Closing the Gap between Inpatient and Outpatient Settings: Integrating Pulmonary Rehabilitation and Technological Advances in the Comprehensive Management of Frail Patients
Abstract
:1. Introduction
2. Pulmonary Rehabilitation: State of the Art
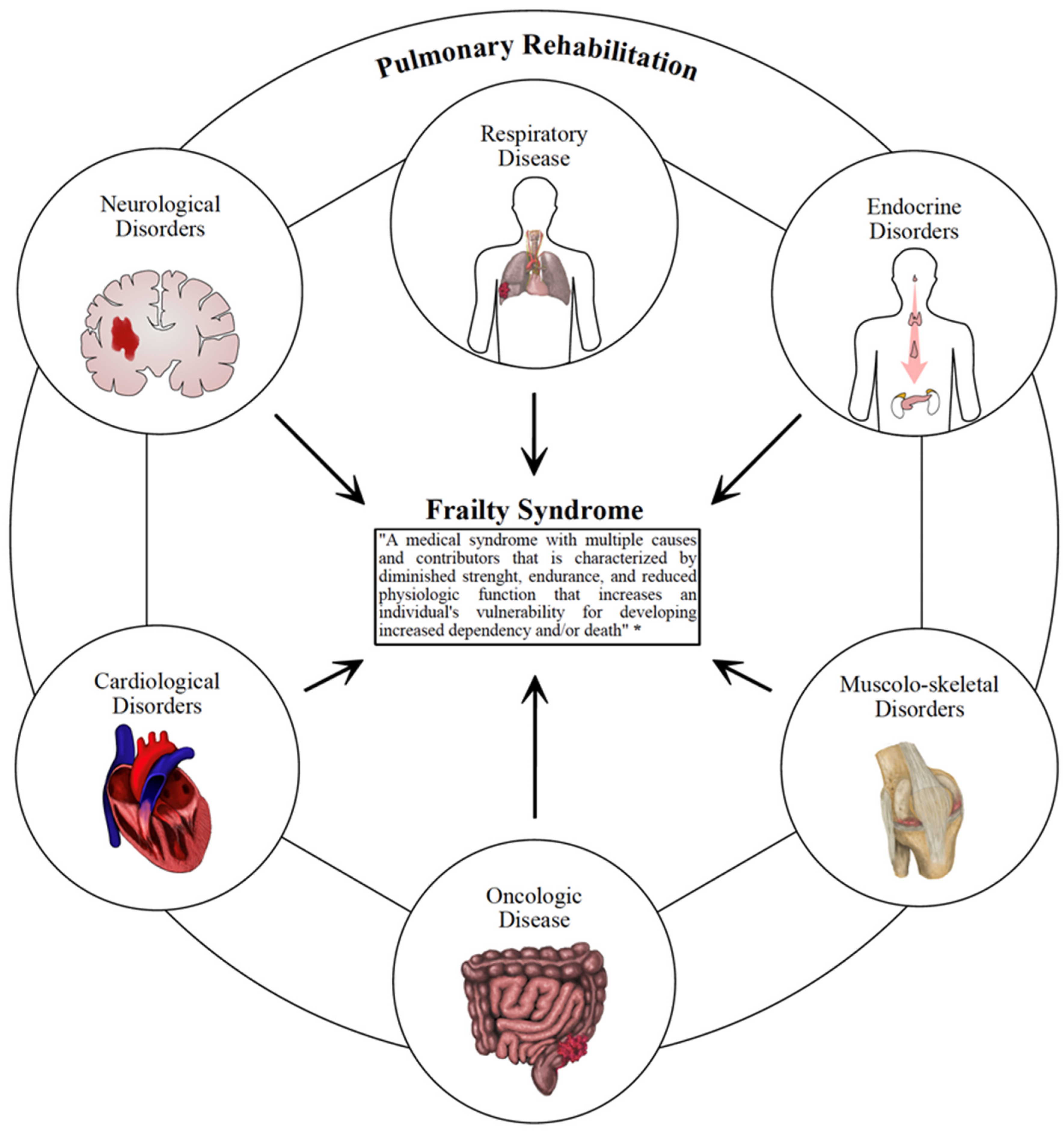
3. Pulmonary Rehabilitation in Frail Patients with Functional Impairment
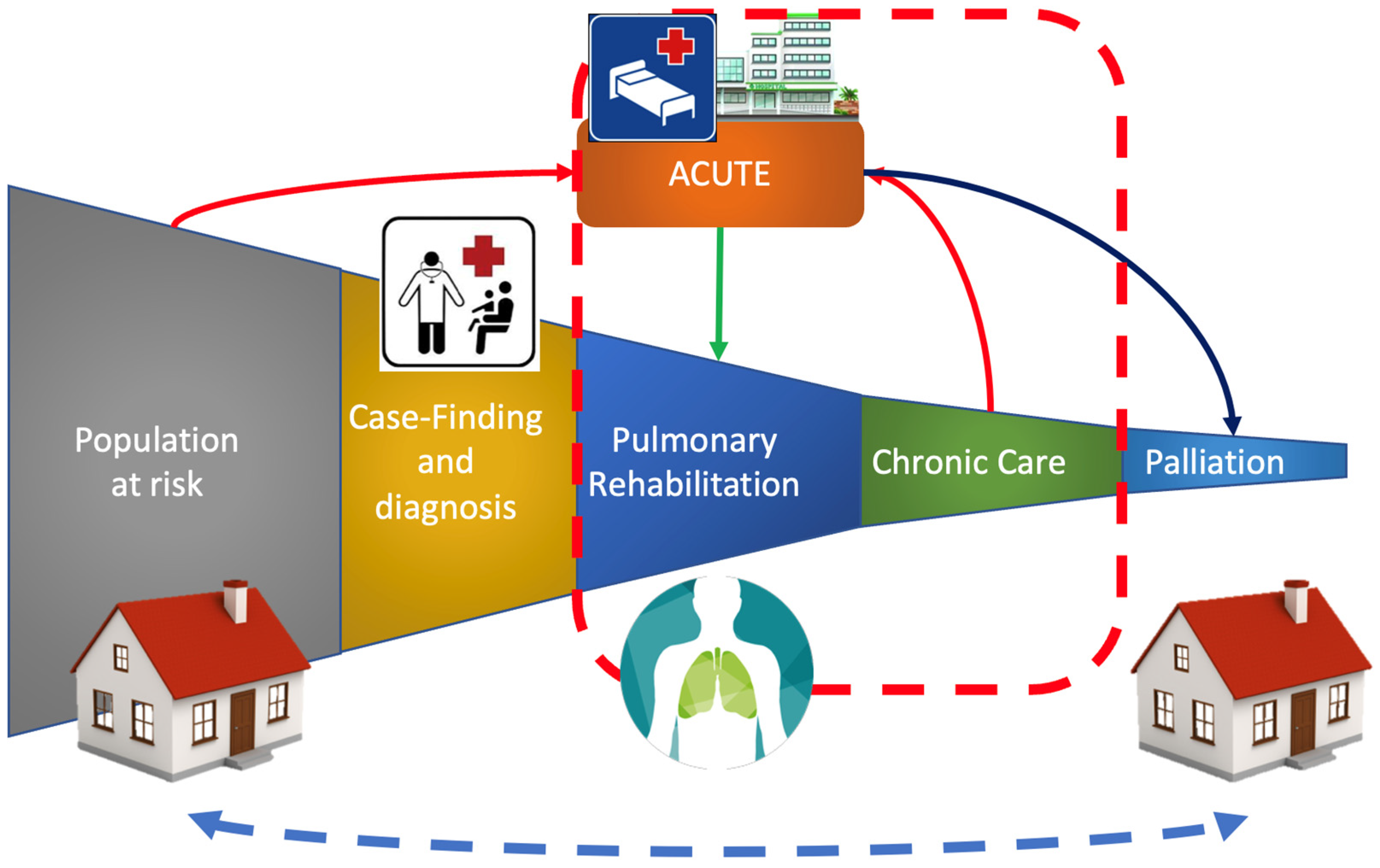
4. Integrating Pulmonary Rehabilitation in Inpatient and Outpatient Settings
5. Sustainable Strategies in the Comprehensive Management of Frail Patients with PR Issues
6. Telemedicine and Technological Innovation to Overcome the Barriers to PR
7. The Role of Training Healthcare Professionals and Patients’ Engagement
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spruit, M.A.; Singh, S.J.; Garvey, C.; Zuwallack, R.; Nici, L.; Rochester, C.; Hill, K.; Holland, A.E.; Lareau, S.C.; Man, W.D.C.; et al. An Official American Thoracic Society/European Respiratory Society Statement: Key Concepts and Advances in Pulmonary Rehabilitation. Am. J. Respir. Crit. Care Med. 2013, 188, e13–e64. [Google Scholar] [CrossRef] [PubMed]
- Garvey, C.; Bayles, M.P.; Hamm, L.F.; Hill, K.; Holland, A.; Limberg, T.M.; Spruit, M.A. Pulmonary Rehabilitation Exercise Prescription in Chronic Obstructive Pulmonary Disease: Review of Selected Guidelines: An Official Statement from The American Association of Cardiovascular and Pulmonary Rehabilitation. J. Cardiopulm. Rehabil. Prev. 2016, 36, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Vestbo, J.; Hurd, S.S.; Agustí, A.G.; Jones, P.W.; Vogelmeier, C.; Anzueto, A.; Barnes, P.J.; Fabbri, L.M.; Martinez, F.J.; Nishimura, M.; et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2013, 187, 347–365. [Google Scholar] [CrossRef]
- Ora, J.; Prendi, E.; Attinà, M.L.; Cazzola, M.; Calzetta, L.; Rogliani, P. Efficacy of respiratory tele-rehabilitation in COPD patients: Systematic review and meta-analysis. Monaldi Arch. Chest Dis. 2022. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Gandullo, E.; Hidalgo-Molina, A.; Montoro-Ballesteros, F.; Morales-González, M.; Muñoz-Ramírez, I.; Arnedillo-Muñoz, A. Inspiratory Muscle Training in Patients with Chronic Obstructive Pulmonary Disease (COPD) as Part of a Respiratory Rehabilitation Program Implementation of Mechanical Devices: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 5564. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, B.; Casey, D.; Devane, D.; Murphy, K.; Murphy, E.; Lacasse, Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2015, 23, CD003793. [Google Scholar] [CrossRef]
- Bernard, S.; Vilarinho, R.; Pinto, I.; Cantante, R.; Coxo, R.; Fonseca, R.; Mayoralas-Alises, S.; Diaz-Lobato, S.; Carvalho, J.; Esteves, C.; et al. Enhance Access to Pulmonary Rehabilitation with a Structured and Personalized Home-Based Program—ReabilitAR: Protocol for Real-World Setting. Int. J. Environ. Res. Public Health 2021, 18, 6132. [Google Scholar] [CrossRef]
- Perumal, S.D. Renewed vision on pulmonary rehabilitation service delivery for chronic obstructive pulmonary disease management beyond COVID-19. Chronic Dis. Transl. Med. 2021, 7, 107–116. [Google Scholar] [CrossRef]
- Burton, M.; Valet, M.; Caty, G.; Aboubakar, F.; Reychler, G. Telerehabilitation physical exercise for patients with lung cancer through the course of their disease: A systematic review. J. Telemed. Telecare 2022, 23, 1357633x221094200. [Google Scholar] [CrossRef]
- He, Y.; Zhao, C.; Liu, Y. Effects of respiratory muscle training on cough function in neurological disorders: A systematic review with meta-analysis. Neurorehabilitation 2021, 48, 441–449. [Google Scholar] [CrossRef]
- Zazzara, M.B.; Vetrano, D.L.; Carfì, A.; Onder, G. Frailty and chronic disease. Panminerva Med. 2019, 61, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Lin, R.; Xu, Z.; Zhang, H. Significance of Pulmonary Rehabilitation in Improving Quality of Life for Subjects with COPD. Respir. Care 2019, 64, 99–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spruit, M.A.; Augustin, I.M.; Vanfleteren, L.E.; Janssen, D.J.; Gaffron, S.; Pennings, H.J.; Smeenk, F.; Pieters, W.; van den Bergh, J.J.; Michels, A.J.; et al. Differential response to pulmonary rehabilitation in COPD: Multidimensional profiling. Eur. Respir. J. 2015, 46, 1625–1635. [Google Scholar] [CrossRef]
- Spruit, M.A.; Pitta, F.; McAuley, E.; Zuwallack, R.L.; Nici, L. Pulmonary Rehabilitation and Physical Activity in Patients with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2015, 192, 924–933. [Google Scholar] [CrossRef]
- Gephine, S.; Saey, D.; Grosbois, J.-M.; Maltais, F.; Mucci, P. Home-based Pulmonary Rehabilitation is Effective in Frail COPD Patients with Chronic Respiratory Failure. Chronic Obstr. Pulm. Dis. J. COPD Found. 2022, 9, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Attwell, L.; Vassallo, M. Response to Pulmonary Rehabilitation in Older People with Physical Frailty, Sarcopenia and Chronic Lung Disease. Geriatrics 2017, 2, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spruit, M.A.; Van’T Hul, A.; Vreeken, H.L.; Beekman, E.; Post, M.H.T.; Meerhoff, G.A.; Van Der Valk, A.-L.; Zagers, C.; Sillen, M.J.H.; Vooijs, M.; et al. Profiling of Patients with COPD for Adequate Referral to Exercise-Based Care: The Dutch Model. Sports Med. 2020, 50, 1421–1429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, A.L.; Hill, C.J.; McDonald, C.F.; Holland, A.E. Pulmonary Rehabilitation in Individuals with Non-Cystic Fibrosis Bronchiectasis: A Systematic Review. Arch. Phys. Med. Rehabil. 2017, 98, 774–782. [Google Scholar] [CrossRef]
- Lee, H.J.; Je, D.I.; Won, S.J.; Paik, D.I.; Bae, K.H. Association between vitamin D deficiency and periodontal status in current smokers. Community Dent. Oral Epidemiol. 2015, 43, 471–478. [Google Scholar] [CrossRef]
- Dowman, L.; Hill, C.J.; Holland, A.E. Pulmonary rehabilitation for interstitial lung disease. Cochrane Database Syst. Rev. 2014, 10, CD006322. [Google Scholar] [CrossRef]
- Morris, N.R.; Kermeen, F.D.; Holland, A.E. Exercise-based rehabilitation programmes for pulmonary hypertension. Cochrane Database Syst. Rev. 2017, 2017, 28099988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soril, L.J.J.; Damant, R.W.; Lam, G.Y.; Smith, M.P.; Weatherald, J.; Bourbeau, J.; Hernandez, P.; Stickland, M.K. The effectiveness of pulmonary rehabilitation for Post-COVID symptoms: A rapid review of the literature. Respir. Med. 2022, 195, 106782. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Shi, H.; Liu, X.; Sun, T.; Wu, J.; Liu, Z. Effect of Pulmonary Rehabilitation for Patients with Post-COVID-19: A Systematic Review and Meta-Analysis. Front. Med. 2022, 9, 837420. [Google Scholar] [CrossRef] [PubMed]
- Holland, A.E.; Cox, N.S.; Houchen-Wolloff, L.; Rochester, C.L.; Garvey, C.; Zuwallack, R.; Nici, L.; Limberg, T.; Lareau, S.C.; Yawn, B.P.; et al. Defining Modern Pulmonary Rehabilitation. An Official American Thoracic Society Workshop Report. Ann. Am. Thorac. Soc. 2021, 18, e12–e29. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, M.; Vogiatzis, I. Personalized exercise training in chronic lung diseases. Respirology 2019, 24, 854–862. [Google Scholar] [CrossRef] [Green Version]
- Kehler, D.S. Age-related disease burden as a measure of population ageing. Lancet Public Health 2019, 4, e123–e124. [Google Scholar] [CrossRef] [Green Version]
- Cho, S.J.; Stout-Delgado, H.W. Aging and Lung Disease. Annu. Rev. Physiol. 2020, 82, 433–459. [Google Scholar] [CrossRef] [Green Version]
- Sharma, G.; Goodwin, J. Effect of aging on respiratory system physiology and immunology. Clin. Interv. Aging 2006, 1, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Cunha, L.L.; Perazzio, S.F.; Azzi, J.; Cravedi, P.; Riella, L.V. Remodeling of the Immune Response with Aging: Immunosenescence and Its Potential Impact on COVID-19 Immune Response. Front. Immunol. 2020, 11, 1748. [Google Scholar] [CrossRef]
- Maddocks, M.; Kon, S.S.C.; Canavan, J.L.; Jones, S.E.; Nolan, C.M.; Labey, A.; Polkey, M.I.; Man, W.D.C. Physical frailty and pulmonary rehabilitation in COPD: A prospective cohort study. Thorax 2016, 71, 988–995. [Google Scholar] [CrossRef] [Green Version]
- Voulgaris, A.; Antoniadou, M.; Agrafiotis, M.; Steiropoulos, P. Respiratory Involvement in Patients with Neuromuscular Diseases: A Narrative Review. Pulm. Med. 2019, 2019, 2734054. [Google Scholar] [CrossRef]
- De Jonge, J.C.; Takx, R.A.P.; Kauw, F.; De Jong, P.A.; Dankbaar, J.W.; Van Der Worp, H.B. Signs of Pulmonary Infection on Admission Chest Computed Tomography Are Associated with Pneumonia or Death in Patients with Acute Stroke. Stroke 2020, 51, 1690–1695. [Google Scholar] [CrossRef]
- De Sire, A.; Ferrillo, M.; Lippi, L.; Agostini, F.; de Sire, R.; Ferrara, P.E.; Raguso, G.; Riso, S.; Roccuzzo, A.; Ronconi, G.; et al. Sarcopenic Dysphagia, Malnutrition, and Oral Frailty in Elderly: A Comprehensive Review. Nutrients 2022, 14, 982. [Google Scholar] [CrossRef] [PubMed]
- Lippi, L.; de Sire, A.; D’Abrosca, F.; Polla, B.; Marotta, N.; Castello, L.M.; Ammendolia, A.; Molinari, C.; Invernizzi, M. Efficacy of Physiotherapy Interventions on Weaning in Mechanically Ventilated Critically Ill Patients: A Systematic Review and Meta-Analysis. Front. Med. 2022, 9, 889218. [Google Scholar] [CrossRef]
- Kubo, H.; Nozoe, M.; Yamamoto, M.; Kamo, A.; Noguchi, M.; Kanai, M.; Mase, K.; Shimada, S. Recovery process of respiratory muscle strength in patients following stroke: A Pilot Study. Phys. Ther. Res. 2020, 23, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Nogues, M.A.; Benarroch, E. Abnormalities of respiratory control and the respiratory motor unit. Neurologist 2008, 14, 273–288. [Google Scholar] [CrossRef] [PubMed]
- Carter, P.; Lagan, J.; Fortune, C.; Bhatt, D.L.; Vestbo, J.; Niven, R.; Chaudhuri, N.; Schelbert, E.B.; Potluri, R.; Miller, C.A. Association of Cardiovascular Disease with Respiratory Disease. J. Am. Coll. Cardiol. 2019, 73, 2166–2177. [Google Scholar] [CrossRef] [PubMed]
- Olsson Möller, U.; Beck, I.; Rydén, L.; Malmström, M. A comprehensive approach to rehabilitation interventions following breast cancer treatment—A systematic review of systematic reviews. BMC Cancer 2019, 19, 472. [Google Scholar] [CrossRef] [Green Version]
- Marthick, M.; McGregor, D.; Alison, J.; Cheema, B.; Dhillon, H.; Shaw, T. Supportive Care Interventions for People with Cancer Assisted by Digital Technology: Systematic Review. J. Med. Internet Res. 2021, 23, e24722. [Google Scholar] [CrossRef]
- Invernizzi, M.; de Sire, A.; Lippi, L.; Venetis, K.; Sajjadi, E.; Gimigliano, F.; Gennari, A.; Criscitiello, C.; Cisari, C.; Fusco, N. Impact of Rehabilitation on Breast Cancer Related Fatigue: A Pilot Study. Front. Oncol. 2020, 10, 556718. [Google Scholar] [CrossRef]
- Hui, D.; Bohlke, K.; Bao, T.; Campbell, T.C.; Coyne, P.J.; Currow, D.C.; Gupta, A.; Leiser, A.L.; Mori, M.; Nava, S.; et al. Management of Dyspnea in Advanced Cancer: ASCO Guideline. J. Clin. Oncol. 2021, 39, 1389–1411. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Ni, Y.; Niu, Y.; Jiang, L. The Clinical Value of Pulmonary Rehabilitation in Reducing Postoperative Complications and Mortality of Lung Cancer Resection: A Systematic Review and Meta-Analysis. Front. Surg. 2021, 8, 685485. [Google Scholar] [CrossRef] [PubMed]
- Tenconi, S.; Mainini, C.; Rapicetta, C.; Braglia, L.; Galeone, C.; Cavuto, S.; Merlo, D.F.; Costi, S.; Paci, M.; Piro, R.; et al. Rehabilitation for lung cancer patients undergoing surgery: Results of the PUREAIR randomized trial. Eur. J. Phys. Rehabil. Med. 2021, 57, 1002–1011. [Google Scholar] [CrossRef] [PubMed]
- Ozmen, I.; Yildirim, E.; Ozturk, M.; Ocakli, B.; Yildiz, R.; Aydin, R.; Karakis, M.; Yilmaz, O.; Aksoy, E. Pulmonary Rehabilitation Reduces Emergency Admission and Hospitalization Rates of Patients with Chronic Respiratory Diseases. Turk. Thorac. J. 2018, 19, 170–175. [Google Scholar] [CrossRef]
- Morley, J.E.; Vellas, B.; van Kan, G.A.; Anker, S.D.; Bauer, J.M.; Bernabei, R.; Cesari, M.; Chumlea, W.C.; Doehner, W.; Evans, J.; et al. Frailty consensus: A call to action. J. Am. Med. Dir. Assoc. 2013, 14, 392–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spitzer, K.A.; Stefan, M.S.; Priya, A.; Pack, Q.R.; Pekow, P.S.; Lagu, T.; Pinto-Plata, V.M.; ZuWallack, R.L.; Lindenauer, P.K. Participation in Pulmonary Rehabilitation after Hospitalization for Chronic Obstructive Pulmonary Disease among Medicare Beneficiaries. Ann. Am. Thorac. Soc. 2019, 16, 99–106. [Google Scholar] [CrossRef]
- Rochester, C.L.; Vogiatzis, I.; Holland, A.E.; Lareau, S.C.; Marciniuk, D.D.; Puhan, M.A.; Spruit, M.A.; Masefield, S.; Casaburi, R.; Clini, E.M.; et al. An Official American Thoracic Society/European Respiratory Society Policy Statement: Enhancing Implementation, Use, and Delivery of Pulmonary Rehabilitation. Am. J. Respir. Crit. Care Med. 2015, 192, 1373–1386. [Google Scholar] [CrossRef] [Green Version]
- Sebio-García, R. Pulmonary Rehabilitation: Time for an Upgrade. J. Clin. Med. 2020, 9, 2742. [Google Scholar] [CrossRef]
- Marques, A.; Jácome, C.; Rebelo, P.; Paixão, C.; Oliveira, A.; Cruz, J.; Freitas, C.; Rua, M.; Loureiro, H.; Peguinho, C.; et al. Improving access to community-based pulmonary rehabilitation: 3R protocol for real-world settings with cost-benefit analysis. BMC Public Health 2019, 19, 676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malaguti, C.; Dal Corso, S.; Janjua, S.; Holland, A.E. Supervised maintenance programmes following pulmonary rehabilitation compared to usual care for chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2021, 8, CD013569. [Google Scholar] [CrossRef] [PubMed]
- Finamore, P.; Scarlata, S.; Delussu, A.S.; Traballesi, M.; Incalzi, R.A.; Laudisio, A. Frailty Impact during and after Pulmonary Rehabilitation. COPD 2021, 18, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Spruit, M.A. Pulmonary rehabilitation. Eur. Respir. Rev. 2014, 23, 55–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, H.; Williams, V.; Curtis, F.; Bridle, C.; Jones, A.W. Facilitators and barriers to physical activity following pulmonary rehabilitation in COPD: A systematic review of qualitative studies. NPJ Prim. Care Respir. Med. 2018, 28, 19. [Google Scholar] [CrossRef] [PubMed]
- Di Vincenzo, F. Exploring the networking behaviors of hospital organizations. BMC Health Serv. Res. 2018, 18, 334. [Google Scholar] [CrossRef] [Green Version]
- Dennett, E.J.; Janjua, S.; Stovold, E.; Harrison, S.L.; McDonnell, M.J.; Holland, A.E. Tailored or adapted interventions for adults with chronic obstructive pulmonary disease and at least one other long-term condition: A mixed methods review. Cochrane Database Syst. Rev. 2021, 7, CD013384. [Google Scholar] [CrossRef]
- Troosters, T.; Blondeel, A.; Janssens, W.; Demeyer, H. The past, present and future of pulmonary rehabilitation. Respirology 2019, 24, 830–837. [Google Scholar] [CrossRef]
- Bonnevie, T.; Smondack, P.; Elkins, M.; Gouel, B.; Medrinal, C.; Combret, Y.; Muir, J.F.; Cuvelier, A.; Prieur, G.; Gravier, F.E. Advanced telehealth technology improves home-based exercise therapy for people with stable chronic obstructive pulmonary disease: A systematic review. J. Physiother. 2021, 67, 27–40. [Google Scholar] [CrossRef]
- Sepúlveda-Loyola, W.; Rodríguez-Sánchez, I.; Pérez-Rodríguez, P.; Ganz, F.; Torralba, R.; Oliveira, D.V.; Rodríguez-Mañas, L. Impact of Social Isolation Due to COVID-19 on Health in Older People: Mental and Physical Effects and Recommendations. J. Nutr. Health Aging 2020, 24, 938–947. [Google Scholar] [CrossRef]
- Ijaz, N.; Buta, B.; Xue, Q.-L.; Mohess, D.T.; Bushan, A.; Tran, H.; Batchelor, W.; Defilippi, C.R.; Walston, J.D.; Bandeen-Roche, K.; et al. Interventions for Frailty among Older Adults with Cardiovascular Disease. J. Am. Coll. Cardiol. 2022, 79, 482–503. [Google Scholar] [CrossRef]
- Trombini, M.; Ferraro, F.; Morando, M.; Regesta, G.; Dellepiane, S. A Solution for the Remote Care of Frail Elderly Individuals via Exergames. Sensors 2021, 21, 2719. [Google Scholar] [CrossRef]
- Md Fadzil, N.H.; Shahar, S.; Rajikan, R.; Singh, D.K.A.; Mat Ludin, A.F.; Subramaniam, P.; Ibrahim, N.; Vanoh, D.; Mohamad Ali, N. A Scoping Review for Usage of Telerehabilitation among Older Adults with Mild Cognitive Impairment or Cognitive Frailty. Int. J. Environ. Res. Public Health 2022, 19, 4000. [Google Scholar] [CrossRef] [PubMed]
- De Sire, A.; Andrenelli, E.; Negrini, F.; Lazzarini, S.G.; Patrini, M.; Ceravolo, M.G. Rehabilitation and COVID-19: The Cochrane Rehabilitation 2020 rapid living systematic review. Update as of August 31st, 2020. Eur. J. Phys. Rehabil. Med. 2021, 56, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, S.; Rutkowska, A.; Kiper, P.; Jastrzebski, D.; Racheniuk, H.; Turolla, A.; Szczegielniak, J.; Casaburi, R. Virtual Reality Rehabilitation in Patients with Chronic Obstructive Pulmonary Disease: A Randomized Controlled Trial. Int. J. Chron. Obstruct. Pulmon. Dis. 2020, 15, 117–124. [Google Scholar] [CrossRef] [Green Version]
- Stafinski, T.; Nagase, F.I.; Avdagovska, M.; Stickland, M.K.; Menon, D. Effectiveness of home-based pulmonary rehabilitation programs for patients with chronic obstructive pulmonary disease (COPD): Systematic review. BMC Health Serv. Res. 2022, 22, 557. [Google Scholar] [CrossRef]
- Mendes Xavier, D.; Lanza Galvão, E.; Aliane Fonseca, A.; de Souza, G.M.; Pereira Lima, V. Effects of Home-Based Pulmonary Rehabilitation on Dyspnea, Exercise Capacity, Quality of Life and Impact of the Disease in COPD Patients: A Systematic Review. COPD 2022, 19, 18–46. [Google Scholar] [CrossRef] [PubMed]
- Michaelchuk, W.; Oliveira, A.; Marzolini, S.; Nonoyama, M.; Maybank, A.; Goldstein, R.; Brooks, D. Design and delivery of home-based telehealth pulmonary rehabilitation programs in COPD: A systematic review and meta-analysis. Int. J. Med. Inform. 2022, 162, 104754. [Google Scholar] [CrossRef] [PubMed]
- Holland, A.E.; Mahal, A.; Hill, C.J.; Lee, A.L.; Burge, A.T.; Cox, N.S.; Moore, R.; Nicolson, C.; O’Halloran, P.; Lahham, A.; et al. Home-based rehabilitation for COPD using minimal resources: A randomised, controlled equivalence trial. Thorax 2017, 72, 57–65. [Google Scholar] [CrossRef] [Green Version]
- Stickland, M.K.; Jourdain, T.; Wong, E.Y.; Rodgers, W.M.; Jendzjowsky, N.G.; Macdonald, G.F. Using Telehealth Technology to Deliver Pulmonary Rehabilitation to Patients with Chronic Obstructive Pulmonary Disease. Can. Respir. J. 2011, 18, 216–220. [Google Scholar] [CrossRef]
- Golmohammadi, K.; Jacobs, P.; Sin, D.D. Economic evaluation of a community-based pulmonary rehabilitation program for chronic obstructive pulmonary disease. Lung 2004, 182, 187–196. [Google Scholar] [CrossRef]
- Almathami, H.K.Y.; Win, K.T.; Vlahu-Gjorgievska, E. Barriers and Facilitators That Influence Telemedicine-Based, Real-Time, Online Consultation at Patients’ Homes: Systematic Literature Review. J. Med. Internet Res. 2020, 22, e16407. [Google Scholar] [CrossRef] [PubMed]
- Gajarawala, S.N.; Pelkowski, J.N. Telehealth Benefits and Barriers. J. Nurse Pract. 2021, 17, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Elbaz, S.; Cinalioglu, K.; Sekhon, K.; Gruber, J.; Rigas, C.; Bodenstein, K.; Naghi, K.; Lavin, P.; Greenway, K.T.; Vahia, I.; et al. A Systematic Review of Telemedicine for Older Adults with Dementia During COVID-19: An Alternative to In-person Health Services? Front. Neurol. 2021, 12, 761965. [Google Scholar] [CrossRef] [PubMed]
- Doraiswamy, S.; Jithesh, A.; Mamtani, R.; Abraham, A.; Cheema, S. Telehealth Use in Geriatrics Care during the COVID-19 Pandemic—A Scoping Review and Evidence Synthesis. Int. J. Environ. Res. Public Health 2021, 18, 1755. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Cako, A.; Urquhart, R.; Straus, S.E.; Wodchis, W.P.; Baker, G.R.; Gagliardi, A.R. Patient engagement in hospital health service planning and improvement: A scoping review. BMJ Open 2018, 8, e018263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mockford, C.; Staniszewska, S.; Griffiths, F.; Herron-Marx, S. The impact of patient and public involvement on UK NHS health care: A systematic review. Int. J. Qual. Health Care 2012, 24, 28–38. [Google Scholar] [CrossRef] [Green Version]
- Prey, J.E.; Woollen, J.; Wilcox, L.; Sackeim, A.D.; Hripcsak, G.; Bakken, S.; Restaino, S.; Feiner, S.; Vawdrey, D.K. Patient engagement in the inpatient setting: A systematic review. J. Am. Med. Inform. Assoc. JAMIA 2014, 21, 742–750. [Google Scholar] [CrossRef]
- Johnson, K.E.; Mroz, T.M.; Abraham, M.; Figueroa Gray, M.; Minniti, M.; Nickel, W.; Reid, R.; Sweeney, J.; Frosch, D.L.; Ness, D.L.; et al. Promoting Patient and Family Partnerships in Ambulatory Care Improvement: A Narrative Review and Focus Group Findings. Adv. Ther. 2016, 33, 1417–1439. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, H.; Manolova, G.; Daskalopoulou, C.; Vitoratou, S.; Prince, M.; Prina, A.M. Prevalence of multimorbidity in community settings: A systematic review and meta-analysis of observational studies. J. Comorbidity 2019, 9, 2235042X1987093. [Google Scholar] [CrossRef]
- Kim, J.W.; Ryu, B.; Cho, S.; Heo, E.; Kim, Y.; Lee, J.; Jung, S.Y.; Yoo, S. Impact of Personal Health Records and Wearables on Health Outcomes and Patient Response: Three-Arm Randomized Controlled Trial. JMIR Mhealth Uhealth 2019, 7, e12070. [Google Scholar] [CrossRef] [PubMed]
- Morgado Areia, C.; Santos, M.; Vollam, S.; Pimentel, M.; Young, L.; Roman, C.; Ede, J.; Piper, P.; King, E.; Gustafson, O.; et al. A Chest Patch for Continuous Vital Sign Monitoring: Clinical Validation Study During Movement and Controlled Hypoxia. J. Med. Internet Res. 2021, 23, e27547. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, C.; Akiguchi, S.; Ohira, M. Development of a Remote Health Monitoring System to Prevent Frailty in Elderly Home-Care Patients with COPD. Sensors 2022, 22, 2670. [Google Scholar] [CrossRef]
- Troosters, T.; Tabin, N.; Langer, D.; Burtin, C.; Chatwin, M.; Clini, E.M.; Emtner, M.; Gosselink, R.; Grant, K.; Inal-Ince, D.; et al. Introduction of the harmonised respiratory physiotherapy curriculum. Breathe 2019, 15, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Chike-Harris, K.E.; Durham, C.; Logan, A.; Smith, G.; DuBose-Morris, R. Integration of Telehealth Education into the Health Care Provider Curriculum: A Review. Telemed. J. e-Health 2021, 27, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Chike-Harris, K.E.; Garber, K.; Derouin, A. Telehealth Educational Resources for Graduate Nurse Faculty. Nurse Educ. 2021, 46, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Sempé, L.; Billings, J.; Lloyd-Sherlock, P. Multidisciplinary interventions for reducing the avoidable displacement from home of frail older people: A systematic review. BMJ Open 2019, 9, e030687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellis, G.; Sevdalis, N. Understanding and improving multidisciplinary team working in geriatric medicine. Age Ageing 2019, 48, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Hardisty, J.; O’Neil, H.; O’Connell, J.; Hancock, R.; Lucas, R.; Parkin, L. Simulating complexity: Providing undergraduate students with exposure in early clinical training to the multidisciplinary management of frail older people. BMJ Simul. Technol. Enhanc. Learn 2019, 5, 116–117. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lippi, L.; D’Abrosca, F.; Folli, A.; Dal Molin, A.; Moalli, S.; Maconi, A.; Ammendolia, A.; de Sire, A.; Invernizzi, M. Closing the Gap between Inpatient and Outpatient Settings: Integrating Pulmonary Rehabilitation and Technological Advances in the Comprehensive Management of Frail Patients. Int. J. Environ. Res. Public Health 2022, 19, 9150. https://doi.org/10.3390/ijerph19159150
Lippi L, D’Abrosca F, Folli A, Dal Molin A, Moalli S, Maconi A, Ammendolia A, de Sire A, Invernizzi M. Closing the Gap between Inpatient and Outpatient Settings: Integrating Pulmonary Rehabilitation and Technological Advances in the Comprehensive Management of Frail Patients. International Journal of Environmental Research and Public Health. 2022; 19(15):9150. https://doi.org/10.3390/ijerph19159150
Chicago/Turabian StyleLippi, Lorenzo, Francesco D’Abrosca, Arianna Folli, Alberto Dal Molin, Stefano Moalli, Antonio Maconi, Antonio Ammendolia, Alessandro de Sire, and Marco Invernizzi. 2022. "Closing the Gap between Inpatient and Outpatient Settings: Integrating Pulmonary Rehabilitation and Technological Advances in the Comprehensive Management of Frail Patients" International Journal of Environmental Research and Public Health 19, no. 15: 9150. https://doi.org/10.3390/ijerph19159150
APA StyleLippi, L., D’Abrosca, F., Folli, A., Dal Molin, A., Moalli, S., Maconi, A., Ammendolia, A., de Sire, A., & Invernizzi, M. (2022). Closing the Gap between Inpatient and Outpatient Settings: Integrating Pulmonary Rehabilitation and Technological Advances in the Comprehensive Management of Frail Patients. International Journal of Environmental Research and Public Health, 19(15), 9150. https://doi.org/10.3390/ijerph19159150










