Involvement of Helicobacter pylori in Preoperative Gastric Findings on a Bariatric Population
Abstract
:1. Introduction
2. Patients and Methods
2.1. Selection of Patients
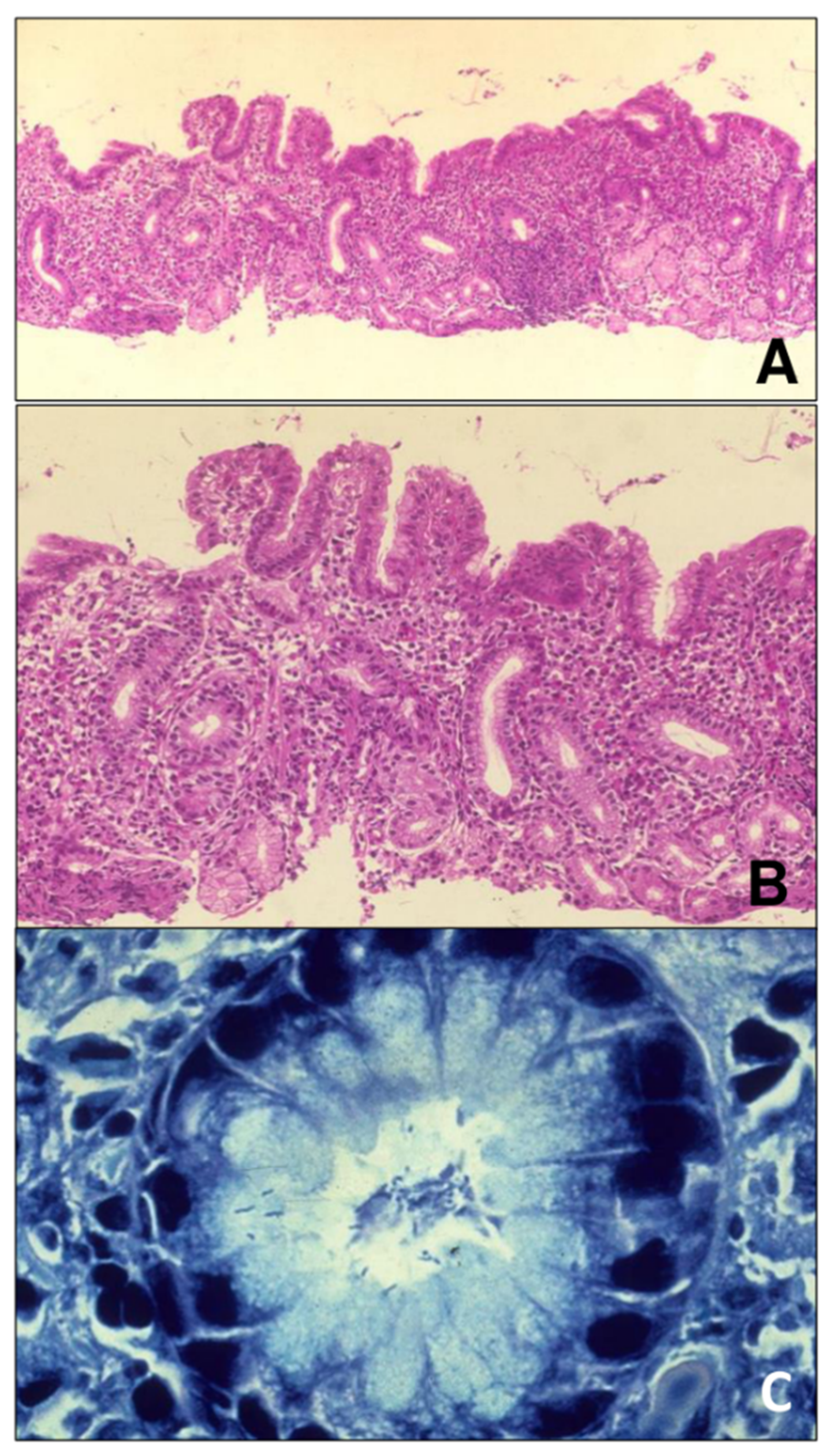
2.2. Description of the Histological Examination and Recorded Variables
2.3. Selection Criteria of the Surgical Technique
2.4. Variables
2.5. Statistical Analysis
3. Results
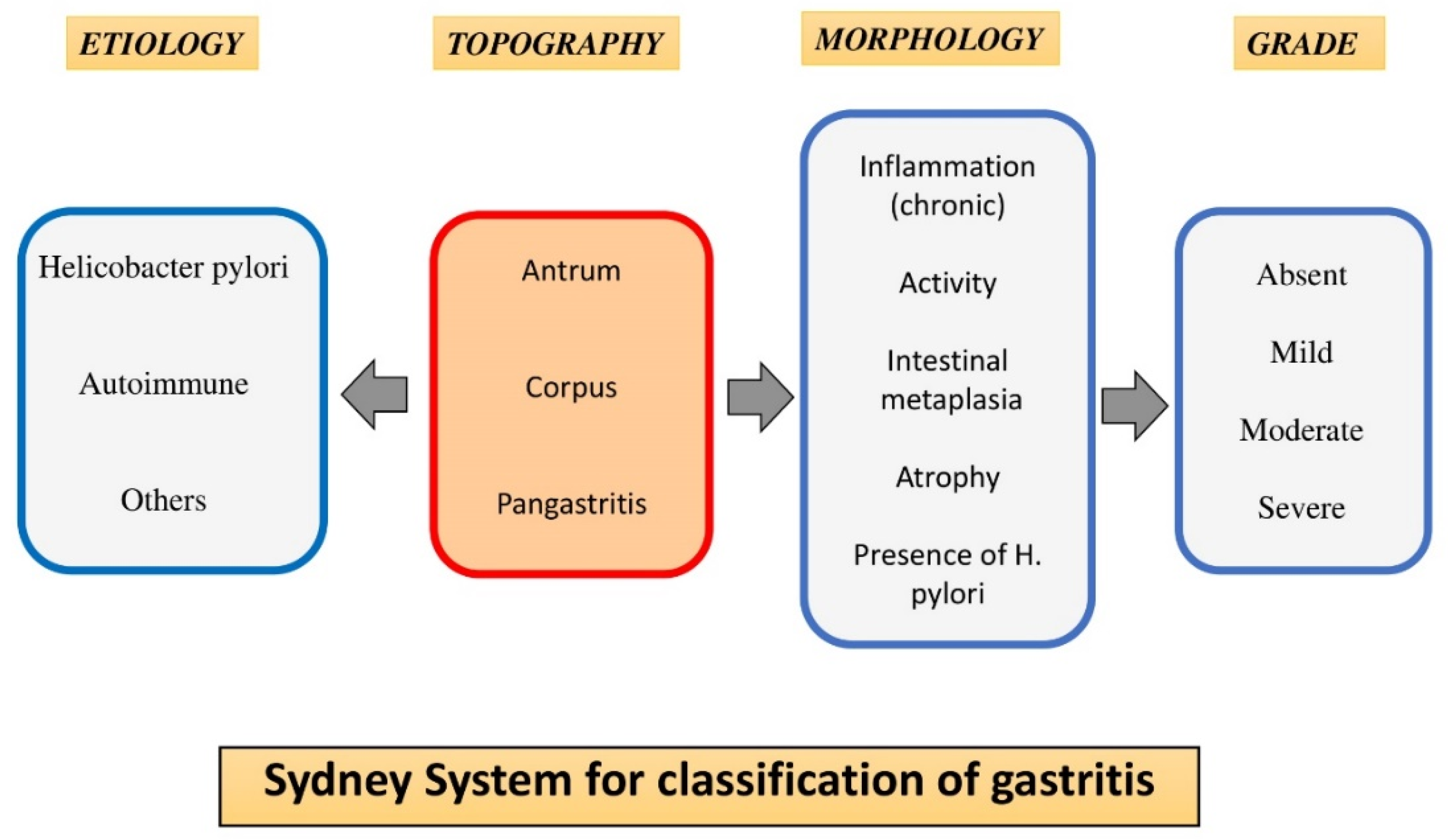
Prevalence of CG, GA, and GIM and Their Association with Hp
4. Discussion
5. Limitations of the Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Garcia Gómez-Heras, S.; Paredes-González, J.; Franco-Rodriguez, R. Introduction to NAFLD and NASH: Etiopathogenesis. In Liver Steatosis and Bariatric Surgery; Ruiz-Tovar, J., Zubiaga, L., Eds.; Nova Science: New York, NY, USA, 2019; pp. 1–28. [Google Scholar]
- Wolfe, B.M.; Kvach, E.; Eckel, R.H. Treatment of Obesity: Weight Loss and Bariatric Surgery. Circ. Res. 2016, 118, 1844–1855. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.C.; Reid, A.S.; Brown, I.S. The pathological findings seen in laparoscopic sleeve gastrectomies for weight loss. Pathology 2016, 48, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Öner, R.I.; Özdaş, S. Histopathological Findings in Morbid Obese Patients Undergoing Laparoscopic Sleeve Gastrectomy: Does H. pylori Infection Effective on Pathological Changes? Obes. Surg. 2018, 28, 3136–3141. [Google Scholar] [CrossRef]
- Adalı, Y.; Binnetoğlu, K.; Eroğlu, H.A.; Kaya, N.; Güvendi, G.F. The relationship Between Histopathologic Findings and Body Mass Index in Sleeve Gastrectomy Materials. Obes. Surg. 2019, 29, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Brutman, J.N.; Sirohi, S.; Davis, J.F. Recent Advances in the Neurobiology of Altered Motivation Following Bariatric Surgery. Curr. Psychiatry Rep. 2019, 9, 117. [Google Scholar] [CrossRef] [PubMed]
- Brown, W.A.; Johari Halim Shah, Y.; Balalis, G.; Bashir, A.; Ramos, A.; Kow, L.; Herrera, M.; Shikora, S.; Campos, G.M.; Himpens, J.; et al. IFSO Position Statement on the Role of Esophago-Gastro-Duodenal Endoscopy Prior to and after Bariatric and Metabolic Surgery Procedures. Obes. Surg. 2021, 30, 3135–3153. [Google Scholar] [CrossRef]
- García-Gómez-Heras, S.; Garcia, A.; Zubiaga, L.; Artuñedo, P.; Ferrigni, C.; Duran, M.; Ruiz-Tovar, J. Prevalence of Endoscopic Findings before Bariatric Surgery and Their Influence on the Selection of the Surgical Technique. Obes. Surg. 2020, 30, 4375–4380. [Google Scholar] [CrossRef]
- Yardimci, E.; Bozkurt, S.; Baskoy, L.; Bektasoglu, H.K.; Gecer, M.O.; Yigman, S.; Akbulut, H.; Coskun, H. Rare Entities of Histopathological Findings in 755 Sleeve Gastrectomy Cases: A Synopsis of Preoperative Endoscopy Findings and Histological Evaluation of the Specimen. Obes. Surg. 2018, 28, 1289–1295. [Google Scholar] [CrossRef] [PubMed]
- Lecube, A.; Valladares, S.; López-Cano, C.; Gutiérrez, L.; Ciudin, A.; Fort, J.M.; Reñé, J.M.; Matias-Guiu, X.; De Torres, I.; Bueno, M.; et al. The Role of Morbid Obesity in the Promotion of Metabolic Disruptions and Non-Alcoholic Steatohepatitis by Helicobacter pylori. PLoS ONE 2016, 11, e0166741. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Sánchez, N.; Pichardo-Bahena, R.; Vásquez-Fernández, F.; Lezama-Mora, J.I.; León-Canales, A.L.; Barredo-Prieto, B.; González-Avila, D.; Ponciano-Rodríguez, G.; Uribe, M. Effect of Helicobacter pylori infection on gastric ghrelin expression and body weight. Rev. Gastroenterol. Mex. 2007, 72, 359–364. [Google Scholar]
- Mala, T. The Gastric Remnant in Roux-en-Y Gastric Bypass: Challenges and Possibilities. J. Clin. Gastroenterol. 2016, 50, 527–531. [Google Scholar] [CrossRef]
- Kim, N. Chemoprevention of gastric cancer by Helicobacter pylori eradication and its underlying mechanism. J. Gastroenterol. Hepatol. 2019, 34, 1287–1295. [Google Scholar] [PubMed] [Green Version]
- Correa, P.; Haenszel, W.; Cuello, C.; Tannenbaum, S.; Archer, M. A model for gastric cancer epidemiology. Lancet 1975, 2, 58–60. [Google Scholar] [CrossRef]
- Huang, R.J.; Choi, A.Y.; Truong, C.D.; Yeh, M.M.; Hwang, J.H. Diagnosis and Management of Gastric Intestinal Metaplasia: Status and Future Directions. Gut Liver 2019, 13, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Sipponen, P.; Price, A.B. The Sydney System for classification of gastritis 20 years ago. J. Gastroenterol. Hepatol. 2011, 26, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, S.R.; Meireles, L.C.; Carrilho-Ribeiro, L.; Velosa, J. The Role of Routine Upper Gastrointestinal Endoscopy before Bariatric Surgery. Obes. Surg. 2016, 26, 2105–2110. [Google Scholar] [CrossRef] [PubMed]
- Peker, K.D.; Sahbaz, N.A.; Seyit, H.; Kones, O.; Gumusoglu, A.Y.; Alis, H. An alternative view on the necessity of EGD before sleeve gastrectomy. Surg. Obes. Relat. Dis. 2017, 13, 1959–1964. [Google Scholar] [CrossRef] [PubMed]
- Ghaderi, I.; Gondal, A.B.; Samamé, J.; Serrot, F.; Galvani, C.A. Preoperative Endoscopic and Radiologic Evaluation of Bariatric Patients: What Do They Add? J. Gastrointest. Surg. 2020, 24, 764–771. [Google Scholar] [CrossRef]
- Martın Garcıa-Almenta, E.; Ruiz-Tovar, J.; Sanchez Santos, R. Guía Clínica En Cirugía Bariatrica; Im3diA Comunicación, S.L.: Albacete, Spain, 2017; ISBN 978-84-697-7104-4. [Google Scholar]
- Sauerland, S.; Angrisani, L.; Belachew, M.; Chevallier, J.M.; Favretti, F.; Finer, N.; Fingerhut, A.; Garcia Caballero, M.; Guisado Macias, J.A.; Mittermair, R.; et al. Evidence-based guidelines of the European Association for Endoscopic Surgery (EAES). Surg. Endosc. 2005, 19, 200–221. [Google Scholar] [CrossRef] [PubMed]
- Kashiwagi, H. Ulcers and gastritis. Endoscopy 2003, 35, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Lei, L.; Xia, C.; Li, H.; Cao, M.; He, S.; Zhang, Z.; Guo, G.; Song, G.; Peng, J.; et al. Sociodemographic disparities in gastric cancer and the gastric precancerous cascade: A population-based study. Lancet Reg. Health West. Pac. 2022, 23, 100437. [Google Scholar] [CrossRef] [PubMed]
- Bas, B.; Dinc, B. Helicobacter pylori-related precancerous lesions in Turkey: A retrospective endoscopic surveillance study. Croat. Med. J. 2020, 61, 319–325. [Google Scholar] [CrossRef]
- Ohkuma, K.; Okada, M.; Murayama, H.; Seo, M.; Maeda, K.; Kanda, M.; Okabe, N. Association of Helicobacter pylori infection with atrophic gastritis and intestinal metaplasia. J. Gastroenterol. Hepatol. 2000, 15, 1105–1112. [Google Scholar] [CrossRef] [PubMed]
- Uotani, T.; Miftahussurur, M.; Yamaoka, Y. Effect of bacterial and host factors on Helicobacter pylori eradication therapy. Expert Opin. Ther. Targets 2015, 19, 1637–1650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, P.I.; Lai, K.H.; Hsu, P.N.; Lo, G.H.; Yu, H.C.; Chen, W.C.; Tsay, F.W.; Lin, H.C.; Tseng, H.H.; Ger, L.P.; et al. Helicobacter pylori Infection and the risk of gastric malignancy. Am. J. Gastroenterol. 2007, 102, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.H.; Yamada, N.; Wu, Y.L.; Wen, M.; Matsuhisa, T.; Matsukura, N. Helicobacter pylori infection, glandular atrophy and intestinal metaplasia in superficial gastritis, gastric erosion, erosive gastritis, gastric ulcer, and early gastric cáncer. World J. Gastroenterol. 2005, 11, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Rugge, M.; Correa, P.; Dixon, M.F.; Fiocca, R.; Hattori, T.; Lechago, J.; Leandro, G.; Price, A.B.; Sipponen, P.; Solcia, E.; et al. Gastric mucosal atrophy: Interobserver consistency using new criteria for classification and grading. Aliment. Pharm. Ther. 2002, 16, 1249–1259. [Google Scholar] [CrossRef] [PubMed]
- Kapadia, C.R. Gastric atrophy, metaplasia, and dysplasia: A clinical perspective. J. Clin. Gastroenterol. 2003, 36, S29–S36. [Google Scholar] [CrossRef]
- De Vries, A.; Kuipers, E.J. Epidemiology of premalignant gastric lesions: Implications for the development of screening and surveillance strategies. Helicobacter 2007, 12, 22–31. [Google Scholar] [CrossRef]
- Wu, M.S.; Chen, C.J.; Lin, J.T. Host–environment interactions: Their impact on progression from gastric inflammation to carcino genesis and on development of new approaches to prevent and treat gastric cancer. Cancer Epidemiol. Biomark. Prev. 2005, 14, 1878–1882. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.J.E.; Kocsmár, I.; Buzás, G.M.; Szirtes, I.; Rusz, O.; Diczházi, C.; Szijártó, A.; Hritz, I.; Schaff, Z.; Kiss, A.; et al. Efficacy of Clarithromycin Depends on the Bacterial Density in Clarithromycin-Heteroresistant Helicobacter pylori Infections: An In Situ Detected Susceptibility and Quantitative Morphometry-Based Retrospective Study. Pathol. Oncol. Res. 2021, 27, 1609863. [Google Scholar] [CrossRef] [PubMed]
- Malfertheiner, P.; Megraud, F.; O’morain, C.A.; Gisbert, J.P.; Kuipers, E.J.; Axon, A.T.; Bazzoli, F.; Gasbarrini, A.; Atherton, J.; Graham, D.Y.; et al. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut 2017, 66, 6–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| N (1040) | |
|---|---|
| Age (years old) | |
| Mean ± SD | 45.4 ± 10.8 |
| Age grouped | |
| ≤50 | 697 |
| >50 | 343 |
| Gender | |
| Male | 344 |
| Female | 696 |
| BMI Kg/m2 | |
| Mean ± SD | 46.8 ± 8.9 |
| 35–39.9 kg/m2 | 214 |
| 40–49.9 kg/m2 | 650 |
| ≥50 kg/m2 | 176 |
| Total | 1040 |
| N (%) | ≤50 Years Old N (%) | >50 Years Old N (%) | Male N (%) | Female N (%) | |
|---|---|---|---|---|---|
| Roux-en-Y gastric bypass (RYGB) | 810 (77.9%) | 231 (28.5%) | 579 (71.5%) | 255 (31.5%) | 555 (68.5%) |
| Sleeve Gastrectomy (SG) | 230 (22.1%) | 66 (28.7%) | 164 (71.3%) | 105 (45.7%) | 125 (54.3%) |
| N (1040) | % | |
|---|---|---|
| Hp positive | 375 | 36.1 |
| Mild Hp | 105 | 28 |
| Moderate Hp | 158 | 42.1 |
| Severe Hp | 112 | 30.9 |
| Hp eradicated | 355 | 94.7 |
| ≤50 Years N (%) 697 (67%) | >50 Years N (%) 343 (33%) | p * | Female N (%) 696 (67%) | Male N (%) 344 (33%) | p * | Gastritis N (%) 489 (47%) | Atrophy N (%) 38 (3.6%) | Metaplasia N (%) 80 (7.7%) | |
|---|---|---|---|---|---|---|---|---|---|
| H. pylori + 375 (36.1%) | 202 (29%) | 173 (50.4%) | 0.001 | 263 (37.8%) | 112 (32.6%) | NS | 375 (76.7%) | 17 (44.7%) | 32 (40%) |
| Mild 105 (28%) | 59 (29.2%) | 46 (26.6%) | NS | 75 (28.5%) | 30 (26.8%) | NS | 105 (28%) | 4 (23.5%) | 0 |
| Moderate 158 (42.1%) | 80 (39.6%) | 78 (45.1%) | 115 (43.7%) | 43 (38.4%) | 158 (42.1%) | 6 (35.3%) | 14 (43.8%) | ||
| Severe 112 (30.9%) | 63 (31.2%) | 49 (28.3%) | 73 (27.8%) | 39 (34.8%) | 112 (30.9%) | 7 (41.2%) | 18 (56.2%) | ||
| H. pylori eradicated 355 (94.7%) | 193 (95.5%) | 162 (93.6%) | NS | 252 (95.8%) | 103 (92%) | NS | 355 (94.7%) | 8 (47.1%) | 12 (37.8%) |
| ≤50 Years N (%) 697 (67%) | >50 Years N (%) 343 (33%) | p * | |
|---|---|---|---|
| Gastritis N (%) 489(47%) | 285 (40.9%) | 132 (38.5%) | NS |
| Atrophy N (%) 38 (3.6%) | 26 (3.7%) | 12 (3.5%) | NS |
| Metaplasia N (%) 80 (7.7%) | 48 (6.9%) | 34 (9.9%) | NS |
| BMI 35–40 N (%) 214 (20.6%) | BMI 41–50 N (%) 650 (62.5%) | BMI >50 N (%) 176 (16.9%) | p * | |
|---|---|---|---|---|
| H. pylori + 375 (36.1%) | 95 (44.4%) | 226 (34.8%) | 54 (30.7%) | 0.006 |
| Mild 105 (28%) | 20 (21.1%) | 74 (32.8%) | 11 (20.4%) | NS |
| Moderate 158 (42.1%) | 55 (57.8%) | 76 (33.6%) | 27 (50%) | NS |
| Severe 112 (20.9%) | 20 (21.1%) | 76 (33.6%) | 16 (29.6%) | NS |
| H. pylori eradicated 355 (94.7%) | 90 (94.7%) | 214 (94.7%) | 51 (94.4%) | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Gómez-Heras, S.; Fernández-Aceñero, M.J.; González, G.; Bolaños-Muñoz, M.d.L.; Franco-Rodríguez, R.; Paredes-González, J.; Ruiz-Tovar, J. Involvement of Helicobacter pylori in Preoperative Gastric Findings on a Bariatric Population. Int. J. Environ. Res. Public Health 2022, 19, 9088. https://doi.org/10.3390/ijerph19159088
García-Gómez-Heras S, Fernández-Aceñero MJ, González G, Bolaños-Muñoz MdL, Franco-Rodríguez R, Paredes-González J, Ruiz-Tovar J. Involvement of Helicobacter pylori in Preoperative Gastric Findings on a Bariatric Population. International Journal of Environmental Research and Public Health. 2022; 19(15):9088. https://doi.org/10.3390/ijerph19159088
Chicago/Turabian StyleGarcía-Gómez-Heras, Soledad, María Jesús Fernández-Aceñero, Gilberto González, María de Lourdes Bolaños-Muñoz, Raquel Franco-Rodríguez, Julio Paredes-González, and Jaime Ruiz-Tovar. 2022. "Involvement of Helicobacter pylori in Preoperative Gastric Findings on a Bariatric Population" International Journal of Environmental Research and Public Health 19, no. 15: 9088. https://doi.org/10.3390/ijerph19159088
APA StyleGarcía-Gómez-Heras, S., Fernández-Aceñero, M. J., González, G., Bolaños-Muñoz, M. d. L., Franco-Rodríguez, R., Paredes-González, J., & Ruiz-Tovar, J. (2022). Involvement of Helicobacter pylori in Preoperative Gastric Findings on a Bariatric Population. International Journal of Environmental Research and Public Health, 19(15), 9088. https://doi.org/10.3390/ijerph19159088






