Rare Earth Elements in Boletus edulis (King Bolete) Mushrooms from Lowland and Montane Areas in Poland
Abstract
:1. Introduction
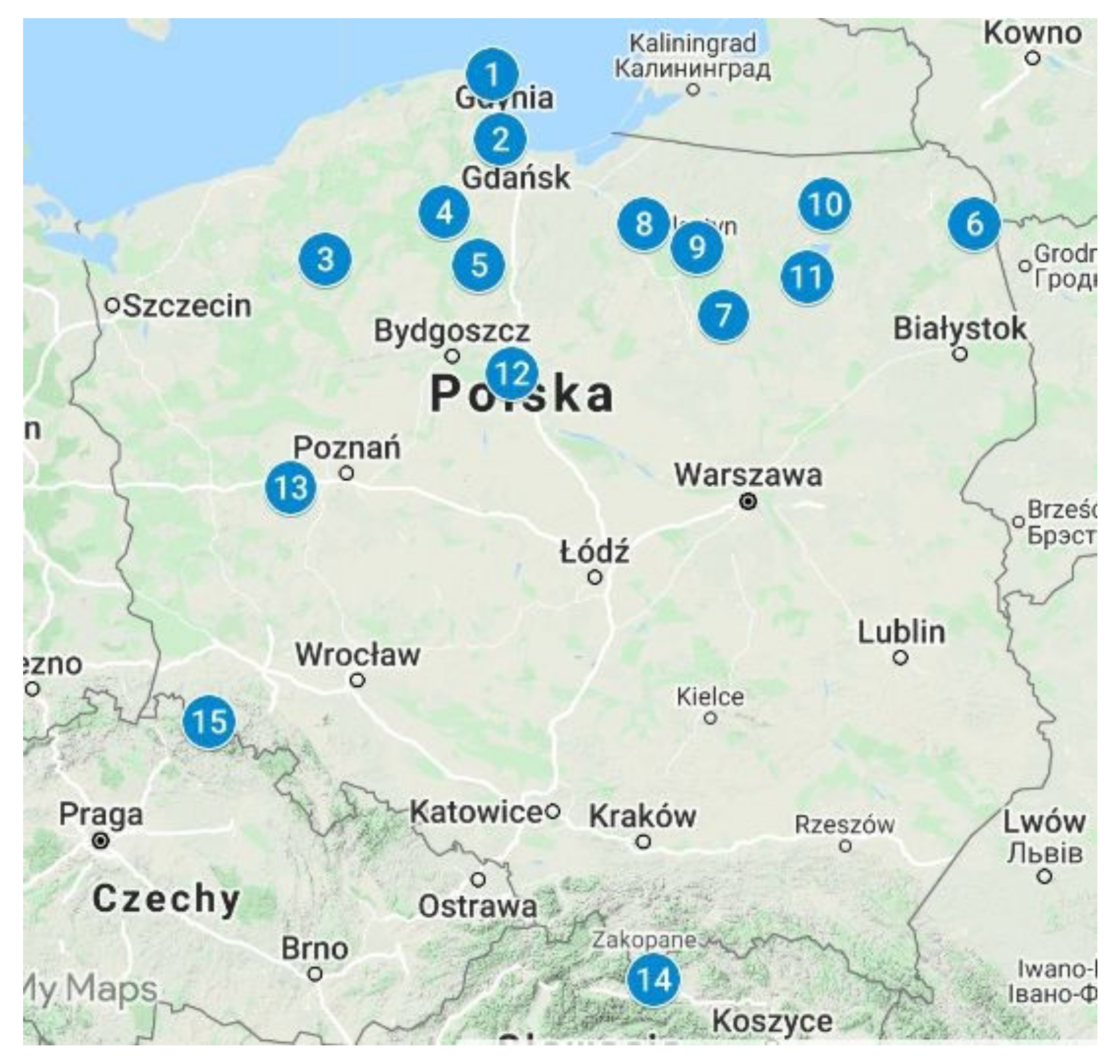
2. Materials and Methods
3. Results and Discussion
3.1. REE Concentration Levels
| Place, Year, Quantity of Specimens and Morphological Part * | La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | 14REE | Sc | Y |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (1) ¶ Seacoast Landscape Park, 2007 (15; c) | 33 | 60 | 6.4 | 23 | 4.5 | 1.1 | 5.9 | 0.85 | 4.8 | 1.0 | 2.9 | 0.5 | 4.3 | 0.7 | 199.95 | 12 | 39 |
| (10) Mazury, Giżycko, 2001 (15; c) | 73 | 140 | 14 | 42 | 6.0 | 1.5 | 5.3 | 1.0 | 5.2 | 1.1 | 2.9 | 0.7 | 3.5 | 0.6 | 348.8 | 13 | 39 |
| (11) Mazury, Piska Forest, 2002 (15; c) | 15 | 27 | 2.3 | 9.9 | 1.5 | 0.5 | 1.7 | 0.3 | 1.3 | 0.3 | 0.7 | 0.2 | 0.6 | 0.2 | 74.3 | 0.8 | 12 |
| (14) Tatra Mountains, 1999 (12; c) | 14 | 30 | 2.9 | 10 | 1.8 | 0.1 | 1.7 | 0.3 | 1.3 | 0.3 | 0.6 | 0.2 | 1.1 | 0.1 | 128.4 | 46 | 18 |
| (15) Sudety Mountains., Kłodzka Dale, 2000 (15; c) | 35 | 70 | 6.7 | 22 | 3.4 | 0.6 | 4.3 | 0.7 | 3.8 | 0.7 | 2.1 | 0.4 | 2.0 | 0.4 | 185.7 | 2.6 | 31 |
| Mean ± SD, (caps 5(72)) # | 34 ± 24 | 65 ± 46 | 6.5 ± 4.7 | 21 ± 13 | 3.4 ± 1.9 | 0.8 ± 0.5 | 3.8 ± 2.0 | 0.6 ± 0.3 | 3.3 ± 1.9 | 0.7 ± 0.4 | 1.8 ± 1.1 | 0.4 ± 0.2 | 2.3 ± 1.6 | 0.4 ± 0.3 | 187 ± 103 | 15 ± 18 | 28 ± 12 |
| Median | 33 | 60 | 6.4 | 22 | 3.4 | 0.6 | 4.3 | 0.7 | 3.8 | 0.7 | 2.1 | 0.4 | 2.0 | 0.4 | 186 | 13 | 31 |
| Range | 14–73 | 27–140 | 2.3–14 | 9.9–42 | 1.5–6.0 | 0.1–1.5 | 1.7–5.9 | 0.3–1.0 | 1.3–5.2 | 0.3–1.1 | 0.6–2.9 | 0.2–0.7 | 0.6–4.3 | 0.1–0.7 | 74–349 | 0.8–46 | 12–39 |
| (1) Seacoast Landscape Park, 2007 (15; s) | 64 | 130 | 13 | 46 | 8.4 | 2.2 | 9.7 | 1.5 | 11 | 2.4 | 8.1 | 1.4 | 9.8 | 1.4 | 433.9 | 40 | 85 |
| (10) Mazury land, Giżycko, 2001 (15; s) | 110 | 220 | 21 | 64 | 6.0 | 0.8 | 5.0 | 0.9 | 4.3 | 1.0 | 2.3 | 0.3 | 2.7 | 0.4 | 477.6 | 3.9 | 35 |
| (11) Mazury land, Piska Forest, 2002 (15; s) | 21 | 40 | 3.7 | 15 | 1.3 | 0.6 | 1.5 | 0.3 | 1.6 | 0.3 | 1.1 | 0.1 | 0.9 | 0.1 | 102.6 | 1.0 | 15 |
| (15) Sudety Mts., Kłodzka Dale, 2000 (15; s) | 49 | 97 | 10 | 36 | 6.6 | 1.1 | 5.4 | 0.9 | 6.7 | 1.3 | 5.0 | 0.8 | 4.4 | 0.6 | 283.7 | 7.9 | 51 |
| Mean ± SD (stipes, 4(60) # | 61 ± 37 | 120 ± 75 | 12 ± 7 | 40 ± 20 | 5.6 ± 3.0 | 1.2 ± 0.7 | 5.4 ± 3.0 | 0.9 ± 0.5 | 5.9 ± 4.0 | 1.3 ± 0.9 | 4.1 ± 3.1 | 0.7 ± 0.6 | 4.5 ± 3.8 | 0.6 ± 0.6 | 324 ± 170 | 13 ± 16 | 47 ± 30 |
| Median | 56 | 110 | 11 | 41 | 6.3 | 0.9 | 5.2 | 1.2 | 5.5 | 1.1 | 3.6 | 0.5 | 3.5 | 0.5 | 359 | 5.9 | 43 |
| Range | 21–110 | 40–220 | 3.7–21 | 15–64 | 1.3–8.4 | 0.6–2.2 | 1.5–9.7 | 0.3–1.5 | 1.6–11 | 0.3–2.4 | 1.1–8.1 | 0.1–1.4 | 0.9–9.8 | 0.1–1.4 | 103–478 | 1.0–40 | 15–85 |
| (1) Seacoast Landscape Park, 2007 (15; w) | 48 | 95 | 9.7 | 34 | 6.4 | 1.6 | 7.8 | 1.2 | 3.9 | 1.7 | 5.5 | 0.9 | 7.0 | 1.0 | 311.7 | 26 | 62 |
| (2) Tricity Landscape Park, Osowa, 2006 (10; w) | 17 | 35 | 3.2 | 11 | 1.4 | 0.4 | 2.3 | 0.27 | 1.5 | 0.2 | 0.6 | 0.1 | 0.8 | 0.1 | 116.8 | 30 | 13 |
| (3) Pomerania, Szczecinek, 2000 (22; w) | 47 | 92 | 8.5 | 29 | 4.1 | 1.3 | 4.0 | 0.9 | 4.8 | 0.7 | 2.5 | 0.6 | 3.1 | 0.4 | 335.9 | 98 | 39 |
| (4) Wdzydze Landscape Park, 1999–2001 (45; w) | 37 | 74 | 7.1 | 26 | 4.6 | 0.8 | 4.0 | 0.6 | 5.4 | 1.0 | 3.2 | 0.5 | 2.9 | 0.5 | 251.6 | 39 | 45 |
| (5) Tuchola Pinewoods, 2000 (15; w) | 69 | 140 | 12 | 41 | 7.5 | 0.9 | 5.7 | 1.2 | 6.3 | 1.4 | 3.6 | 0.6 | 4.1 | 0.6 | 398.9 | 52 | 53 |
| (6) Augustów Primeval Forest, 2000 (16; w) | 150 | 270 | 26 | 79 | 10 | 3.4 | 8.4 | 1.4 | 7.2 | 1.6 | 5.4 | 0.6 | 4.3 | 0.7 | 758 | 120 | 70 |
| (7) Warmia land, Puchałowo, 2001 (15; w) | 53 | 95 | 9.6 | 25 | 3.5 | 1.0 | 5.0 | 0.6 | 4.1 | 1.0 | 1.4 | 0.3 | 1.8 | 0.3 | 235.5 | 8.9 | 25 |
| (8) Warmia, Morąg, 1998 (30; w) § | 300 | 630 | 63 | 240 | 44 | 9.7 | 36 | 6.2 | 33 | 7.3 | 21 | 3.5 | 24 | 3.6 | 1796.3 | 65 | 310 |
| (9) Warmia, Olsztyn, 1999 (19; w) | 58 | 110 | 11 | 45 | 6.8 | 1.2 | 6.6 | 1.0 | 5.5 | 1.3 | 3.7 | 0.5 | 3.9 | 0.6 | 310.1 | 14 | 41 |
| (10) Mazury, Giżycko, 2001 (15; w) | 91 | 180 | 17 | 53 | 6.0 | 1.1 | 5.1 | 0.9 | 4.7 | 1.0 | 2.6 | 0.5 | 3.1 | 0.5 | 470.5 | 67 | 37 |
| (11) Mazury, Piska Forest, 2002 (15; w) | 18 | 33 | 3.0 | 12 | 1.4 | 0.5 | 1.6 | 0.3 | 1.4 | 0.3 | 0.9 | 0.1 | 0.7 | 0.1 | 86.7 | 0.4 | 13 |
| (12) Kujawy, Toruń forests, 1999 (16; w) | 96 | 180 | 19 | 63 | 13 | 2.2 | 13 | 2.0 | 13 | 3.1 | 11 | 1.5 | 11 | 1.6 | 565.4 | 26 | 110 |
| (13) Greater Poland, Porażyn, 2008 (13; w) | 24 | 41 | 3.6 | 14 | 2.3 | 0.5 | 2.2 | 0.4 | 2.0 | 0.3 | 1.7 | 0.2 | 1.4 | 0.2 | 174.8 | 61 | 20 |
| (15) Sudety Mountains, Kłodzka Dale, 2000 (15; w) | 42 | 83 | 8.3 | 29 | 5.0 | 0.8 | 4.8 | 0.8 | 5.2 | 1.0 | 3.5 | 0.6 | 3.2 | 0.5 | 234.9 | 5.2 | 42 |
| Mean ± SD (whole fruiting bodies, 14(261) # | 75 ± 74 | 150 ± 150 | 14 ± 15 | 50 ± 58 | 8.3 ± 11.0 | 1.8 ± 2.4 | 7.6 ± 8.7 | 1.3 ± 1.5 | 7.0 ± 8.0 | 1.6 ± 1.8 | 4.8 ± 5.4 | 0.8 ± 0.9 | 5.1 ± 6.1 | 0.8 ± 0.9 | 327 ± 186 | 44 ± 36 | 63 ± 76 |
| Median | 51 | 95 | 9.7 | 32 | 5.5 | 1.1 | 5.1 | 0.9 | 5.0 | 1.0 | 3.4 | 0.6 | 3.2 | 0.5 | 310 | 35 | 42 |
| Range | 17–300 | 33–630 | 3.0–63 | 11–240 | 1.4–44 | 0.4–9.7 | 1.6–36 | 0.27–6.5 | 1.4–33 | 0.2–7.3 | 0.6–21 | 0.1–3.5 | 0.7–24 | 0.1–3.6 | 86.7–758 | 0.4–120 | 13–310 |
3.2. LREE and HREE in B. edulis
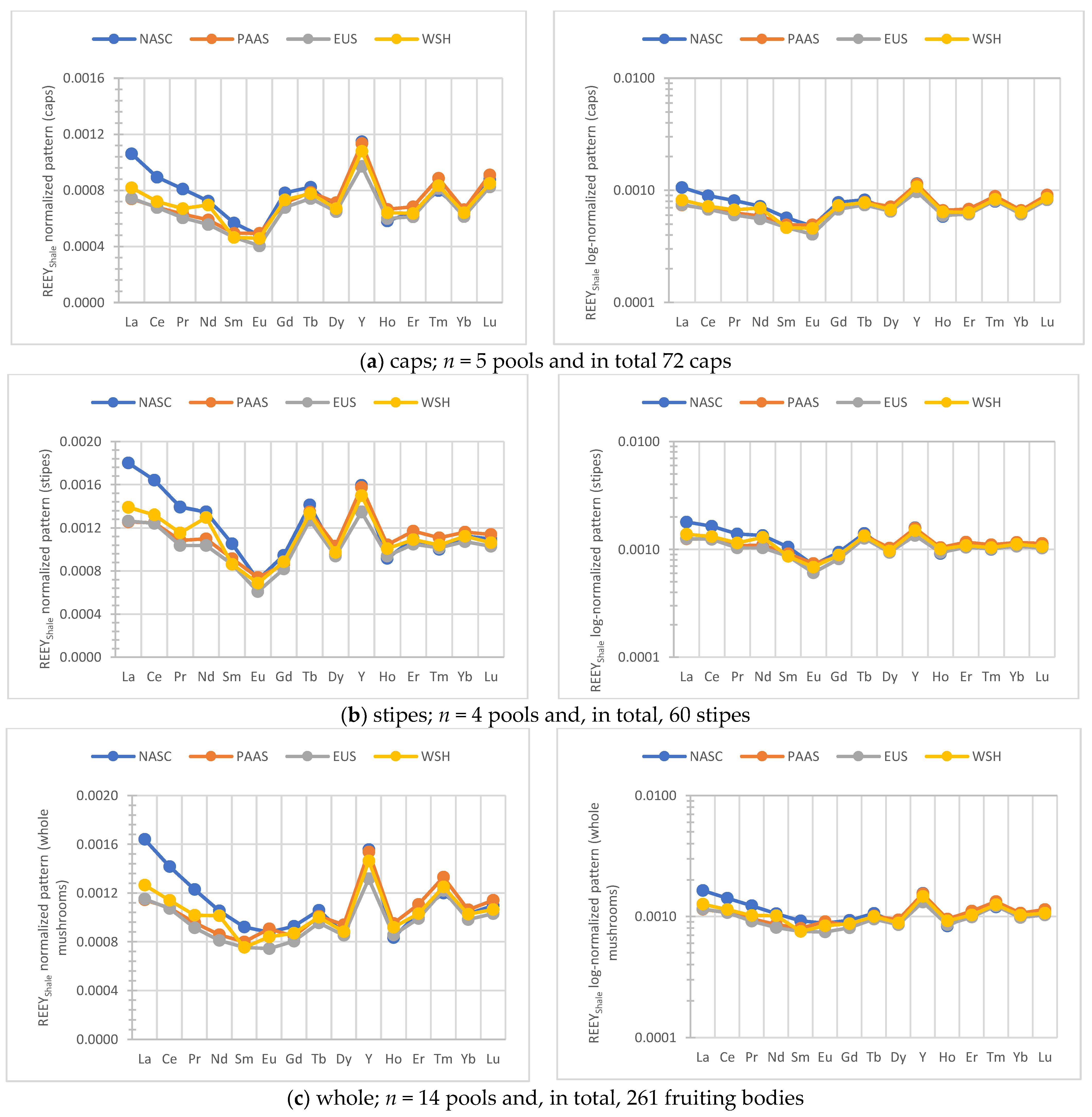
3.3. Shale Normalized Patterns of REEY in B. edulis
3.4. REE Intakes from Bolete Meals
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Migaszewski, Z.M.; Gałuszka, A. The characteristics, occurrence, and feochemical behavior of rare earth elements in the environment: A review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 429–471. [Google Scholar] [CrossRef]
- Rim, K.T. A book review; “Rare earth elements in human and environmental health; at the crossroads between toxicity and safety”. J. Appl. Biol. Chem. 2017, 60, 207–211. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Chen, Z.; Chen, Z.; Zhang, Y. A human health risk assessment of rare earth elements in soil and vegetables from a mining area in Fujian Province, Southeast China. Chemosphere 2013, 93, 1240–1246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vukojević, V.; Durđić, S.; Stefanović, V.; Trifković, J.; Čakmak, D.; Veljko Perović, V.; Mutić, J. Scandium, yttrium, and lanthanide contents in soil from Serbia and their accumulation in the mushroom Macrolepiota procera (Scop.) Singer. Environ. Sci. Pollut. Res. 2019, 26, 5422–5434. [Google Scholar] [CrossRef]
- Balaram, V. Rare earth elements: A review of applications, occurrence, exploration, analysis, recycling, and environmental impact. Geosci. Front. 2019, 10, 1285–1303. [Google Scholar] [CrossRef]
- Gałuszka, A.; Migaszewski, Z.M. Extremely high levels of trace elements in aerial parts of plants naturally growing in the Wiśniówka acid mine drainage area (south-central Poland). In Risk to Opportunity, Proceedings of the ICARD/IMWA, Pretoria, South Africa, 10–14 September 2018; Wolkersdorfer, C., Sartz, L., Weber, A., Burgess, J., Tremblay, G., Eds.; Tshwane University of Technology: Pretoria, South Africa, 2018; Volume 2, pp. 598–603. [Google Scholar]
- Klinger, J.M. A historical geography of rare earth elements: From discovery to the atomic age. Extract. Ind. Soc. 2015, 2, 572–580. [Google Scholar] [CrossRef]
- Li, J.X.; Hong, M.; Yin, X.Q. Accumulation and geochemical characteristics of exogenous rare earths in soil of leeward area of tailings dam of Baotou Iron and Steel (Group) Company. Chin. Rare Earths 2008, 29, 57–62. [Google Scholar]
- Li, J.X.; Hong, M.; Yin, X.Q.; Liu, J.L. Effects of the accumulation of the rare earth elements on soil macrofauna community. J. Rare Earths 2010, 28, 957–964. [Google Scholar] [CrossRef]
- Migaszewski, Z.M.; Gałuszka, A.; Dołęgowska, S. Rare earth and trace element signatures for assessing an impact of rock mining and processing on the environment: Wiśniówka case study, south-central Poland. Environ. Sci. Pollut. Res. 2016, 23, 24943–24959. [Google Scholar] [CrossRef]
- Ichihashi, H.; Morita, H.; Tatsukawa, R. Rare earth elements (REEs) in naturally grown plants in relation to their variation in soils. Environ. Pollut. 1992, 76, 157–162. [Google Scholar] [CrossRef]
- Gałuszka, A.; Migaszewski, Z.M.; Pelc, A.; Trembaczowski, A.; Dołęgowska, S.; Michalik, A. Trace elements and stable sulfur isotopes in plants of acid mine drainage area: Implications for revegetation of degraded land. J. Environ. Sci. 2020, 94, 128–136. [Google Scholar] [CrossRef]
- Wang, L.; Liang, T. Accumulation and fractionation of rare earth elements in atmospheric particulates around a mine tailing in Baotou, China. Atmos. Environ. 2014, 88, 23–29. [Google Scholar] [CrossRef]
- Franus, W.; Wiatros-Motyka, M.M.; Wdowin, M. Coal fly ash as a resource for rare earth elements. Environ. Sci. Pollut. Res. 2015, 22, 9464–9474. [Google Scholar] [CrossRef] [Green Version]
- Feng, J.; Zhang, H.; Zhu, W.F.; Liu, C.Q.; Xu, S.Q.; Wu, D.S. Bio-effect of rare earths in the high background region I. Some blood biochemical indices from population resided in light REE district. J. Rare Earths 2000, 18, 356–359. [Google Scholar]
- Ma, J.Y.; Young, S.H.; Mercer, R.R.; Barger, M.; Schwegler-Berry, D.; Ma, J.K.; Castranova, V. Interactive effects of cerium oxide and diesel exhaust nanoparticles on inducing pulmonary fibrosis. Toxicol. Appl. Pharmacol. 2014, 278, 135–147. [Google Scholar] [CrossRef] [Green Version]
- Pagano, G.; Aliberti, F.; Guida, M.; Oral, R.; Siciliano, A.; Trifuoggi, M.; Tommasi, F. Rare earth elements in human and animal health: State of art and research prioritties. Environ. Res. 2015, 142, 215–228. [Google Scholar] [CrossRef]
- Rare Earth Element. Available online: https://en.wikipedia.org/wiki/Rare_earth_element (accessed on 27 May 2022).
- Hirano, S.; Suzuki, K.T. Exposure, metabolism, and toxicity of rare earths and related compounds. Environ. Health Perspect. 1996, 104 (Suppl. 1), 85–95. [Google Scholar] [CrossRef]
- Falandysz, J.; Borovička, J. Macro and trace mineral constituents and radionuclides in mushrooms: Health benefits and risks. Appl. Microbiol. Biotechnol. 2013, 97, 477–501. [Google Scholar] [CrossRef] [Green Version]
- Falandysz, J.; Szymczyk, K.; Ichihashi, H.; Bielawski, L.; Gucia, M.; Frankowska, A.; Yamasaki, S.-I. ICP/MS and ICP/AES elemental analysis (38 elements) of edible wild mushrooms growing in Poland. Food Addit. Contam. 2001, 18, 503–513. [Google Scholar] [CrossRef]
- Borovička, J.; Kubrová, J.; Rohovec, J.; Řanda, Z.; Dunn, C.E. Uranium, thorium and rare earth elements in macrofungi: What are the genuine concentrations? Biometals 2011, 24, 837–845. [Google Scholar] [CrossRef]
- Zocher, A.-L.; Kraemer, D.; Merschel, G.; Bau, M. Distribution of major and trace elements in the bolete mushroom Suillus luteus and the bioavailability of rare earth elements. Chem. Geol. 2018, 483, 491–500. [Google Scholar] [CrossRef]
- Falandysz, J.; Sapkota, A.; Mędyk, M.; Feng, X. Rare earth elements in parasol mushroom Macrolepiota procera. Food Chem. 2017, 221, 24–28. [Google Scholar] [CrossRef]
- Bau, M.; Schmidt, K.; Pack, A.; Bendel, V.; Kraemer, D. The European shale: An improved data set for normalisation of rare earth element and yttrium concentrations in environmental and biological samples from Europe. Appl. Geochem. 2018, 90, 142–149. [Google Scholar] [CrossRef]
- Grawunder, A.; Gube, M. Element distribution in fruiting bodies of Lactarius pubescens with focus on rare earth elements. Chemosphere 2018, 208, 614–625. [Google Scholar] [CrossRef]
- Laessoe, T.; Del Conte, A.; Lincoff, G. The Mushroom Book. How to Identify, Gather and Cook Wild Mushrooms and Other Fungi; ADK Publishing Inc.: New York, NY, USA, 1996. [Google Scholar]
- Petkovšek, S.A.S.; Pokorny, B. Lead and cadmium in mushrooms from the vicinity of two large emission sources in Slovenia. Sci. Total Environ. 2013, 443, 944–954. [Google Scholar] [CrossRef]
- Zhang, D.; Frankowska, A.; Jarzyńska, G.; Kojta, A.K.; Drewnowska, M.; Wydmańska, D.; Bielawski, L.; Wang, J.; Falandysz, J. Metals of King Bolete (Boletus edulis) collected at the same site over two years. Afr. J. Agric. Res. 2010, 5, 3050–3055. [Google Scholar]
- Collin-Hansen, C.; Andersen, R.A.; Steinnes, E. Damage to DNA and lipids in Boletus edulis exposed to heavy metals. Mycol. Res. 2005, 109, 1386–1396. [Google Scholar] [CrossRef]
- Frankowska, A.; Ziółkowska, J.; Bielawski, L.; Falandysz, J. Profile and bioconcentration of minerals by King Bolete (Boletus edulis) from the Płocka Dale in Poland. Food Addit. Contam. B 2010, 3, 1–6. [Google Scholar] [CrossRef]
- Cocchi, L.; Kluza, K.; Zalewska, T.; Apanel, A.; Falandysz, J. Radioactive caesium (134Cs and 137Cs) in mushrooms of the genus Boletus from the Reggio Emilia in Italy and Pomerania in Poland. Isot. Environ. Health Stud. 2017, 53, 620–627. [Google Scholar] [CrossRef]
- Falandysz, J.; Saba, M.; Strumińska-Parulska, D. 137Caesium, 40K and total K in Boletus edulis at different maturity stages: Effect of braising and estimated radiation dose intake. Chemosphere 2021, 268, 129336. [Google Scholar] [CrossRef]
- Falandysz, J.; Zalewska, D.; Saniewski, M.; Fernandes, A.R. An evaluation of the occurrence and trends in 137Cs and 40K radioactivity in King Bolete Boletus edulis mushrooms in Poland during 1995–2019. Environ. Sci. Pollut. Res. 2021, 28, 32405–32415. [Google Scholar] [CrossRef] [PubMed]
- Dimitrijevic, M.V.; Mitic, V.D.; Cvetkovic, J.S.; Stankov Jovanovic, V.P.; Mutic, J.J.; Nikolic Mandic, S.D. Update on element content profiles in eleven wild edible mushrooms from family Boletaceae. Eur. Food Res. Technol. 2016, 242, 1–10. [Google Scholar] [CrossRef]
- Falandysz, J. Nutritional and other trace elements and their associations in raw King Bolete mushrooms, Boletus edulis. Int. J. Environ. Res. Public Health 2022, 19, 417. [Google Scholar] [CrossRef] [PubMed]
- Rutkowska, M.; Falandysz, J.; Saba, M.; Szefer, P.; Misztal-Szkudlińska, M.; Konieczka, P. A method for the analysis of methylmercury and total Hg in fungal matrices. Appl. Microbiol. Biotechnol. 2022, 106, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Niewiadomski, A.; Tołoczko, W. Characteristics of soil cover in Poland with special attention paid to the Łódź region. In Natural Environment of Poland and Its Protection in Łódź University Geographical Research; Kobojek, E., Marszał, T., Eds.; Łódź University Press: Łódź, Poland, 2014; pp. 75–99. ISBN 978-83-7969-134-0. [Google Scholar]
- Stijve, T.; Noorloos, T.; Byrne, A.R.; Steikovec, Z.; Goessler, W. High selenium levels in edible Albatrellus mushrooms. Dtsch. Lebensm.-Rundsch. 1998, 94, 275–279. [Google Scholar]
- Falandysz, J.; Frankowska, A.; Jarzyńska, G.; Dryżałowska, A.; Kojta, A.K.; Zhang, D. Survey on composition and bioconcentration potential of 12 metallic elements in King Bolete (Boletus edulis) mushroom that emerged at 11 spatially distant sites. J. Environ. Sci. Health Part B 2011, 46, 231–246. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Grégoire, D.C. Determination of trace elements in twenty six Chinese geochemistry reference materials by inductively coupled plasma-mass spectrometry. Geostand. Newslett. 2000, 24, 51–63. [Google Scholar] [CrossRef]
- Shi, W.; Feng, X.; Zhang, G.; Ming, L.; Yin, R.; Zhao, Z.; Wang, J. High-precision measurement of mercury isotope ratios of atmospheric deposition over the past 150 years recorded in a peat core taken from Hongyuan, Sichuan Province, China. Chin. Sci. Bull. 2011, 56, 877–882. [Google Scholar] [CrossRef] [Green Version]
- Dołęgowska, S.; Migaszewski, Z.M. Anomalous concentrations of rare earth elements in the moss-soil system from south—Central Poland. Environ. Pollut. 2013, 178, 33–40. [Google Scholar] [CrossRef]
- US EPA. Guidance for Assessing Chemical Contaminant, Data for Use in Fish Advisories; United State Environmental Protection Agency: Washington, DC, USA, 2000.
- Zhu, W.F.; Xu, S.Q.; Shao, P.P.; Zhang, H.; Feng, J.; Wu, D.L.; Yang, W.J. Investigation on intake allowance of rare earth—A study on bio-effect of rare earth in South Jiangxi. Chin. Environ. Sci. 1997, 1, 63–65. [Google Scholar]
- Zhu, W.F.; Xu, S.Q.; Shao, P.P.; Zhang, H.; Wu, D.S.; Yang, W.J.; Feng, J. Bioelectrical activity of the central nervous system among populations in a rare earth element area. Biol. Trace Elem. Res. 1997, 57, 71–77. [Google Scholar] [CrossRef]
- Strumińska-Parulska, D.; Falandysz, J.; Moniakowska, A. Beta-emitting radionuclides in wild mushrooms and potential radiotoxicity for their consumers. Trends Food Sci. Technol. 2021, 114, 672–683. [Google Scholar] [CrossRef]
- Chen, Z.; Zhu, X. Accumulation of rare earth elements in bone and its toxicity and potential hazard to health. J. Ecol. Rural Environ. 2008, 24, 88–91. [Google Scholar]
- Tyler, G. Rare earth elements in soil and plant systems—A review. Plant Soil 2004, 267, 191–206. [Google Scholar] [CrossRef]
- Falandysz, J.; Kunito, T.; Kubota, R.; Bielawski, L.; Mazur, A.; Falandysz, J.J.; Tanabe, S. Multivariate characterization of elements accumulated in King Bolete Boletus edulis mushroom at lowland and high mountain regions. J. Environ. Sci. Health Part A 2008, 43, 1692–1699. [Google Scholar] [CrossRef]
- Mędyk, M.; Treu, R.; Falandysz, J. Accumulation of minerals by Leccinum scabrum from two large forested areas in Central Europe: Notecka Wilderness and Tuchola Forest (Pinewoods). Chem. Biodivers. 2020, 17, e2000264. [Google Scholar] [CrossRef]
- Yoshida, S.; Muramatsu, Y. Determination of major and trace elements in mushroom, plant and soil samples collected from Japanese forests. Int. J. Environ. Anal. Chem. 1997, 67, 49–58. [Google Scholar] [CrossRef]
- Falandysz, J.; Brzostowski, A. Mercury and its bioconcentration factors in Poison Pax (Paxillus involutus) from various sites in Poland. J. Environ. Sci. Health Part A 2007, 42, 1095–1100. [Google Scholar] [CrossRef]
- Falandysz, J.; Kunito, T.; Kubota, R.; Lipka, K.; Mazur, A.; Falandysz, J.J.; Tanabe, S. Selected elements in Fly agaric Amanita muscaria. J. Environ. Sci. Health Part A 2007, 42, 1615–1623. [Google Scholar] [CrossRef]
- Aruguete, D.M.; Altstad, J.H.; Mueller, G.M. Accumulation of several heavy metals and lanthanides in mushrooms (Agaricales) from the Chicago region. Sci. Total Environ. 1998, 224, 43–56. [Google Scholar] [CrossRef]
- Drewnowska, M.; Falandysz, J.; Chudzińska, M.; Hanć, A.; Saba, M.; Barałkiewicz, D. Leaching of arsenic and sixteen metallic elements from Amanita fulva mushrooms after food processing. LWT–Food Sci. Technol. 2017, 84, 861–866. [Google Scholar] [CrossRef]
- Saba, M.; Falandysz, J. The effects of different cooking modes on the 137Cs, 40K, and total K content in Boletus edulis (King Bolete) mushrooms. Environ. Sci. Pollut. Res. 2021, 28, 12441–12446. [Google Scholar] [CrossRef] [PubMed]
- Pankavec, S. Proces litowania pieczarki dwuzarodnikowej (Agaricus bisporus) różnymi solami litu na podłożu—gromadzenie, wpływ przetwarzania i uwalnianie litu in vitro. Ph.D. Thesis, University of Gdańsk, Gdańsk, Poland, 2022. [Google Scholar]
- Falandysz, J.; Zhang, J.; Mędyk, M.; Zhang, X. Mercury in stir-fried and raw mushrooms from the Boletaceae family from the geochemically anomalous region in the Midu county, China. Food Control. 2019, 102, 17–21. [Google Scholar] [CrossRef]
- Falandysz, J.; Zhang, J.; Saniewski, M. 137Cs, 40K and K in raw and stir-fried mushrooms from the Boletaceae family from the Midu region in Yunnan, Southwest China. Environ. Sci. Pollut. Res. 2020, 27, 32509–32517. [Google Scholar] [CrossRef]
- Falandysz, J.; Saba, M.; Rutkowska, M.; Konieczka, P. Total mercury and monomethylmercury (MeHg) in braised and crude Boletus edulis carpophores during various developmental stages. Environ. Sci. Pollut. Res. 2022, 29, 3107–3115. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falandysz, J.; Nnorom, I.C.; Mędyk, M. Rare Earth Elements in Boletus edulis (King Bolete) Mushrooms from Lowland and Montane Areas in Poland. Int. J. Environ. Res. Public Health 2022, 19, 8948. https://doi.org/10.3390/ijerph19158948
Falandysz J, Nnorom IC, Mędyk M. Rare Earth Elements in Boletus edulis (King Bolete) Mushrooms from Lowland and Montane Areas in Poland. International Journal of Environmental Research and Public Health. 2022; 19(15):8948. https://doi.org/10.3390/ijerph19158948
Chicago/Turabian StyleFalandysz, Jerzy, Innocent Chidi Nnorom, and Małgorzata Mędyk. 2022. "Rare Earth Elements in Boletus edulis (King Bolete) Mushrooms from Lowland and Montane Areas in Poland" International Journal of Environmental Research and Public Health 19, no. 15: 8948. https://doi.org/10.3390/ijerph19158948
APA StyleFalandysz, J., Nnorom, I. C., & Mędyk, M. (2022). Rare Earth Elements in Boletus edulis (King Bolete) Mushrooms from Lowland and Montane Areas in Poland. International Journal of Environmental Research and Public Health, 19(15), 8948. https://doi.org/10.3390/ijerph19158948








