had multicollinearity with urbanization level | |||||||
| Northern Taiwan | Reference | Had multicollinearity with urbanization level | ||||||
| Middle Taiwan | 1.340 | 1.296 | 1.386 | <0.001 | Had multicollinearity with urbanization level | |||
| Southern Taiwan | 1.189 | 1.147 | 1.233 | <0.001 | Had multicollinearity with urbanization level | |||
| Eastern Taiwan | 1.922 | 1.824 | 2.026 | <0.001 | Had multicollinearity with urbanization level | |||
| Outlets islands | 1.738 | 1.477 | 2.088 | <0.001 | Had multicollinearity with urbanization level | |||
| Urbanization level | ||||||||
| 1 (The highest) | 0.673 | 0.647 | 0.700 | <0.001 | 0.814 | 0.779 | 0.851 | <0.001 |
| 2 | 0.725 | 0.699 | 0.752 | <0.001 | 0.827 | 0.796 | 0.860 | <0.001 |
| 3 | 0.833 | 0.786 | 0.881 | <0.001 | 0.872 | 0.823 | 0.923 | <0.001 |
| 4 (The lowest) | Reference | Reference | ||||||
| Level of care | ||||||||
| Hospital center | 0.626 | 0.603 | 0.650 | <0.001 | 0.636 | 0.609 | 0.664 | <0.001 |
| Regional hospital | 0.778 | 0.752 | 0.805 | <0.001 | 0.774 | 0.747 | 0.801 | <0.001 |
| Local hospital | Reference | Reference | ||||||
| Sleep Disorders (With vs. Without) | With | Without | Ratio | Adjusted OR | 95%CI | 95%CI | p | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Stratified | Exposure | PYs | Rate (per 103 PYs) | Exposure | PYs | Rate (per 103 PYs) | |||||
| Total | 5173 | 288,330.74 | 17.94 | 16,230 | 2,377,934.41 | 6.83 | 2.629 | 1.440 | 1.392 | 1.489 | <0.001 |
| Gender | |||||||||||
| Male | 3206 | 166,695.38 | 19.23 | 9977 | 1,390,636.46 | 7.17 | 2.681 | 1.469 | 1.410 | 1.598 | <0.001 |
| Female | 1967 | 121,635.36 | 16.17 | 6253 | 987,297.95 | 6.33 | 2.553 | 1.399 | 1.252 | 1.444 | <0.001 |
| Age group | |||||||||||
| 18–44 | 555 | 39,942.92 | 13.89 | 1318 | 221,566.17 | 5.95 | 2.336 | 1.280 | 1.137 | 1.304 | <0.001 |
| 45–64 | 1773 | 97,440.27 | 18.20 | 5307 | 749,270.05 | 7.08 | 2.569 | 1.407 | 1.345 | 1.469 | <0.001 |
| ≥65 | 2845 | 150,947.55 | 18.85 | 9605 | 1,407,098.19 | 6.83 | 2.761 | 1.513 | 1.460 | 1.678 | <0.001 |
| Married | |||||||||||
| Without | 810 | 38,801.75 | 20.88 | 2916 | 306,989.47 | 9.50 | 2.198 | 1.204 | 1.102 | 1.385 | 0.001 |
| With | 2098 | 144,232.45 | 14.55 | 5925 | 1,066,714.45 | 5.55 | 2.619 | 1.435 | 1.374 | 1.571 | <0.001 |
| Divorce | 1144 | 62,067.42 | 18.43 | 4433 | 642,980.13 | 6.89 | 2.673 | 1.529 | 1.411 | 1.601 | <0.001 |
| Spouse death | 1121 | 43,121.08 | 26.00 | 2956 | 360,245.12 | 8.21 | 3.168 | 1.671 | 1.520 | 1.798 | <0.001 |
| Unknown | 0 | 108.04 | 0.00 | 0 | 1005.24 | 0.00 | - | - | - | - | - |
| Education | |||||||||||
| Elementary/junior high school | 907 | 73,201.45 | 12.39 | 3373 | 506,601.45 | 6.66 | 1.861 | 1.019 | 0.984 | 1.127 | 0.298 |
| (Vocational) high school | 2021 | 119,454.24 | 16.92 | 6345 | 1,000,845.06 | 6.34 | 2.669 | 1.462 | 1.349 | 1.602 | 0.011 |
| Univeristy/college/graduate | 2245 | 94,248.57 | 23.82 | 6512 | 797,715.11 | 8.16 | 2.918 | 1.598 | 1.445 | 1.798 | 0.006 |
| Others | 0 | 1426.48 | 0.00 | 0 | 72,772.79 | 0.00 | - | - | - | - | - |
| Insured premium (NT$) | |||||||||||
| <18,000 | 5101 | 284,012.19 | 17.96 | 15,901 | 2,332,537.19 | 6.82 | 2.635 | 1.495 | 1.407 | 1.572 | 0.005 |
| 18,000–34,999 | 55 | 3594.56 | 15.30 | 240 | 37,892.06 | 6.33 | 2.416 | 1.302 | 1.215 | 1.480 | 0.010 |
| ≥35,000 | 17 | 723.99 | 23.48 | 89 | 7505.16 | 11.86 | 1.980 | 1.082 | 1.009 | 1.133 | 0.042 |
| DM | |||||||||||
| Without | 4506 | 248,115.42 | 18.16 | 12,674 | 1,797,644.23 | 7.05 | 2.576 | 1.411 | 1.364 | 1.459 | <0.001 |
| With | 667 | 40,215.32 | 16.59 | 3556 | 580,290.18 | 6.13 | 2.707 | 1.483 | 1.433 | 1.533 | <0.001 |
| HT | |||||||||||
| Without | 4230 | 241,960.51 | 17.48 | 11,192 | 1,570,819.26 | 7.12 | 2.454 | 1.344 | 1.299 | 1.390 | <0.001 |
| With | 943 | 46,370.23 | 20.34 | 5038 | 807,115.15 | 6.24 | 3.258 | 1.785 | 1.725 | 1.845 | <0.001 |
| Depression | |||||||||||
| Without | 5070 | 286,741.71 | 17.68 | 15,819 | 2,348,030.54 | 6.74 | 2.624 | 1.338 | 1.290 | 1.402 | <0.001 |
| With | 103 | 1589.03 | 64.82 | 411 | 29,903.87 | 13.74 | 4.716 | 2.854 | 2.497 | 2.671 | <0.001 |
| Stroke | |||||||||||
| Without | 4964 | 277,924.03 | 17.86 | 14,340 | 2,071,846.60 | 6.92 | 2.581 | 1.414 | 1.367 | 1.463 | <0.001 |
| With | 209 | 10,406.71 | 20.08 | 1890 | 306,087.81 | 6.17 | 3.252 | 1.782 | 1.722 | 1.897 | <0.001 |
| Dementia | |||||||||||
| Without | 5146 | 287,148.59 | 17.92 | 15,871 | 2,333,477.43 | 6.80 | 2.635 | 1.443 | 1.382 | 1.487 | <0.001 |
| With | 27 | 1182.15 | 22.84 | 359 | 44,456.98 | 8.08 | 2.828 | 1.519 | 1.498 | 1.601 | <0.001 |
| CKD | |||||||||||
| Without | 4986 | 281,046.24 | 17.74 | 15,758 | 2,322,854.16 | 6.78 | 2.615 | 1.433 | 1.372 | 1.479 | <0.001 |
| With | 187 | 7284.50 | 25.67 | 472 | 55,080.25 | 8.57 | 2.996 | 1.641 | 1.586 | 1.708 | <0.001 |
| Season | |||||||||||
| Spring | 997 | 62,060.76 | 16.06 | 3489 | 516,204.92 | 6.76 | 2.377 | 1.302 | 1.259 | 1.346 | <0.001 |
| Summer | 1242 | 70,358.21 | 17.65 | 3961 | 568,305.95 | 6.97 | 2.533 | 1.387 | 1.341 | 1.435 | <0.001 |
| Autumn | 1699 | 84,211.27 | 20.18 | 4930 | 728,515.28 | 6.77 | 2.981 | 1.633 | 1.579 | 1.689 | <0.001 |
| Winter | 1235 | 71,700.50 | 17.22 | 3850 | 564,908.26 | 6.82 | 2.527 | 1.384 | 1.338 | 1.432 | <0.001 |
| Urbanization level | |||||||||||
| 1 (The highest) | 1720 | 101,539.67 | 16.94 | 4420 | 661,751.90 | 6.68 | 2.536 | 1.389 | 1.343 | 1.469 | <0.001 |
| 2 | 2370 | 135,786.88 | 17.45 | 6921 | 1,069,017.83 | 6.47 | 2.696 | 1.477 | 1.428 | 1.571 | <0.001 |
| 3 | 190 | 12,557.40 | 15.13 | 1100 | 198,584.58 | 5.54 | 2.732 | 1.496 | 1.411 | 1.598 | <0.001 |
| 4 (The lowest) | 893 | 38,446.79 | 23.23 | 3789 | 448,580.10 | 8.45 | 2.750 | 1.506 | 1.402 | 1.672 | <0.001 |
| Level of care | |||||||||||
| Hospital center | 2153 | 147,056.04 | 14.64 | 3898 | 709,805.57 | 5.49 | 2.666 | 1.420 | 1.305 | 1.477 | <0.001 |
| Regional hospital | 2161 | 112,013.98 | 19.29 | 7564 | 1,128,912.29 | 6.70 | 2.879 | 1.557 | 1.425 | 1.701 | <0.001 |
| Local hospital | 859 | 29,260.72 | 29.36 | 4768 | 539,216.55 | 8.84 | 3.320 | 1.820 | 1.558 | 1.978 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, F.-C.; Hsu, C.-H.; Chung, C.-H.; Pu, T.-W.; Chang, P.-K.; Lin, T.-C.; Jao, S.-W.; Chen, C.-Y.; Chien, W.-C.; Hu, J.-M. The Combination of Sleep Disorders and Depression Significantly Increases Cancer Risk: A Nationwide Large-Scale Population-Based Study. Int. J. Environ. Res. Public Health 2022, 19, 9266. https://doi.org/10.3390/ijerph19159266
Hsu F-C, Hsu C-H, Chung C-H, Pu T-W, Chang P-K, Lin T-C, Jao S-W, Chen C-Y, Chien W-C, Hu J-M. The Combination of Sleep Disorders and Depression Significantly Increases Cancer Risk: A Nationwide Large-Scale Population-Based Study. International Journal of Environmental Research and Public Health. 2022; 19(15):9266. https://doi.org/10.3390/ijerph19159266
Chicago/Turabian StyleHsu, Fang-Chin, Chih-Hsiung Hsu, Chi-Hsiang Chung, Ta-Wei Pu, Pi-Kai Chang, Tzu-Chiao Lin, Shu-Wen Jao, Chao-Yang Chen, Wu-Chien Chien, and Je-Ming Hu. 2022. "The Combination of Sleep Disorders and Depression Significantly Increases Cancer Risk: A Nationwide Large-Scale Population-Based Study" International Journal of Environmental Research and Public Health 19, no. 15: 9266. https://doi.org/10.3390/ijerph19159266
APA StyleHsu, F.-C., Hsu, C.-H., Chung, C.-H., Pu, T.-W., Chang, P.-K., Lin, T.-C., Jao, S.-W., Chen, C.-Y., Chien, W.-C., & Hu, J.-M. (2022). The Combination of Sleep Disorders and Depression Significantly Increases Cancer Risk: A Nationwide Large-Scale Population-Based Study. International Journal of Environmental Research and Public Health, 19(15), 9266. https://doi.org/10.3390/ijerph19159266






