Sociodemographic and Health-Related Factors Influencing Drug Intake among the Elderly Population
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Explanatory Variables
2.3. Measures
2.4. Statistical Analysis
3. Results
3.1. Antihypertensive Drugs
3.2. Diuretics
3.3. Painkillers
3.4. Anticoagulants
3.5. Antidepressants
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, E.S.; Lee, P.S.S.; Xie, Y.; Ryan, B.L.; Fortin, M.; Stewart, M. The prevalence of multimorbidity in primary care: A comparison of two definitions of multimorbidity with two different lists of chronic conditions in Singapore. BMC Public Health 2021, 21, 1409. [Google Scholar] [CrossRef]
- World Health Organization. Medication Safety in Polypharmacy; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Kornholt, J.; Christensen, M.B. Prevalence of polypharmacy in Denmark. Dan. Med. J. 2020, 67. [Google Scholar]
- Masnoon, N.; Shakib, S.; Kalisch-Ellett, L.; Caughey, G.E. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017, 17, 230. [Google Scholar] [CrossRef] [PubMed]
- Stoehr, G.P.; Lu, S.Y.; Lavery, L.; Bilt, J.V.; Saxton, J.A.; Chang, C.C.; Ganguli, M. Factors associated with adherence to medication regimens in older primary care patients: The Steel Valley Seniors Survey. Am. J. Geriatr. Pharmacother. 2008, 6, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Midão, L.; Giardini, A.; Menditto, E.; Kardas, P.; Costa, E. Polypharmacy prevalence among older adults based on the survey of health, ageing and retirement in Europe. Arch. Gerontol. Geriatr. 2018, 78, 213–220. [Google Scholar] [CrossRef]
- Corsonello, A.; Pedone, C.; Incalzi, R.A. Age-related pharmacokinetic and pharmacodynamic changes and related risk of adverse drug reactions. Curr. Med. Chem. 2010, 17, 571–584. [Google Scholar] [CrossRef]
- Hajjar, E.R.; Hanlon, J.T.; Sloane, R.J.; Lindblad, C.I.; Pieper, C.F.; Ruby, C.M.; Branch, L.C.; Schmader, K.E. Unnecessary drug use in frail older people at hospital discharge. J. Am. Geriatr. Soc. 2005, 53, 1518–1523. [Google Scholar] [CrossRef]
- Skoog, J.; Midlöv, P.; Beckman, A.; Sundquist, J.; Halling, A. Indication for pharmacological treatment is often lacking: A cross-sectional study on the quality of drug therapy among the elderly. BMC Geriatr. 2015, 15, 117. [Google Scholar] [CrossRef]
- Rochon, P.A.; Gurwitz, J.H. The prescribing cascade revisited. Lancet 2017, 389, 1778–1780. [Google Scholar] [CrossRef]
- Sternberg, S.A.; Guy-Alfandary, S.; Rochon, P.A. Prescribing cascades in older adults. CMAJ 2021, 193, E215. [Google Scholar] [CrossRef]
- Cybulski, M.; Cybulski, L.; Krajewska-Kulak, E.; Orzechowska, M.; Cwalina, U. Preferences and attitudes of older adults of Bialystok, Poland toward the use of over-the-counter drugs. Clin. Interv. Aging 2018, 13, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed on 4 October 2021).
- Katz, S.; Ford, A.B.; Moskowitz, R.W.; Jackson, B.A.; Jaffe, M.W. Studies of illness in the aged. The Index of ADL: A standarized measure of biological and psychosocial function. JAMA 1963, 185, 914–919. [Google Scholar] [CrossRef] [PubMed]
- Lawton, M.P.; Brody, E.M. Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist 1969, 9, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Hodkinson, H.M. Evaluation of a mental test score for assessment of mental impairment in the elderly. Age Ageing 1972, 1. Available online: http://ageing.oxfordjournals.org/cgi/reprint/1/4/233 (accessed on 21 January 2022). [CrossRef]
- Yesavage, J.A.; Brink, T.L.; Rose, T.L.; Lum, O.; Huang, V.; Adey, M.B.; Leirer, V.O. Development and validation of a geriatric depression screening scale: A preliminary report. J. Psychiatr. Res. 1983, 17, 37–49. [Google Scholar] [CrossRef]
- Segal, D.L.; June, A.; Payne, M.; Coolidge, F.L.; Yochim, B. Development and initial validation of a self-report assessment tool for anxiety among older adults: The Geriatric Anxiety Scale. J. Anxiety Disord. 2010, 24, 709–714. [Google Scholar] [CrossRef]
- Lubben, J. Assessing social networks among elderly populations. Fam. Community Health 1988, 11, 42–52. [Google Scholar] [CrossRef]
- De Jong Gierveld, J.; Kamphuis, F. The development of a Rasch-type loneliness scale. Appl. Psychol. Meas. 1985, 9, 289–299. [Google Scholar] [CrossRef]
- Guigoz, Y.; Vellas, B.; Garry, P.J. Mini Nutritional Assessment: A practical assessment tool for grading the nutritional state of elderly patients. Facts Res. Gerontol. 1994, 4 (Suppl. 2), 15–59. [Google Scholar]
- Hales, C.M.; Servais, J.; Martin, C.B.; Kohen, D. Prescription Drug Use Among Adults Aged 40–79 in the United States and Canada. NCHS Data Brief No. 347. 2019. Available online: https://www.cdc.gov/nchs/products/databriefs/db347.htm (accessed on 21 January 2022).
- Statista 2020, Share of Households in Which Medicines and Supplements Were Purchased in Poland in 2020. Available online: https://www.statista.com/statistics/1100331/poland-share-of-households-which-bought-medicines-and-supplements/ (accessed on 22 January 2022).
- Seymour, R.M.; Routledge, P.A. Important drug-drug interactions in the elderly. Drugs Aging 1998, 12, 485–494. [Google Scholar] [CrossRef]
- Hayes, B.D.; Klein-Schwartz, W.; Barrueto, F., Jr. Polypharmacy and the geriatric patient. Clin. Geriatr. Med. 2007, 23, 371–390. [Google Scholar] [CrossRef] [PubMed]
- Unutmaz, G.D.; Soysal, P.; Tuven, B.; Isik, A.T. Costs of medication in older patients: Before and after comprehensive geriatric assessment. Clin. Interv. Aging 2018, 13, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Sergi, G.; De Rui, M.; Sarti, S.; Manzato, E. Polypharmacy in the elderly: Can comprehensive geriatric assessment reduce inappropriate medication use? Drugs Aging 2011, 28, 509–518. [Google Scholar] [CrossRef]
- Murman, D.L. The Impact of Age on Cognition. In Seminars in Hearing; Thieme Medical Publishers: New York, NY, USA, 2015; Volume 36, pp. 111–121. [Google Scholar] [CrossRef]
- National Institute of Drug Abuse. Overview. Misuse of Prescription Drugs Research Report. 2020. Available online: https://nida.nih.gov/publications/research-reports/misuse-prescription-drugs/overview (accessed on 12 March 2022).
- Ruscin, M.; Linnebur, S.A. Aging and Drugs. Available online: https://www.msdmanuals.com/home/older-people%E2%80%99s-health-issues/aging-and-drugs/aging-and-drugs (accessed on 2 February 2022).
- Hruby, A.; Hu, F.B. The Epidemiology of Obesity: A Big Picture. Pharmacoeconomics 2015, 33, 673–689. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Adult Obesity Causes & Consequences. Available online: https://www.cdc.gov/obesity/adult/causes.html (accessed on 2 February 2022).
- Rabkin, J.G.; Charles, E.; Kass, F. Hypertension and DSM-III depression in psychiatric outpatients. Am. J. Psychiatry 1983, 140, 1072–1074. [Google Scholar]
- Jokisalo, E.; Enlund, H.; Halonen, P.; Takala, J.; Kumpusalo, E. Factors related to poor control of blood pressure with antihypertensive drug therapy. Blood Press. 2003, 12, 49–55. [Google Scholar]
- Barton, D.A.; Dawood, T.; Lambert, E.A.; Esler, M.D.; Haikerwal, D.; Brenchley, C.; Socratous, F.; Kaye, D.M.; Schlaich, M.P.; Hickie, I.; et al. Sympathetic activity in major depressive disorder: Identifying those at increased cardiac risk? J. Hypertens. 2007, 25, 2117–2124. [Google Scholar] [CrossRef] [PubMed]
- Djernes, J.K. Prevalence and predictors of depression in populations of elderly: A review. Acta Psychiatr. Scand. 2006, 113, 372–387. [Google Scholar] [CrossRef]
- Miletic, B.; Lekic, A.; Courteney, U. Depression in Elderly with Different Comorbidities—Just a Small Problem or Something More? Psychiatr. Danub. 2021, 33 (Suppl. 4), 471–474. [Google Scholar]
- Yohannes, A.M.; Baldwin, R.C. Medical comorbidities in late-life depression. Psychiatr. Times 2008, 25, 52–55. [Google Scholar]
- Kovess-Masféty, V.; Alonso, J.; de Graaf, R.; Demyttenaere, K. A European approach to rural-urban differences in mental health: The ESEMeD 2000 comparative study. Can. J. Psychiatry 2005, 50, 926–936. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, K.; Murray, A.; Booth, T. Do urban environments increase the risk of anxiety, depression and psychosis? An epidemiological study. J. Affect. Disord. 2013, 150, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Lau, S.W.; Tandon, V.; Kumi, K.; Pfuma, E.; Abernethy, D.R. Geriatric drug evaluation: Where are we now and where should we be in the future? Arch. Intern. Med. 2011, 171, 937. [Google Scholar] [CrossRef] [PubMed]
- Boyd, C.M.; Darer, J.; Boult, C.; Fried, L.P.; Boult, L.; Wu, A.W. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: Implications for pay for performance. JAMA 2005, 294, 716. [Google Scholar] [CrossRef]
- Saraf, A.A.; Petersen, A.W.; Simmons, S.F. Medications associated with geriatric syndromes and their prevalence in older hospitalized adults discharged to skilled nursing facilities. J. Hosp. Med. 2016, 11, 694–700. [Google Scholar] [CrossRef]
- Nightingale, G.; Hajjar, E.; Swartz, K.; Andrel-Sendecki, J.; Chapman, A. Evaluation of a pharmacist-led medication assessment used to identify prevalence of and associations with polypharmacy and potentially inappropriate medication use among ambulatory senior adults with cancer. J. Clin. Oncol. 2015, 33, 1453–1459. [Google Scholar] [CrossRef]
- Whitman, A.; DeGregory, K.; Morris, A.; Mohile, S.; Ramsdale, E. Pharmacist-led medication assessment and deprescribing intervention for older adults with cancer and polypharmacy: A pilot study. Supportive Care 2018, 26. [Google Scholar] [CrossRef]
- Halli-Tierney, A.D.; Scarbrough, C.; Carroll, D. Polypharmacy: Evaluating Risks and Deprescribing. Am. Fam. Physician 2019, 100, 32–38. [Google Scholar]
- Steinman, M.A.; Hanlon, J.T. Managing medications in clinically complex elders: “There’s got to be a happy medium”. JAMA 2010, 304, 1592–1601. [Google Scholar] [CrossRef]

| Feature (Variable) | Statistics |
|---|---|
| Gender | |
| Women | 290 (58.0%) |
| Men | 210 (42.0%) |
| Age (years) | |
| 60–64 | 141 (28.2%) |
| 65–69 | 128 (25.6%) |
| 70 and more | 231 (46.2%) |
| Domicile | |
| Village | 110 (22.0%) |
| City up to 20,000 inhabitants | 56 (11.2%) |
| A city with 20,000 to 100,000 inhabitants | 136 (27.2%) |
| A city with 100,000 to 200,000 inhabitants | 62 (12.4%) |
| A city with 200,000 to 400,000 inhabitants | 39 (7.8%) |
| A city with over 400,000 inhabitants | 97 (19.4%) |
| Household size | |
| I live alone | 108 (21.6%) |
| I live with my partner | 202 (40.4%) |
| I live with my partner and our children | 117 (23.4%) |
| I live alone with my children | 35 (7.0%) |
| I live with a family | 29 (5.8%) |
| A different situation | 9 (1.8%) |
| Education | |
| Primary | 8 (1.6%) |
| Vocational | 105 (21.0%) |
| Secondary | 245 (49.0%) |
| Higher | 142 (28.4%) |
| Body mass (kg) | |
| M ± SD | 78.5 ± 15.7 |
| Me (IQR) | 76 (67–88) |
| Min–Max | 48–140 |
| Body height (cm) | |
| M ± SD | 169 ± 9 |
| Me (IQR) | 168 (163–175) |
| Min–Max | 141–210 |
| BMI (kg/m2) | |
| M ± SD | 27.4 ± 4.6 |
| Me (IQR) | 27 (24–30) |
| Min–Max | 19–46 |
| Net income per person in the household per month | |
| <500 PLN | 5 (1.0%) |
| 501–1000 PLN | 24 (4.8%) |
| 1001–2000 PLN | 188 (37.6%) |
| 2001–3000 PLN | 158 (31.6%) |
| Above 3000 PLN | 110 (2.0%) |
| Refusal | 15 (3.0%) |
| Chronic Diseases: | Statistics |
|---|---|
| Coronary artery disease | 63 (12.6%) |
| Diabetes | 74 (14.8%) |
| Asthma | 43 (8.6%) |
| COPD | 33 (6.6%) |
| Heart failure | 71 (14.2%) |
| Kidney failure | 20 (4.0%) |
| Physician-diagnosed gastroesophageal reflux disease (GERD) | 68 (13.6%) |
| Vaccinations: | Statistics |
| He/she was vaccinated against the flu in 2019 | 62 (12.4%) |
| He/she was vaccinated against the flu in 2020 | 51 (10.2%) |
| Avoids vaccination because of possible complications | 164 (32.8%) |
| You want to get vaccinated against the flu, but it is difficult due to the lack of a vaccine in pharmacies | 104 (20.8%) |
| The primary care physician recommended flu and pneumococcal immunization | 81 (16.2%) |
| He/she knows about flu vaccine reimbursement for seniors | 259 (51.8%) |
| Questionnaire Questions | Statistics |
|---|---|
| 1. How many drugs are you currently taking? | |
| 1–3 | 301 (60.2%) |
| 4–6 | 151 (30.2%) |
| 7–10 | 40 (8.0%) |
| >10 | 8 (1.6%) |
| 2. Which group of medications do they belong to? | |
| Hypertension drugs | 255 (51.0%) |
| Diuretics | 78 (15.6%) |
| Painkillers | 230 (46.0%) |
| Anticoagulants | 87 (17.4%) |
| Antidepressants | 78 (15.6%) |
| 3. Have you been prescribed all the medications by the same doctor? | |
| Yes | 352 (70.4%) |
| No | 148 (29.6%) |
| 4. How many different doctors prescribed the medications you are taking? | |
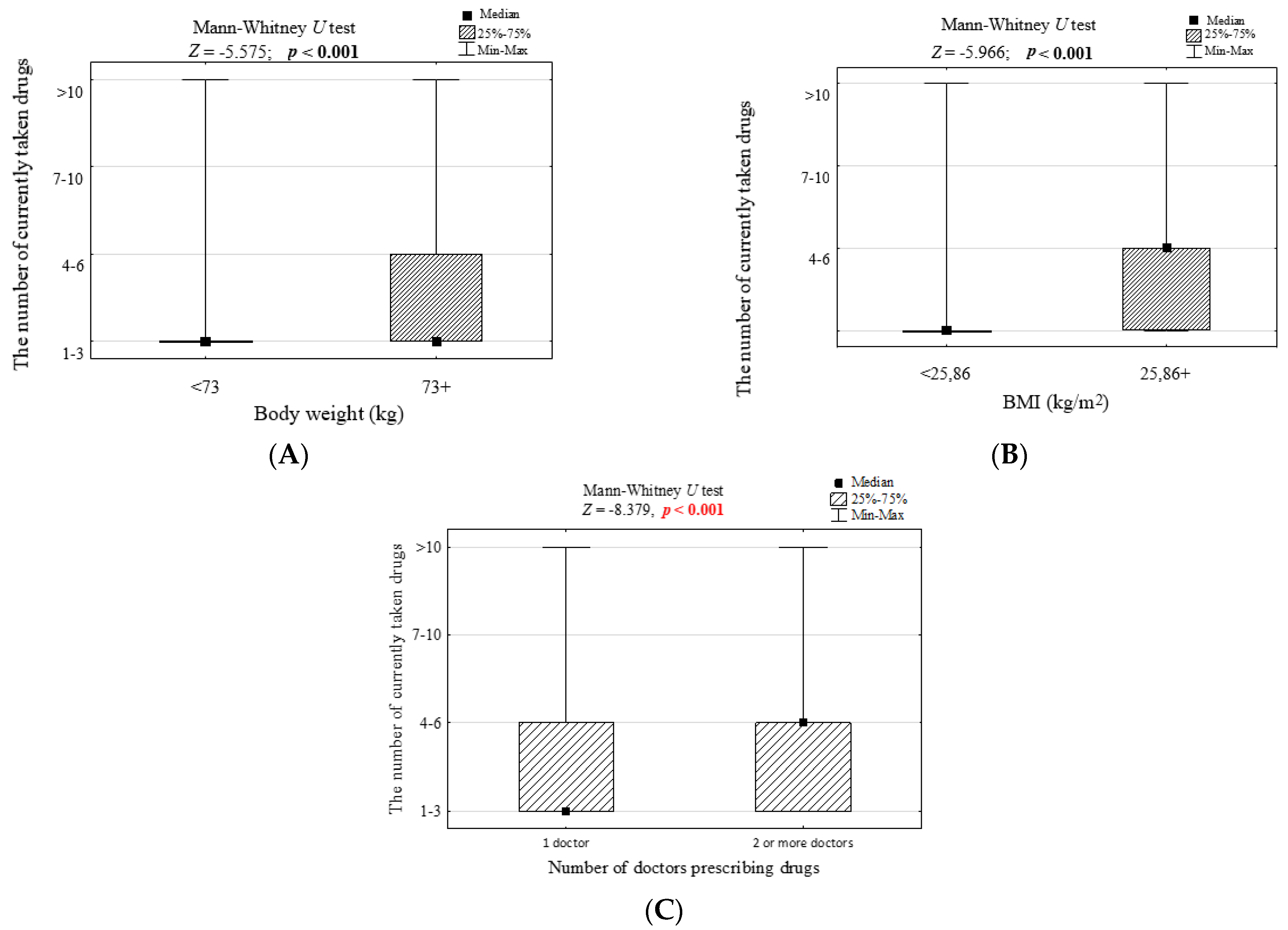
| 1 | 352 (70.4%) |
| 2 | 82 (16.4%) |
| 3 | 52 (10.4%) |
| 4 | 10 (2.0%) |
| 5 and more | 4 (0.8%) |
| 5. Do you inform your family doctor about all new medications? | |
| Yes | 391 (78.2%) |
| No | 109 (21.8%) |
| 6. Do you buy drugs and/or supplements without a prescription? | |
| Yes | 378 (75.6%) |
| No | 122 (24.4%) |
| 7. Please select over-the-counter medications/supplements: | |
| Painkillers (paracetamol, ibuprofen, acetylsalicylic acid, metamizole, ketoprofen, diclofenac) | 305 (61.0%) |
| Drugs for heartburn (proton pump inhibitors, for example: omeprazole, pantoprazole, etc.) | 132 (26.4%) |
| Herbal (St. John’s wort, ginseng, Ginkgo biloba) | 155 (31.0%) |
| Vitamins (C, B, D) | 345 (69.0%) |
| Other (magnesium, potassium, calcium, zinc, selenium) | 96 (19.2%) |
| Predictors of Taking More than 3 Drugs a Day | Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|---|
| Number of Drugs | p | OR (95% CI) | OR (95% CI) | ||||
| 4 or More n = 199 | 1–3 n = 301 | ||||||
| n | % | n | % | ||||
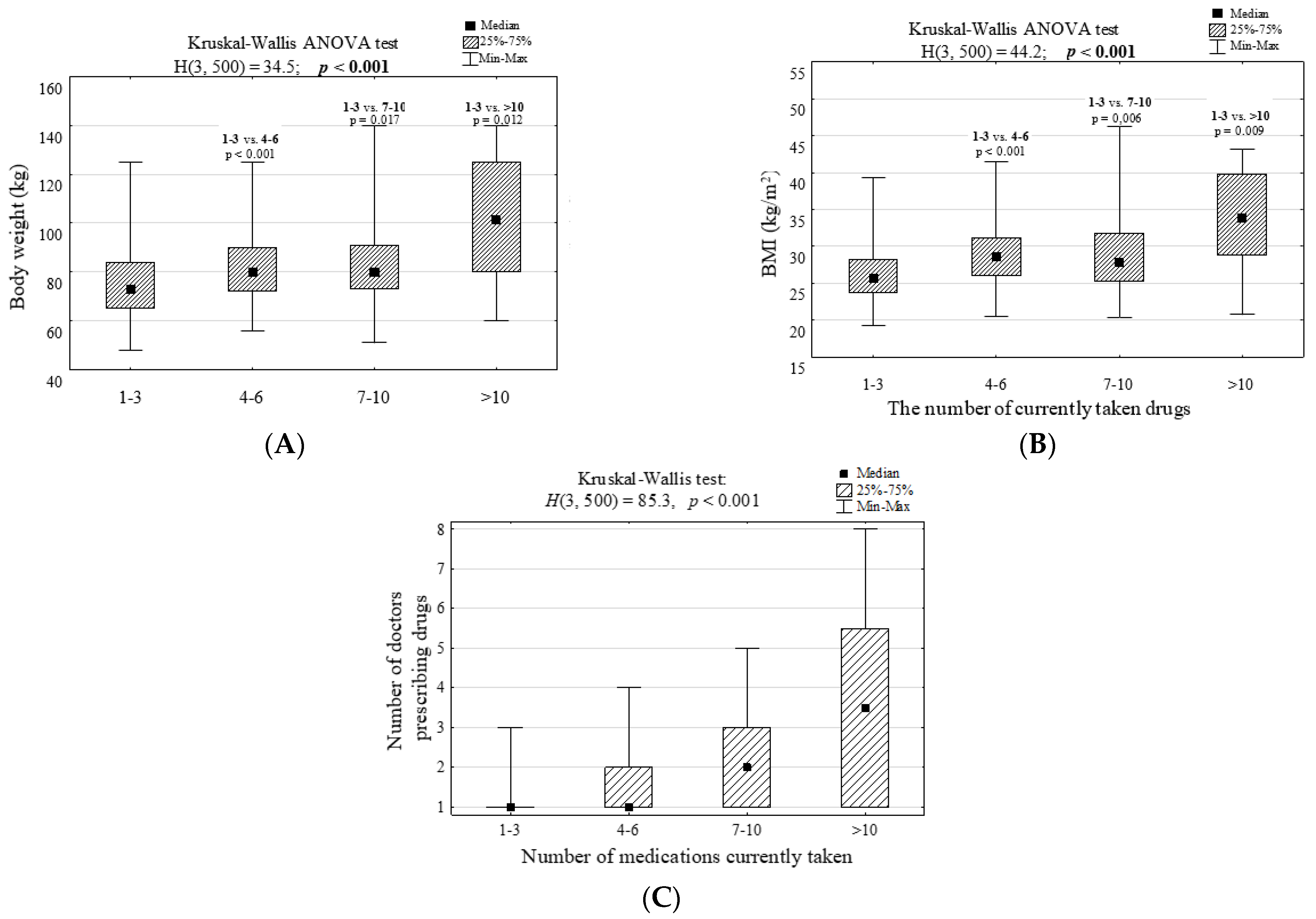
| Body mass > 73 kg | 150 | 75.4 | 152 | 50.5 | <0.001 | 3.00 (2.02–4.45) | 1.48 (0.83–2.61) |
| BMI > 25.9 kg/m2 | 152 | 76.4 | 146 | 48.5 | <0.001 | 3.43 (2.31–5.11) | 2.68 (1.50–4.77) |
| ADL < 5 pkt. | 195 | 98.0 | 298 | 99.0 | 0.444 | 0.49 (0.11–2.22) | 1.18 (0.16–8.62) |
| IADL < 24 pkt. | 78 | 39.2 | 140 | 46.5 | 0.118 | 0.74 (0.52–1.07) | 1.52 (0.92–2.50) |
| GDS-15 > 5 pkt. | 105 | 52.8 | 144 | 47.8 | 0.315 | 1.22 (0.85–1.74) | 1.28 (0.72–2.25) |
| GAS-10 > 7 pkt. | 101 | 50.8 | 100 | 33.2 | <0.001 | 2.07 (1.44–2.99) | 1.46 (0.90–2.36) |
| LSND-6 < 15 pkt. | 105 | 52.8 | 144 | 47.8 | 0.315 | 1.22 (0.85–1.74) | 0.94 (0.60–1.49) |
| MNA < 12 pkt. | 46 | 23.1 | 36 | 12.0 | 0.001 | 2.21 (1.37–3.57) | 1.83 (0.99–3.38) |
| CAD | 54 | 27.1 | 9 | 3.0 | <0.001 | 12.1 (5.80–25.2) | 6.77 (2.86–16.1) |
| Diabetes | 51 | 25.6 | 23 | 7.6 | <0.001 | 4.17 (2.45–7.08) | 3.23 (1.75–5.95) |
| Asthma | 30 | 15.1 | 13 | 4.3 | <0.001 | 3.93 (2.00–7.75) | 4.87 (2.13–11.1) |
| COPD | 20 | 10.1 | 13 | 4.3 | 0.016 | 2.48 (1.20–5.10) | 0.37 (0.14–1.02) |
| Heart failure | 56 | 28.1 | 15 | 5.0 | <0.001 | 7.47 (4.08–13.7) | 3.38 (1.59–7.19) |
| Kidney failure | 12 | 6.0 | 8 | 2.7 | 0.066 | 2.35 (0.94–5.86) | 1.62 (0.49–5.35) |
| GERD | 39 | 19.6 | 29 | 9.6 | 0.002 | 2.29 (1.36–3.84) | 1.93 (1.03–3.62) |
| Feature (Variable) | He/She Is Taking Medication for High Blood Pressure | p-Value | OR (95% CI) | Multivariate Logistic Regression | |||
|---|---|---|---|---|---|---|---|
| Yes n = 255 | No n = 245 | OR (95% CI) | |||||
| Body weight ≥ 75 kg | 169 | 66.3 | 111 | 45.3 | <0.001 | 2.37 (1.65–3.41) | 1.03 (0.63–1.66) |
| BMI ≥ 29.0 kg/m2 | 118 | 46.3 | 46 | 18.8 | <0.001 | 3.73 (2.49–5.58) | 3.12 (1.85–5.27) |
| IADL < 23 pts | 64 | 25.1 | 40 | 16.3 | 0.020 | 1.72 (1.10–2.67) | 1.18 (0.71–1.97) |
| AMTS < 9 pts | 65 | 25.5 | 39 | 15.9 | 0.011 | 1.81 (1.16–2.81) | 1.70 (1.04–2.78) |
| GDS ≥ 3 pts | 173 | 67.8 | 138 | 56.3 | 0.010 | 1.64 (1.14–2.36) | 1.20 (0.76–1.90) |
| GAS ≥ 6 pts | 161 | 63.1 | 129 | 52.7 | 0.019 | 1.54 (1.08–2.20) | 1.08 (0.69–1.72) |
| CAD | 54 | 21.2 | 9 | 3.7 | <0.001 | 7.04 (3.39–14.6) | 4.05 (1.79–9.21) |
| Diabetes | 58 | 22.7 | 16 | 6.5 | <0.001 | 4.21 (2.35–7.57) | 2.88 (1.53–5.43) |
| COPD | 24 | 9.4 | 9 | 3.7 | 0.011 | 2.72 (1.24–5.99) | 1.58 (0.64–3.95) |
| Heart failure | 58 | 22.7 | 13 | 5.3 | <0.001 | 5.25 (2.80–9.87) | 2.46 (1.18–5.13) |
| Feature (Variable) | He/She Is Taking Diuretics | p-Value | OR (95% CI) | Multivariate Logistic Regression | |||
|---|---|---|---|---|---|---|---|
| Yes n = 78 | No n = 422 | OR (95% CI) | |||||
| n | % | n | % | ||||
| Body weight ≥ 73 kg | 61 | 78.2 | 241 | 57.1 | <0.001 | 2.69 (1.52–4.77) | 2.52 (1.21–5.26) |
| BMI ≥ 25.6 kg/m2 | 57 | 73.1 | 249 | 59.0 | 0.022 | 1.89 (1.10–3.23) | 0.92 (0.46–1.86) |
| IADL < 23 pts | 27 | 34.6 | 77 | 18.2 | 0.002 | 2.37 (1.40–4.02) | 1.63 (0.92–2.91) |
| MNA < 14 pts | 55 | 70.5 | 234 | 55.5 | 0.017 | 1.92 (1.14–3.24) | 1.73 (0.97–3.08) |
| CAD | 24 | 30.8 | 39 | 9.2 | <0.001 | 4.36 (2.44–7.82) | 3.31 (1.64–6.68) |
| Diabetes | 20 | 25.6 | 54 | 12.8 | 0.005 | 2.35 (1.31–4.21) | 1.56 (0.83–2.96) |
| Heart failure | 21 | 26.9 | 50 | 11.8 | 0.001 | 2.74 (1.53–4.90) | 1.10 (0.54–2.26) |
| Feature (Variable) | He/She Is Taking Painkillers | p-Value | OR (95% CI) | Multivariate Logistic Regression | |||
|---|---|---|---|---|---|---|---|
| Yes n = 230 | No n = 270 | OR (95% CI) | |||||
| Higher education | 50 | 21.7 | 92 | 34.1 | 0.003 | 0.54 (0.36–0.80) | 0.81 (0.51–1.27) |
| Net income up to 2.000 PLN | 116 | 50.4 | 101 | 37.4 | 0.004 | 1.70 (1.19–2.43) | 1.47 (0.98–2.21) |
| BMI ≥ 25.8 kg/m2 | 150 | 65.2 | 150 | 55.6 | 0.028 | 1.50 (1.04–2.15) | 1.54 (1.04–2.29) |
| IADL < 24 pts | 80 | 34.8 | 59 | 21.9 | 0.001 | 1.91 (1.28–2.83) | 1.44 (0.93–2.23) |
| GDS ≥ 4 pts | 138 | 60.0 | 120 | 44.4 | 0.001 | 1.88 (1.31–2.68) | 0.84 (0.52–1.36) |
| GAS ≥ 9 pts | 114 | 49.6 | 66 | 24.4 | <0.001 | 3.04 (2.08–4.44) | 2.59 (1.58–4.26) |
| LSNS < 12 pts | 91 | 39.6 | 76 | 28.1 | 0.008 | 1.67 (1.15–2.43) | 1.48 (0.96–2.28) |
| GLS < 13 pts | 111 | 48.3 | 82 | 30.4 | <0.001 | 2.14 (1.48–3.08) | 2.08 (1.38–3.13) |
| MNA < 14 pts | 149 | 64.8 | 140 | 51.9 | 0.004 | 1.71 (1.19–2.45) | 1.07 (0.70–1.63) |
| Asthma | 26 | 11.3 | 17 | 6.3 | 0.047 | 1.90 (1.00–3.59) | 1.53 (0.76–3.09) |
| Feature (Variable) | He/She is Taking Anticoagulants | p-Value | OR (95% CI) | Multivariate Logistic Regression | |||
|---|---|---|---|---|---|---|---|
| Yes n = 87 | No n = 413 | OR (95% CI) | |||||
| Female | 40 | 46.0 | 250 | 60.5 | 0.017 | 0.55 (0.35–0,88) | 0.88 (0.51-1.53) |
| Lives with a partner or family | 49 | 56.3 | 182 | 44.1 | 0.044 | 1.64 (1.03–2.61) | 1.56 (0.93–2.62) |
| Body weight ≥ 81 kg | 50 | 57.5 | 136 | 32.9 | <0.001 | 2.75 (1.72–4.41) | 2.16 (1.09–4.27) |
| BMI ≥ 27.2 kg/m2 | 51 | 58.6 | 177 | 42.9 | 0.009 | 1.89 (1.18–3.02) | 0.95 (0.49–1.84) |
| CAD | 29 | 33.3 | 34 | 8.2 | <0.001 | 5.57 (3.16–9.83) | 1.97 (0.97–3.99) |
| COPD | 14 | 16.1 | 19 | 4.6 | <0.001 | 3.98 (1.91–8.29) | 2.11 (0.89–5.01) |
| Heart failure | 35 | 40.2 | 36 | 8.7 | <0.001 | 7.05 (4.07–12.2) | 4.41 (2.27–8.56) |
| Feature (Variable) | He/She Is Taking Antidepressants | p-Value | OR (95% CI) | Multivariate Logistic Regression | |||
|---|---|---|---|---|---|---|---|
| Yes n = 78 | No n = 422 | OR (95% CI) | |||||
| Lives in a city of over 400.000 inhabitants | 24 | 30.8 | 73 | 17.3 | 0.008 | 2.12 (1.23–3.66) | 2.18 (1.20–3.94) |
| GDS-15 ≥ 4 pts | 61 | 78.2 | 197 | 46.7 | <0.001 | 4.10 (2.32–7.25) | 1.95 (0.96–3.94) |
| GAS-10 ≥ 8 pts | 57 | 73.1 | 144 | 34.1 | <0.001 | 5.24 (3.06–8.99) | 2.91 (1.49–5.70) |
| GLS < 12 pts | 19 | 24.4 | 57 | 13.5 | 0.024 | 2.06 (1.15–3.71) | 1.11 (0.58–2.13) |
| MNA < 13 pts | 48 | 61.5 | 129 | 30.6 | <0.001 | 3.63 (2.20–6.00) | 2.64 (1.54–4.53) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pietraszek, A.; Agrawal, S.; Dróżdż, M.; Makuch, S.; Domański, I.; Dudzik, T.; Dudek, K.; Sobieszczańska, M. Sociodemographic and Health-Related Factors Influencing Drug Intake among the Elderly Population. Int. J. Environ. Res. Public Health 2022, 19, 8766. https://doi.org/10.3390/ijerph19148766
Pietraszek A, Agrawal S, Dróżdż M, Makuch S, Domański I, Dudzik T, Dudek K, Sobieszczańska M. Sociodemographic and Health-Related Factors Influencing Drug Intake among the Elderly Population. International Journal of Environmental Research and Public Health. 2022; 19(14):8766. https://doi.org/10.3390/ijerph19148766
Chicago/Turabian StylePietraszek, Alicja, Siddarth Agrawal, Mateusz Dróżdż, Sebastian Makuch, Igor Domański, Tomasz Dudzik, Krzysztof Dudek, and Małgorzata Sobieszczańska. 2022. "Sociodemographic and Health-Related Factors Influencing Drug Intake among the Elderly Population" International Journal of Environmental Research and Public Health 19, no. 14: 8766. https://doi.org/10.3390/ijerph19148766
APA StylePietraszek, A., Agrawal, S., Dróżdż, M., Makuch, S., Domański, I., Dudzik, T., Dudek, K., & Sobieszczańska, M. (2022). Sociodemographic and Health-Related Factors Influencing Drug Intake among the Elderly Population. International Journal of Environmental Research and Public Health, 19(14), 8766. https://doi.org/10.3390/ijerph19148766







