Replacing Sedentary Time with Physically Active Behaviour Predicts Improved Body Composition and Metabolic Health Outcomes
Abstract
:1. Introduction
2. Methods
2.1. Participants
2.2. Physical Activity
2.3. Anthropometry and Body Composition
2.4. Metabolic Biomarkers
2.5. Dietary Analysis
2.6. Statistical Analysis
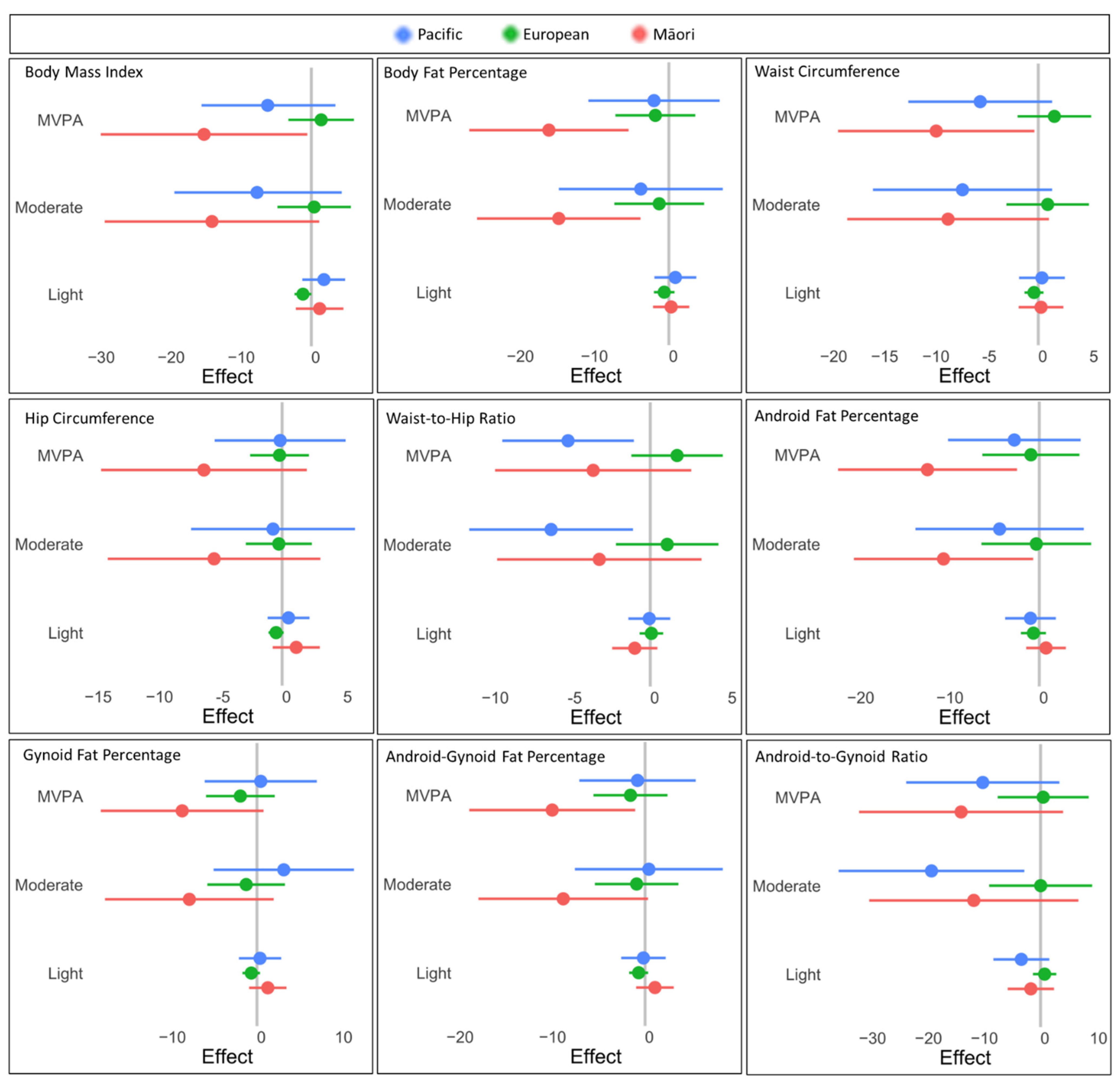
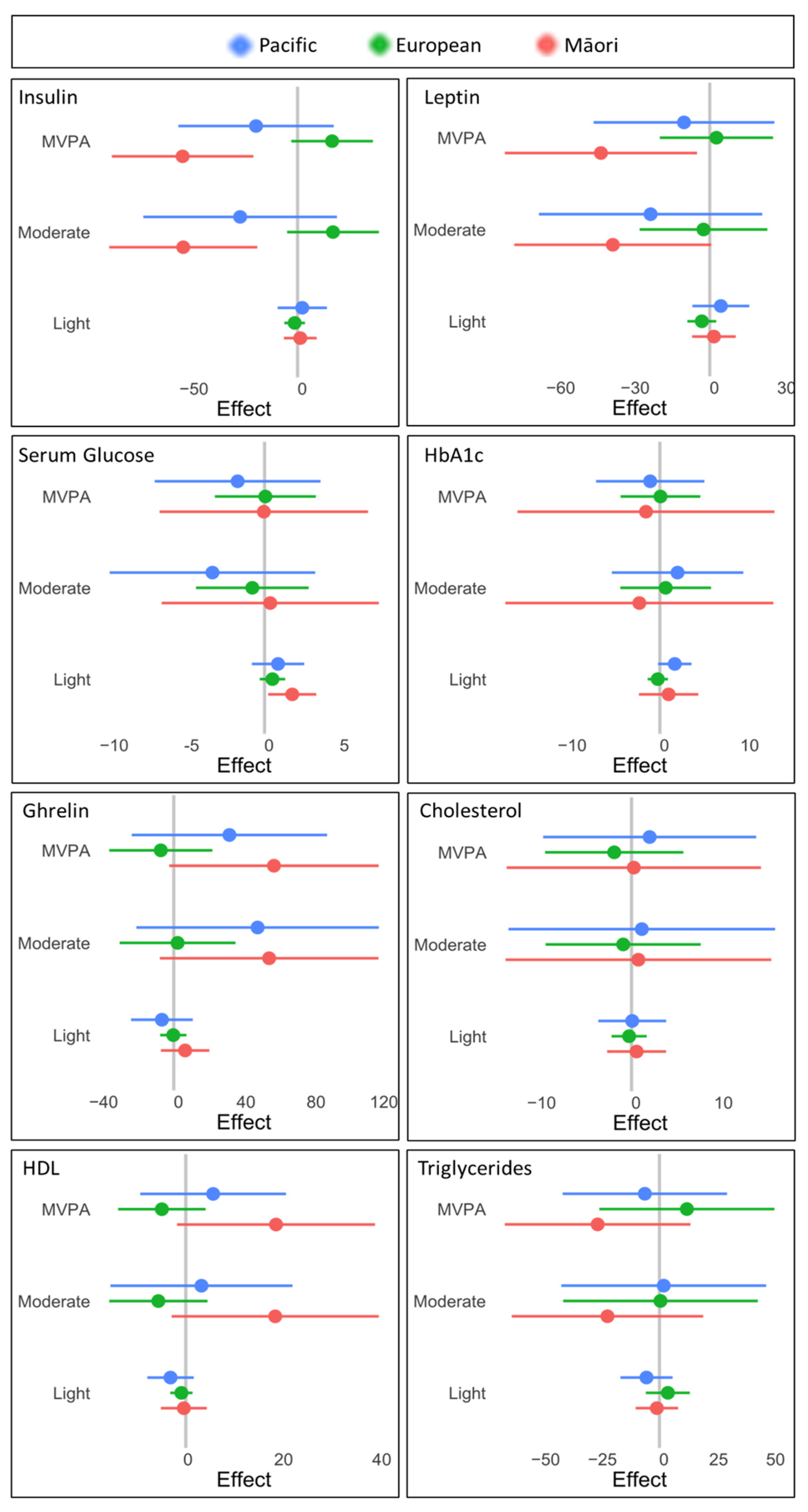
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Camhi, S.M.; Bray, G.A.; Bouchard, C.; Greenway, F.L.; Johnson, W.D.; Newton, R.L.; Ravussin, E.; Ryan, D.H.; Smith, S.R.; Katzmarzyk, P.T. The relationship of waist circumference and BMI to visceral, subcutaneous, and total body fat: Sex and race differences. Obesity 2011, 19, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Carroll, J.F.; Chiapa, A.L.; Rodriquez, M.; Phelps, D.R.; Cardarelli, K.M.; Vishwanatha, J.K.; Bae, S.; Cardarelli, R. Visceral fat, waist circumference, and BMI: Impact of race/ethnicity. Obesity 2008, 16, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Rush, E.C.; Freitas, I.; Plank, L.D. Body size, body composition and fat distribution: Comparative analysis of European, Maori, Pacific Island and Asian Indian adults. Br. J. Nutr. 2009, 102, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Sluyter, J.D.; Schaaf, D.; Scragg, R.K.R.; Plank, L.D. Body mass index and percent body fat in a New Zealand multi-ethnic adolescent population. Int. J. Pediatr. Obes. 2011, 6, 36–44. [Google Scholar] [CrossRef]
- Karnes, J.H.; Arora, A.; Feng, J.; Steiner, H.E.; Sulieman, L.; Boerwinkle, E.; Clark, C.; Cicek, M.; Cohn, E.; Gebo, K.; et al. Racial, ethnic, and gender differences in obesity and body fat distribution: An All of Us Research Program demonstration project. PLoS ONE 2021, 16, e0255583. [Google Scholar] [CrossRef]
- Ronn, P.F.; Andersen, G.S.; Lauritzen, T.; Christensen, D.L.; Aadahl, M.; Carstensen, B.; Jorgensen, M.E. Ethnic differences in anthropometric measures and abdominal fat distribution: A cross-sectional pooled study in Inuit, Africans and Europeans. J. Epidemiol. Community Health 2017, 71, 536–543. [Google Scholar] [CrossRef]
- Ministry of Health. Annual Data Explorer 2020/21: New Zealand Health Survey; Ministry of Health: Wellington, New Zealand, 2021.
- World Health Organization. World Health Organization: Obesity and Overweight Fact Sheet No. 311; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Sattelmair, J.; Pertman, J.; Ding, E.L.; Kohl, H.W., III; Haskell, W.L.; Lee, I.M. Dose response between physical activity and risk of coronary heart disease: A meta-analysis. Circulation 2011, 124, 789–795. [Google Scholar] [CrossRef] [Green Version]
- Grontved, A.; Pan, A.; Mekary, R.A.; Stampfer, M.J.; Willett, W.C.; Manson, J.E.; Hu, F.B. Muscle-strengthening and conditioning activities and risk of type 2 diabetes: A prospective study in two cohorts of US women. PLoS Med. 2014, 11, e1001587. [Google Scholar] [CrossRef]
- Mekary, R.A.; Willett, W.C.; Hu, F.B.; Ding, E.L. Isotemporal substitution paradigm for physical activity epidemiology and weight change. Am. J. Epidemiol. 2009, 170, 519–527. [Google Scholar] [CrossRef] [Green Version]
- Healy, G.N.; Winkler, E.A.H.; Owen, N.; Anuradha, S.; Dunstan, D.W. Replacing sitting time with standing or stepping: Associations with cardio-metabolic risk biomarkers. Eur. Heart J. 2015, 36, 2643–2649. [Google Scholar] [CrossRef] [Green Version]
- Buman, M.P.; Winkler, E.A.H.; Kurka, J.M.; Hekler, E.B.; Baldwin, C.M.; Owen, N.; Ainsworth, B.E.; Healy, G.N.; Gardiner, P.A. Reallocating time to sleep, sedentary behaviours, or active behaviours: Associations with cardiovascular disease risk biomarkers, NHANES 2005–2006. Am. J. Epidemiol. 2014, 179, 323–334. [Google Scholar] [CrossRef] [PubMed]
- De Lorenzo, A.; Bianchi, A.; Maroni, P.; Iannarelli, A.; Di Daniele, N.; Iacopino, L.; Di Renzo, L. Adiposity rather than BMI determines metabolic risk. Int. J. Cardiol. 2013, 166, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Bastien, M.; Poirier, P.; Lemieux, I.; Despres, J.P. Overview of epidemiology and contribution of obesity to cardiovascular disease. Prog. Cardiovasc. Dis. 2014, 56, 369–381. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Recommendations on Physical Activity for Health; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- OECD. Obesity Among Adults, 2013 (or Nearest Year); OECD Publishing: Paris, France, 2015. [Google Scholar]
- OECD. Obesity Update; OECD: Paris, France, 2017. [Google Scholar]
- Kruger, R.; Shultz, S.P.; McNaughton, S.A.; Russell, A.P.; Firestone, R.T.; George, L.; Beck, K.L.; Conlon, C.A.; von Hurst, P.R.; Breier, B.H.; et al. Predictors and risks of body fat profiles in young New Zealand European, Māori and Pacific women: Study protocol for the women’s EXPLORE study. SpringerPlus 2015, 4, 128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. BMI Classification. Available online: http://apps.who.int/bmi/index.jsp?introPage=intro_3.html (accessed on 14 January 2016).
- Okorodudu, D.O.; Jumean, M.F.; Montori, V.M.; Romero-Corral, A.; Somers, V.K.; Erwin, P.J.; Lopez-Jimenez, F. Diagnostic performance of body mass index to identify obesity as defined by body adiposity: A systematic review and meta-analysis. Int. J. Obes. 2010, 34, 791–799. [Google Scholar] [CrossRef] [Green Version]
- Tudor-Locke, C.; Camhi, S.M.; Troiano, R.P. A catalog of rules, variables, and definitions applied to accelerometer data in the National Health and Nutrition Examination Survey, 2003–2006. Prev. Chronic Dis. 2012, 9, E113. [Google Scholar] [CrossRef] [Green Version]
- Barreira, T.V.; Schuna, J.M., Jr.; Mire, E.F.; Katzmarzyk, P.T.; Chaput, J.-P.; Leduc, G.; Tudor-Locke, C. Identifying children’s nocturnal sleep using 24-h waist accelerometry. Med. Sci. Sports Exerc. 2015, 47, 937–943. [Google Scholar] [CrossRef]
- Troiano, R.P.; Berrigan, D.; Dodd, K.W.; Masse, L.C.; Tilert, T.; McDowell, M. Physical activity in the United States measured by accelerometer. Med. Sci. Sports Exerc. 2008, 40, 181–188. [Google Scholar] [CrossRef]
- Marfell-Jones, M.; Olds, T.; Stewart, A.; Carter, L. International Standards in Anthropometric Measurement; ISAK: Potchefstroom, South Africa, 2006. [Google Scholar]
- Siri, W.E. Body composition from fluid spaces and density. In Analysis of Methods. Techniques for Measuring Body Composition; Brozek, A.H.J., Ed.; Academy of Sciences, National Research Council: Washington, DC, USA, 1961; pp. 223–244. [Google Scholar]
- Beck, K.L.; Houston, Z.L.; McNaughton, S.A.; Kruger, R. Development and evaluation of a food frequency questionnaire to assess nutrient intakes of adult women in New Zealand. Nutr. Diet. 2020, 77, 253–259. [Google Scholar] [CrossRef] [Green Version]
- New Zealand Institute for Crop & Food Research. FOODfiles: Data Files of the New Zealand Food Composition Database; New Zealand Institute for Crop & Food Research: Auckland, New Zealand, 2006. [Google Scholar]
- Jorge, M.L.M.P.; de Oliveira, V.N.; Resende, N.M.; Paraiso, L.F.; Calixto, A.; Diniz, A.L.D.; Resende, E.S.; Ropelle, E.R.; Carvalheira, J.B.; Espindola, F.S.; et al. The effects of aerobic, resistance, and combined exercise on metabolic control, inflammatory markers, adipocytokines, and muscle insulin signaling in patients with type 2 diabetes mellitus. Metabolism 2011, 60, 1244–1252. [Google Scholar] [CrossRef]
- Ryan, B.J.; Schleh, M.W.; Ahn, C.; Ludzki, A.C.; Gillen, J.B.; Varshney, P.; Van Pelt, D.W.; Pitchford, L.M.; Chenevert, T.L.; Gioscia-Ryan, R.A.; et al. Moderate-Intensity Exercise and High-Intensity Interval Training Affect Insulin Sensitivity Similarly in Obese Adults. J. Clin. Endocrinol. Metab. 2020, 105, e2941–e2959. [Google Scholar] [CrossRef] [PubMed]
- Kleist, B.; Wahrburg, U.; Stehle, P.; Schomaker, R.; Greiwing, A.; Stoffel-Wagner, B.; Egert, S. Moderate Walking Enhances the Effects of an Energy-Restricted Diet on Fat Mass Loss and Serum Insulin in Overweight and Obese Adults in a 12-Week Randomized Controlled Trial. J. Nutr. 2017, 147, 1875–1884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabriel, B.M.; Zierath, J.R. The limits of exercise physiology: From performance to health. Cell Metab. 2017, 25, 1000–1011. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; Sparks, L.M. Metabolic flexibility in health and disease. Cell Metab. 2017, 25, 1027–1036. [Google Scholar] [CrossRef] [Green Version]
- Ministry of Health. 2014/15 New Zealand Health Survey: Results for Adults; Part 1: Health Status, Health Behaviours and Risk Factors; Ministry of Health: Wellington, New Zealand, 2015.
- Mathieu, P.; Poirier, P.; Pibarot, P.; Lemieux, I.; Despres, J.P. Visceral obesity the link among inflammation, hypertension, and cardiovascular disease. Hypertension 2009, 53, 577–584. [Google Scholar] [CrossRef] [Green Version]
- Coutinho, T.; Goel, K.; Corrêa de Sá, D.; Carter, R.E.; Hodge, D.O.; Kragelund, C.; Kanaya, A.M.; Zeller, M.; Park, J.S.; Kober, L.; et al. Combining body mass index with measures of central obesity in the assessment of mortality in subjects with coronary disease: Role of “Normal Weight Central Obesity”. J. Am. Coll. Cardiol. 2013, 61, 553–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rush, E.C.; Plank, L.D.; Coward, W.A. Energy expenditure of young Polynesian and European women in New Zealand and relations to body composition. Am. J. Clin. Nutr. 1999, 69, 43–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Dyck, D.; Cerin, E.; De Bourdeaudhuij, I.; Hinckson, E.; Reis, R.S.; Davey, R.; Sarmiento, O.L.; Mitas, J.; Troelsen, J.; MacFarlane, D.; et al. International study of objectively measured physical activity and sedentary time with body mass index and obesity: IPEN adult study. Int. J. Obes. 2015, 39, 199–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitaker, K.M.; Odegaard, A.O.; Jacobs, D.R.; Sidney, S.; Pereira, M.A. Sedentary behaviour, physical activity, and abdominal adipose tissue deposition. Med. Sci. Sports Exerc. 2017, 49, 450–458. [Google Scholar] [CrossRef] [Green Version]
- Neely, A.; Bray, M. Racial/ethnic Differences in Body Composition Measures and Exercise Parameters in the TIGER Study (P21-046-19). Curr. Dev. Nutr. 2019, 3, nzz041. [Google Scholar] [CrossRef] [Green Version]
- Migueles, J.H.; Cadenas-Sanchez, C.; Alcantara, J.M.A.; Leal-Martín, J.; Mañas, A.; Ara, I.; Glynn, N.W.; Shiroma, E.J. Calibration and Cross-Validation of Accelerometer Cut-Points to Classify Sedentary Time and Physical Activity from Hip and Non-Dominant and Dominant Wrists in Older Adults. Sensors 2021, 21, 3326. [Google Scholar] [CrossRef]
- Beale, C.; Rauff, E.L.; O’Brien, W.J.; Shultz, S.P.; Fink, P.W.; Kruger, R. Are all Sedentary Behaviours Equal? An Examination of Sedentary Behaviour and Associations with Indicators of Disease Risk Factors in Women. Int. J. Environ. Res. Public Health 2020, 17, 2643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tudor-Locke, C.; Schuna, J.M.J.; Han, H.; Aguiar, E.J.; Green, M.A.; Busa, M.A.; Larrivee, S.; Johnson, W.D. Step-based physical activity metrics and cardiometabolic risk: NHANES 2005-06. Med. Sci. Sports Exerc. 2017, 49, 283–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vella, C.A.; Allison, M.A.; Cushman, M.; Jenny, N.S.; Miles, M.P.; Larsen, B.; Lakoski, S.G.; Michos, E.D.; Blaha, M.J. Physical activity and adiposity-related inflammation: The MESA. Med. Sci. Sports Exerc. 2017, 49, 915–921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cloostermans, L.; Wendel-Vos, W.; Doornbos, G.; Howard, B.; Craig, C.L.; Kivimaeki, M.; Tabak, A.G.; Jefferis, B.J.; Ronkainen, K.; Brown, W.J.; et al. Independent and combined effects of physical activity and body mass index on the development of type 2 diabetes: A meta-analysis of 9 prospective cohort studies. Int. J. Behav. Nutr. Phys. Act. 2015, 12, 147. [Google Scholar] [CrossRef] [Green Version]
- Ross, R.; Janssen, I.; Dawson, J.; Kungl, A.M.; Kuk, J.L.; Wong, S.L.; Nguyen-Duy, T.B.; Lee, S.; Kilpatrick, K.; Hudson, R. Exercise-induced reduction in obesity and insulin resistance in women: A randomized controlled trial. Obes. Res. 2004, 12, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Keating, S.E.; Machan, E.A.; O’Connor, H.T.; Gerofi, J.A.; Sainsbury, A.; Caterson, I.D.; Johnson, N.A. Continuous exercise but not high intensity interval training improves fat distribution in overweight adults. J. Obes. 2014, 2014, 12. [Google Scholar] [CrossRef]
- Drenowatz, C.; Prasad, V.K.; Hand, G.A.; Shook, R.P.; Blair, S.N. Effects of moderate and vigorous physical activity on fitness and body composition. J. Behav. Med. 2016, 39, 624–632. [Google Scholar] [CrossRef]
- Kozey-Keadle, S.; Libertine, A.; Lyden, K.; Staudenmayer, J.; Freedson, P.S. Validation of wearable monitors for assessing sedentary behaviour. Med. Sci Sports Exerc. 2011, 43, 1561–1567. [Google Scholar] [CrossRef] [Green Version]
- Ekblom-Bak, E.; Ekblom, Ö.; Bergström, G.; Börjesson, M. Isotemporal substitution of sedentary time by physical activity of different intensities and bout lengths, and its associations with metabolic risk. Eur. J. Prev. Cardiol. 2016, 23, 967–974. [Google Scholar] [CrossRef]
- Hamer, M.; Stamatakis, E.; Steptoe, A. Effects of substituting sedentary time with physical activity on metabolic risk. Med. Sci. Sports Exerc. 2014, 46, 1946–1950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blair, S.N.; LaMonte, M.J.; Nichaman, M.Z. The evolution of physical activity recommendations: How much is enough? Am. J. Clin. Nutr. 2004, 79, 913–920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Brien, W.J.; Shultz, S.P.; Firestone, R.T.; George, L.; Breier, B.H.; Kruger, R. Exploring the challenges in obtaining physical activity data from women using hip-worn accelerometers. Eur. J. Sport Sci. 2017, 17, 922–930. [Google Scholar] [CrossRef] [PubMed]
| Healthy Reference Range | Total (n = 175) | Māori (n = 37) | Pacific (n = 54) | European (n = 84) | |
|---|---|---|---|---|---|
| Physical characteristics | |||||
| Age (y) | 32.4 ± 8.6 | 31.9 ± 8.5 | 31.6 ± 9.2 | 33.1 ± 8.3 | |
| Body mass (kg) | 86.4 ± 14.9 | 90.2 ± 17.0 | 93.1 ± 15.1 | 80.5 ± 11.2 ‡* | |
| Height (cm) | 166.5 ± 6.6 | 166.2 ± 4.3 | 167.4 ± 5.6 | 166.0 ± 6.9 | |
| BMI (kg/m2) | 22.0–24.9 | 31.2 ± 5 | 32.6 ± 5.8 | 33.2 ± 5.2 | 29.2 ± 3.7 ‡* |
| Body fat (%) | 18.0–29.9 | 39.7 ± 5.7 | 40.9 ± 5.8 | 40.2 ± 5.5 | 38.9 ± 5.7 |
| Waist (cm) | <80.0 | 90.8 ± 10.4 | 93.0 ± 11.0 | 95.0 ± 10.8 | 87.1 ± 8.6 ‡* |
| Waist-to-hip ratio | <0.8 | 0.8 ± 0.1 | 0.80 ± 0.06 | 0.81 ± 0.06 | 0.79 ± 0.06 * |
| Android fat (%) | 38.8 ± 5.2 | 40.5 (37.0, 44.5) | 39.3 (37.1, 43.0) | 38.3 (33.1, 41.4) ‡ | |
| Gynoid fat (%) | 39.5 ± 4.2 | 39.3 (37.4, 42.1) | 38.8 (37.1, 41.3) | 39.9 (36.4, 43.2) | |
| Metabolic health markers | |||||
| Systolic BP (mmHg) | <130 | 119 ± 11 | 119 ± 12 | 121 ± 10 | 117 ± 10 |
| Diastolic BP (mmHg) | <80 | 75 ± 9 | 77 ± 10 | 76 ± 10 | 74 ± 8 |
| HbA1c (mmol/mol) | <40.0 | 29.3 ± 3.9 | 30.4 ± 4.9 | 30.8 ± 3.2 | 27.8 ± 3.3 ‡* |
| Fasting glucose (mmol/L) | 3.5–5.4 | 4.8 ± 0.4 | 4.86 ± 0.47 | 4.88 ± 0.42 | 4.71 ± 0.41 |
| Insulin (mU/L) | 3.0–25.0 | 15.1 ± 9.5 | 16.7 ± 8.3 | 19.6 ± 11.8 | 11.3 ± 6.3 ‡* |
| Cholesterol (mmol/L) | <5.0 | 4.6 ± 0.9 | 4.59 ± 0.71 | 4.25 ± 0.83 | 4.81 ± 0.95 * |
| HDL-c (mmol/L) | >1.0 | 1.4 ± 0.4 | 1.31 ± 0.32 | 1.36 ± 0.34 | 1.55 ± 0.35 ‡* |
| Triglycerides (mmol/L) | <2.0 | 1.1 ± 0.9 | 1.34 ± 0.64 | 1.03 ± 0.64 | 1.06 ± 1.03 |
| LDL-c (mmol/L) | <3.4 | 2.7 ± 0.8 | 2.68 ± 0.71 | 2.42 ± 0.73 | 2.83 ± 0.92 * |
| Chol:HDL ratio | <4.5 | 3.4 ± 0.9 | 3.69 ± 0.92 | 3.26 ± 0.80 | 3.26 ± 0.93 ‡ |
| Activity Intensity (min/Day) | ||||||
|---|---|---|---|---|---|---|
| n | Sedentary | Light | Moderate | Vigorous | MVPA | |
| Māori | 37 | 450 ± 56 | 328 ± 80 | 25 ± 16 | 0 (0, 2) | 21 (16, 35) |
| Pacific | 54 | 490 ± 67 ‡ | 306 ± 66 | 23 ± 16 | 0 (0, 1) | 22 (11, 32) |
| European | 84 | 452 ± 75 * | 329 ± 82 | 34 ± 16 ‡* | 1 (0, 3) | 35 (23, 48) ‡* |
| p-value | 0.004 | 0.202 | <0.001 | 0.611 | <0.001 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Brien, W.J.; Rauff, E.L.; Shultz, S.P.; Sloughter, M.; Fink, P.W.; Breier, B.; Kruger, R. Replacing Sedentary Time with Physically Active Behaviour Predicts Improved Body Composition and Metabolic Health Outcomes. Int. J. Environ. Res. Public Health 2022, 19, 8760. https://doi.org/10.3390/ijerph19148760
O’Brien WJ, Rauff EL, Shultz SP, Sloughter M, Fink PW, Breier B, Kruger R. Replacing Sedentary Time with Physically Active Behaviour Predicts Improved Body Composition and Metabolic Health Outcomes. International Journal of Environmental Research and Public Health. 2022; 19(14):8760. https://doi.org/10.3390/ijerph19148760
Chicago/Turabian StyleO’Brien, Wendy J., Erica L. Rauff, Sarah P. Shultz, McLean Sloughter, Philip W. Fink, Bernhard Breier, and Rozanne Kruger. 2022. "Replacing Sedentary Time with Physically Active Behaviour Predicts Improved Body Composition and Metabolic Health Outcomes" International Journal of Environmental Research and Public Health 19, no. 14: 8760. https://doi.org/10.3390/ijerph19148760
APA StyleO’Brien, W. J., Rauff, E. L., Shultz, S. P., Sloughter, M., Fink, P. W., Breier, B., & Kruger, R. (2022). Replacing Sedentary Time with Physically Active Behaviour Predicts Improved Body Composition and Metabolic Health Outcomes. International Journal of Environmental Research and Public Health, 19(14), 8760. https://doi.org/10.3390/ijerph19148760







