Interobserver Agreement and Reference Intervals for Biventricular Myocardial Deformation in Full-Term, Healthy Newborns: A 2D Speckle-Tracking Echocardiography-Based Strain Analysis
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
3.1. Sample Characteristics
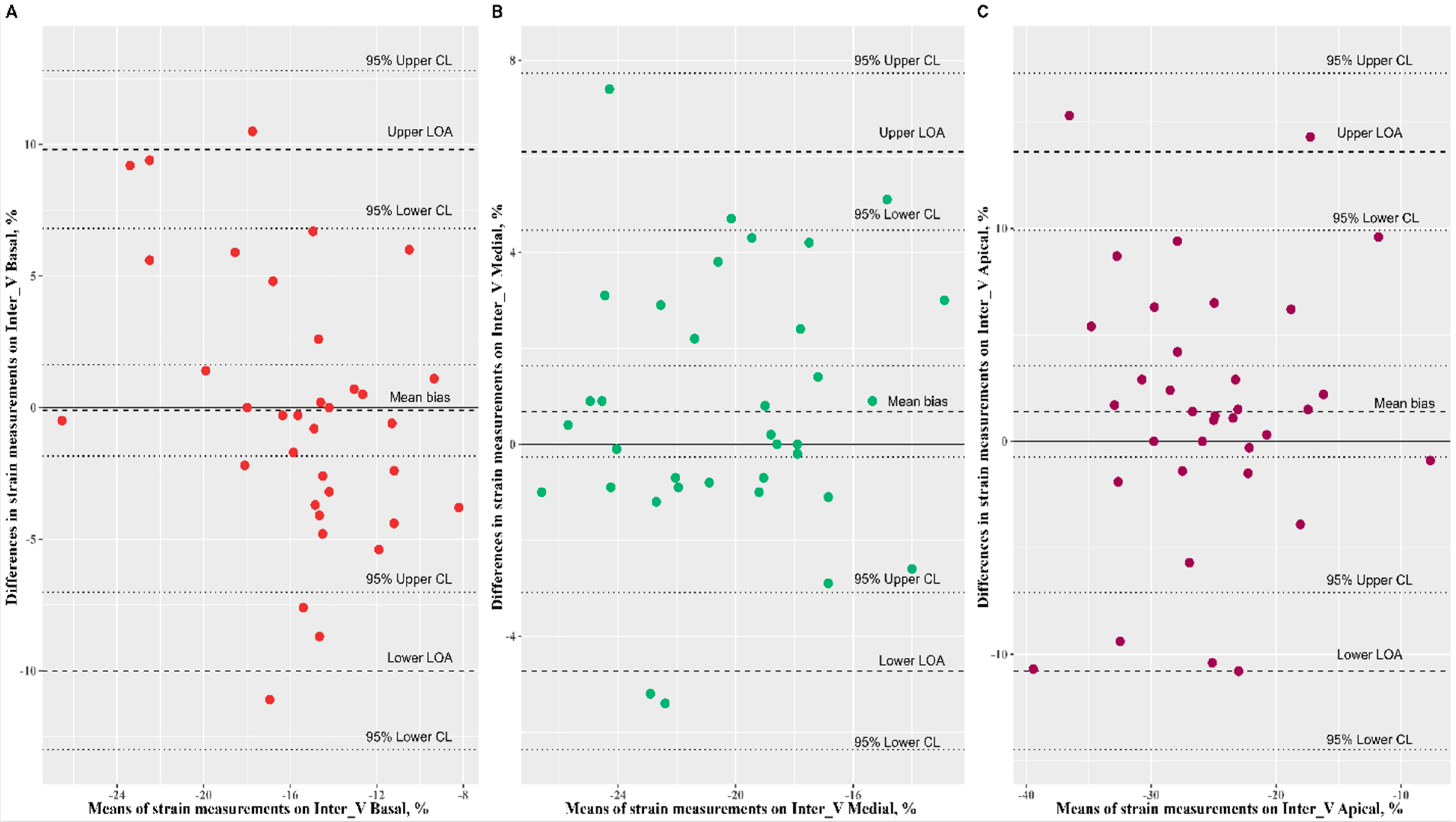
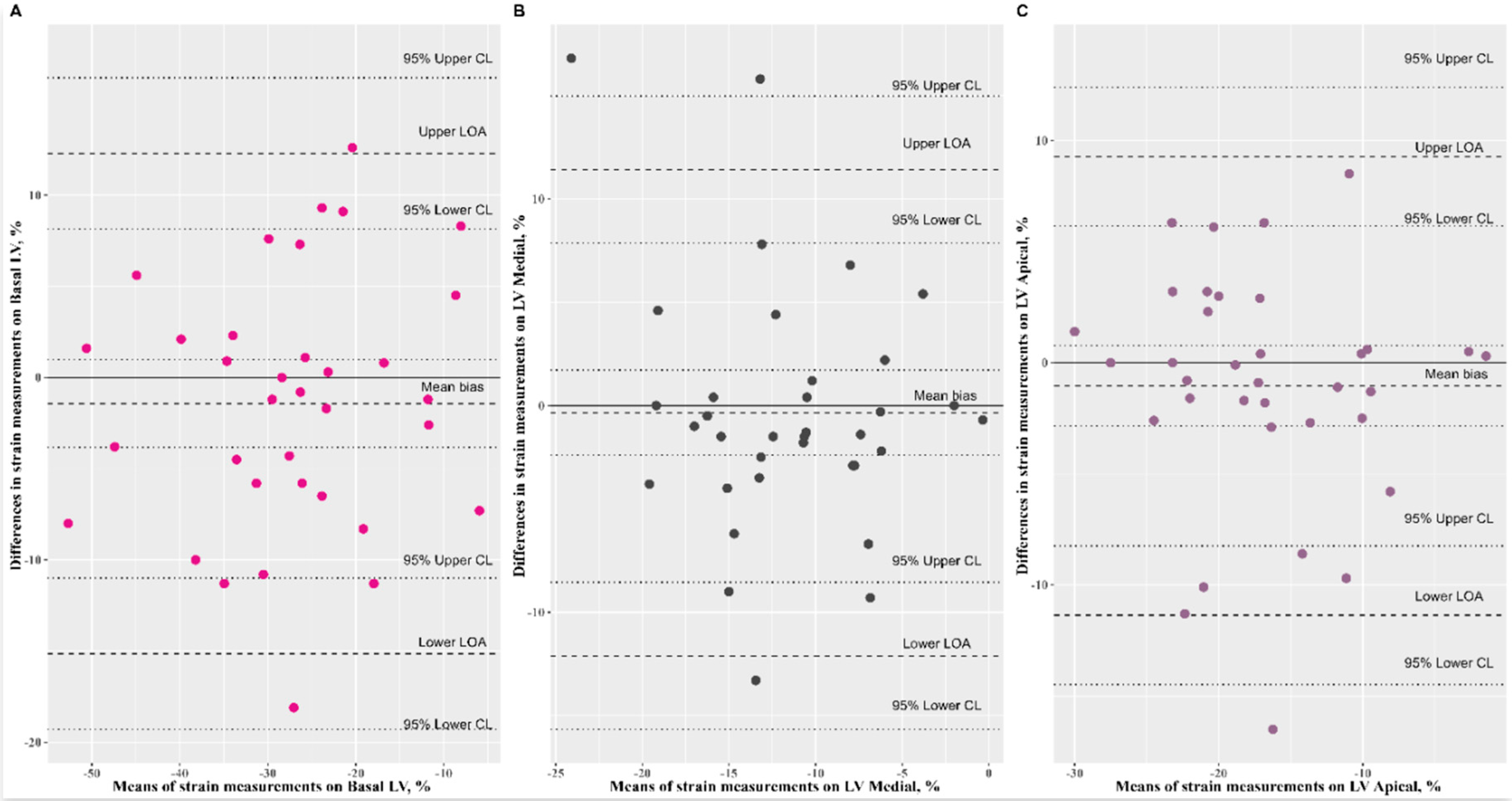
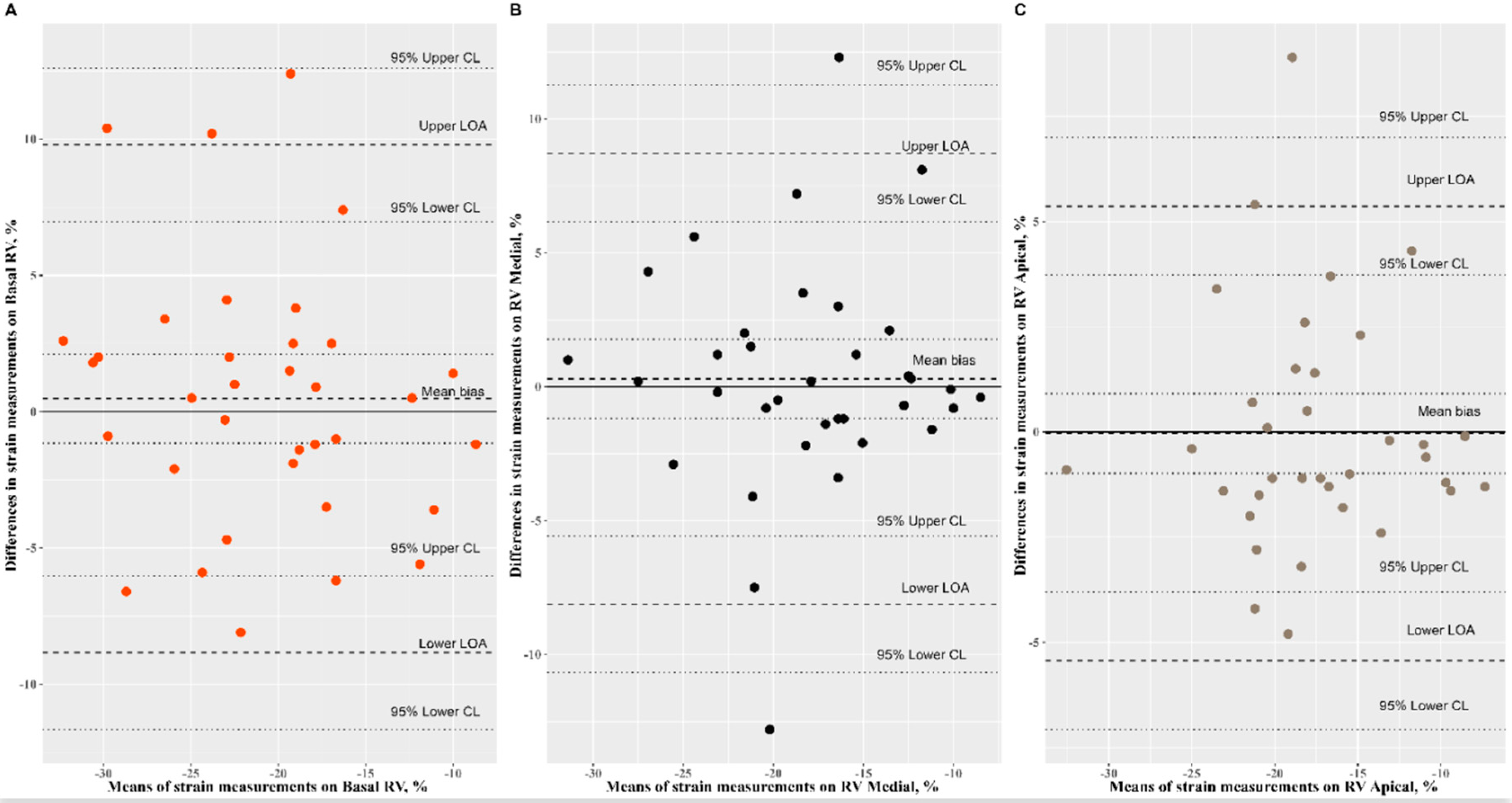
3.2. Reproducibility Analysis of Speckle-Tracking Parameters
3.3. Speckle-Tracking Strain Distribution
3.4. Speckle-Tracking Cardiac Parameters in Relation with Age, Gestational Age, Gender, and Weight
3.5. Reference Interval Estimation
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salte, I.M.; Østvik, A.; Smistad, E.; Melichova, D.; Nguyen, T.M.; Karlsen, S.; Brunvand, H.; Haugaa, K.H.; Edvardsen, T.; Lovstakken, L.; et al. Artificial Intelligence for Automatic Measurement of Left Ventricular Strain in Echocardiography. JACC Cardiovasc. 2021, 14, 1918–1928. [Google Scholar] [CrossRef]
- Jashari, H.; Rydberg, A.; Ibrahimi, P.; Bajraktari, G.; Kryeziu, L.; Jashari, F.; Henein, M.Y. Normal ranges of left ventricular strain in children: A meta-analysis. Cardiovasc. Ultrasound 2015, 13, 37. [Google Scholar] [CrossRef] [PubMed]
- Reisner, S.A.; Lysyansky, P.; Agmon, Y.; Mutlak, D.; Lessick, J.; Friedman, Z. Global longitudinal strain: A novel index of left ventricular systolic function. J. Am. Soc. Echocardiogr. 2004, 17, 630–633. [Google Scholar] [CrossRef] [PubMed]
- Khan, U.; Omdal, T.R.; Matre, K.; Greve, G. What is Left Ventricular Strain in Healthy Neonates? A Systematic Review and Meta-analysis. Pediatric Cardiol. 2020, 41, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Levy, P.T.; El-Khuffash, A.; Patel, M.D.; Breatnach, C.R.; James, A.T.; Sanchez, A.A.; Abuchabe, C.; Rogal, S.R.; Holland, M.R.; McNamara, P.J.; et al. Maturational Patterns of Systolic Ventricular Deformation Mechanics by Two-Dimensional Speckle-Tracking Echocardiography in Preterm Infants over the First Year of Age. J. Am. Soc. Echocardiogr. 2017, 30, 685–698. [Google Scholar] [CrossRef] [PubMed]
- Levy, P.T.; Holland, M.R.; Sekarski, T.J.; Hamvas, A.; Singh, G.K. Feasibility and reproducibility of systolic right ventricular strain measurement by speckle-tracking echocardiography in premature infants. J. Am. Soc. Echocardiogr. 2013, 26, 1201–1213. [Google Scholar] [CrossRef][Green Version]
- De Waal, K.; Lakkundi, A.; Othman, F. Speckle tracking echocardiography in very preterm infants: Feasibility and reference values. Early Hum. Dev. 2014, 90, 275–279. [Google Scholar] [CrossRef]
- Nasu, Y.; Oyama, K.; Nakano, S.; Matsumoto, A.; Soda, W.; Takahashi, S.; Chida, S. Longitudinal systolic strain of the bilayered ventricular septum during the first 72 hours of life in preterm infants. J. Echocardiogr. 2015, 13, 90–99. [Google Scholar] [CrossRef]
- El-Khuffash, A.; Schubert, U.; Levy, P.T.; Nestaas, E.; de Boode, W.P. European Special Interest Group ‘Neonatologist Performed Echocardiography’ (NPE). Deformation imaging and rotational mechanics in neonates: A guide to image acquisition, measurement, interpretation, and reference values. Pediatric Res. 2018, 84, 30–45. [Google Scholar] [CrossRef]
- Bunting, K.V.; Steeds, R.P.; Slater, L.T.; Rogers, J.K.; Gkoutos, G.V.; Kotecha, D. A Practical Guide to Assess the Reproducibility of Echocardiographic Measurements. J. Am. Soc. Echocardiogr. 2019, 32, 1505–1515. [Google Scholar] [CrossRef]
- Horn, P.S.; Feng, L.; Li, Y.; Pesce, A.J. Effect of outliers and nonhealthy individuals on reference interval estimation. Clin Chem. 2001, 47, 2137–2145. [Google Scholar] [CrossRef]
- Horn, P.S.; Pesce, A.J. Reference intervals: An update. Clin. Chim. Acta 2003, 334, 15–23. [Google Scholar] [CrossRef]
- Horn, P.S.; Pesce, A.J.; Copeland, B.E. A robust approach to reference interval estimation and evaluation. Clin. Chem. 1998, 44, 622–631. [Google Scholar] [CrossRef] [PubMed]
- Horn, P.S.; Pesce, A.J.; Copeland, B.E. Reference interval computation using robust vs parametric and nonparametric analyses. Clin. Chem. 1999, 45, 2284–2285. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria, 2022. Available online: https://www.R-project.org/ (accessed on 13 May 2022).
- Elkiran, O.; Karakurt, C.; Kocak, G.; Karadag, A. Tissue Doppler, strain, and strain rate measurements assessed by two-dimensional speckle-tracking echocardiography in healthy newborns and infants. Cardiol. Young 2014, 24, 201–211. [Google Scholar] [CrossRef]
- Lorch, S.M.; Ludomirsky, A.; Singh, G.K. Maturational and growth-related changes in left ventricular longitudinal strain and strain rate measured by two-dimensional speckle tracking echocardiography in healthy pediatric population. J. Am. Soc. Echocardiogr. 2008, 21, 1207–1215. [Google Scholar] [CrossRef]
- Marcus, K.A.; Mavinkurve-Groothuis, A.M.; Barends, M.; van Dijk, A.; Feuth, T.; de Korte, C.; Kapusta, L. Reference values for myocardial two-dimensional strain echocardiography in a healthy pediatric and young adult cohort. J. Am. Soc. Echocardiogr. 2011, 24, 625–636. [Google Scholar] [CrossRef]
- Schubert, U.; Müller, M.; Norman, M.; Abdul-Khaliq, H. Transition from fetal to neonatal life: Changes in cardiac function assessed by speckle-tracking echocardiography. Early Hum. Dev. 2013, 89, 803–808. [Google Scholar] [CrossRef]
- Klitsie, L.M.; Roest, A.A.; Haak, M.C.; Blom, N.A.; Ten Harkel, A.D. Longitudinal follow-up of ventricular performance in healthy neonates. Early Hum. Dev. 2013, 89, 993–997. [Google Scholar] [CrossRef]
- Struijk, P.C.; Mathews, V.J.; Loupas, T.; Stewart, P.A.; Clark, E.B.; Steegers, E.A.; Wladimiroff, J.W. Blood pressure estimation in the human fetal descending aorta. Ultrasound Obs. Gynecol. 2008, 32, 673–681. [Google Scholar] [CrossRef]
- Carasso, S.; Cohen, O.; Mutlak, D.; Adler, Z.; Lessick, J.; Reisner, S.A.; Rakowski, H.; Bolotin, G.; Agmon, Y. Differential effects of afterload on left ventricular long- and short-axis function: Insights from a clinical model of patients with aortic valve stenosis undergoing aortic valve replacement. Am. Heart J. 2009, 158, 540–545. [Google Scholar] [CrossRef] [PubMed]


| Neonates Characteristics | n = 103 |
|---|---|
| Age (days), median (IQR) | 3 (2, 4) |
| Gestational age (weeks) | 39 (38, 40) |
| Gender, n (%) | |
| Boy | 59 (57.28) |
| Girl | 44 (42.72) |
| Head_Circumference (cm) | 34.50 (33.50, 35.00) |
| Birth_Length (cm) | 54.20 ± 2.70 |
| Birth._Weight (g) | 3431.75 ± 467.50 |
| Apgar Score 1 min | 9 (9, 10) |
| Apgar Score 5 min | 10 (10, 10) |
| C-section (yes) | 22 (21.36) |
| Diagnosis | |
| Patients diagnosed with PFO ) | 35 (33.98) |
| Patients diagnosed with small ostium secundum ASD | 52 (50.49) |
| Patients diagnosed with small muscular VSD | 4 (3.88) |
| Patients diagnosed with Bicuspid Aortic Valve | 12 (11.65) |
| Systolic blood pressure (mmHg) | 72 (65, 76) |
| Diastolic blood pressure (mmHg) | 37 (35, 45) |
| Heart rate (beats per minute) | 134 (128, 138) |
| Echocardiographic parameters | |
| EF (%) | 68.73 ± 7.90 |
| TAPSE (mm) | 9.06 ± 1.76 |
| MAPSE (mm) | 6.80 (6.05, 7.95) |
| Echocardiographic Variables | Observer 1 | Observer 2 | Interobserver Reproducibility (n = 35) | |||
|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | ICC (95% CI) | Systematic Bias (95% CI) Bland–Altman | Intercept (95% CI) Passing–Bablock | Slope (95% CI) Passing–Bablock | |
| LV basal (%) | −28.01 ± 12.44 | −26.59 ± 11.54 | 0.83 (0.69, 0.91) | −1.43 (−3.83, 0.98) | 0.13 (−3.98, 3.78) | 0.98 (0.75, 1.23) |
| LV medial (%) | −11.72 ± 5.58 | −11.38 ± 6.51 | 0.52 (0.22, 0.72) | −0.34 (−2.40, 1.72) | 1.30 (−1.50, 5.81) | 1.00 (0.75, 1.38) |
| LV apical (%) | −17.36 ± 6.83 | −16.31 ± 7.26 | 0.72 (0.51, 0.85) | −1.05 (−2.85, 0.76) | 2.44 (−0.70, 8.25) | 1.11 (0.92, 1.43) |
| Inter V basal (%) | −15.60 ± 3.82 | −15.50 ± 5.48 | 0.43 (0.12, 0.67) | −0.10 (−1.84, 1.63) | 10.25 (0.01, 28.43) | 1.63 (0.98, 2.79) |
| Inter V medial (%) | −19.93 ± 3.88 | −20.62 ± 3.66 | 0.73 (0.52, 0.85) | 0.68 (−0.27, 1.63) | −1.41 (−6.31, 4.09) | 0.94 (0.70, 1.22) |
| Inter V apical (%) | −24.68 ± 7.75 | −26.08 ± 7.30 | 0.65 (0.42, 0.81) | 1.40 (−0.74, 3.54) | −0.90 (−8.04, 8.46) | 1.02 (0.71, 1.40) |
| RV basal (%) | −20.67 ± 6.21 | −21.15 ± 6.95 | 0.74 (0.55, 0.86) | 0.48 (−1.16, 2.11) | 2.03 (−2.65, 9.62) | 1.13 (0.88, 1.47) |
| RV medial (%) | −17.93 ± 5.89 | −18.22 ± 5.76 | 0.73 (0.53, 0.86) | 0.29 (−1.18, 1.77) | 0.68 (−2.85, 3.76) | 1.03 (0.82, 1.22) |
| RV apical (%) | −17.50 ± 5.41 | −17.46 ± 5.44 | 0.87 (0.77, 0.94) | −0.04 (−0.98, 0.91) | 0.62 (−1.60, 2.94) | 0.99 (0.86, 1.15) |
| LV pGLS (%) | −19.08 ± 3.66 | −18.94 ± 3.32 | 0.70 (0.48, 0.84) | −0.14 (−1.08, 0.80) | −1.65 (−6.30, 8.34) | 0.90 (0.66, 1.42) |
| RVFWSL (%) | −19.01 ± 5.67 | −19.26 ± 5.67 | 0.78 (0.61, 0.88) | 0.24 (−1.08, 1.56) | 0.85 (−2.96, 5.26) | 1.04 (0.84, 1.28) |
| RV4CSL (%) | −16.52 ± 3.99 | −16.34 ± 3.79 | 0.79 (0.62, 0.89) | −0.18 (−1.06, 0.70) | 0.13 (−3.98, 3.78) | 0.98 (0.75, 1.23) |
| Measurements | Descriptive Statistics | |||
|---|---|---|---|---|
| Mean (SD) | Range (Min, Max) | Median (IQR) | Distribution | |
| LV basal (%) | −29.43 (10.34) | −56.70 to −3.90 | −28.80 (−36.00, −22.90) | Gaussian |
| LV medial (%) | −12.17 (5.19) | −23.50 to −0.70 | −12.10 (−16.00, −8.85) | Gaussian |
| LV apical (%) | −18.72 (7.15) | −35.30 to −1.30 | −18.50 (−23.25, −14.20) | Gaussian |
| Inter V basal (%) | −15.28 (5.23) | −40.40 to −5.60 | −14.70 (−17.90, −12.10) | nonGaussian |
| Inter V medial (%) | −19.57 (3.37) | −27.30 to −11.40 | −19.30 (−21.85, −17.45) | Gaussian |
| Inter V apical (%) | −26.66 (6.61) | −45.90 to −7.00 | −26.50 (−30.05, −22.95) | nonGaussian |
| RV basal (%) | −21.07 (6.06) | −40.02 to −0.10 | −21.10 (−24.50, −18.10) | Gaussian |
| RV medial (%) | −18.18 (5.06) | −30.09 to −7.1 | −17.70 (−21.55, −16.05) | Gaussian |
| RV apical (%) | −17.89 (4.64) | −33.00 to −0.20 | −18.40 (−21.05, −14.65) | Gaussian |
| LV pGLS (%) | −19.85 (2.87) | −30.30 to −9.10 | −20.10 (−21.50, −18.10) | nonGaussian |
| RVFWSL (%) | −19.38 (4.94) | −31.50 to −5.30 | −19.60 (−22.80, −17.10) | Gaussian |
| RV4CSL (%) | −16.97 (3.66) | −27.10 to −8.00 | −16.70 (−18.80, −15.00) | Gaussian |
| Measurements | RI Type | 95% RI with Outlier Removal | 95% RI without Outlier Removal | ||||
|---|---|---|---|---|---|---|---|
| RI (LL–UL) | 90% CI for LL | 90% CI for UL | RI (LL–UL) | 90% CI for LL | 90% CI for UL | ||
| LV basal (%) (a) | Parametric | −48.53 to −10.30 | −51.26 to −45.80 | −13.03 to −7.57 | −49.70 to −9.16 | −52.56 to −46.83 | −12.03 to −6.30 |
| Robust | −48.66 to −9.66 | −51.81 to −45.92 | −12.33 to −7.06 | −49.79 to −8.47 | −53.09 to −46.93 | −11.24 to −5.41 | |
| LV medial (%) | Parametric | Na | Na | Na | −22.34 to −1.99 | −23.78 to −20.90 | −3.43 to −0.56 |
| Robust | Na | Na | Na | −22.58 to −1.87 | −23.98 to −21.26 | −3.16 to −0.35 | |
| LV apical (%) | Parametric | Na | Na | Na | −32.74 to −4.70 | −34.72 to −30.76 | −6.68 to −2.72 |
| Robust | Na | Na | Na | −33.09 to −4.54 | −35.21 to −31.19 | −6.32 to −2.43 | |
| Inter V basal (%) (b) | Nonparametric | −26.90 to −7.41 | −29.83 to −26.29 | −8.31 to −6.38 | −27.20 to −6.42 | −29.88 to −14.00 | −7.24 to −5.16 |
| Inter V medial (%) | Parametric | Na | Na | Na | −26.17 to −12.98 | −27.10 to −25.24 | −13.91 to −12.98 |
| Robust | Na | Na | Na | −26.30 to −12.83 | −27.34 to −25.35 | −13.69 to −12.83 | |
| Inter V apical (%) | Nonparametric | −37.48 to −15.37 | −40.44 to −35.85 | −16.44 to −12.94 | −44.26 to −9.30 | −51.12 to −42.62 | −11.60 to −2.96 |
| RV basal (%) (b) | Parametric | −31.58 to −10.87 | −33.06 to −30.09 | −12.35 to −9.38 | −32.95 to −9.20 | −34.62 to −31.27 | −10.88 to −7.52 |
| Robust | −31.81 to −10.73 | −33.40 to −30.43 | −12.12 to −9.06 | −33.30 to −9.16 | −35.67 to −31.66 | −10.87 to −6.77 | |
| RV medial (%) (c) | Parametric | −27.25 to −9.93 | −28.51 to −25.99 | −11.19 to −8.67 | −32.95 to −9.20 | −34.62 to −31.27 | −10.88 to −7.52 |
| Robust | −27.33 to −9.47 | −28.82 to −25.95 | −10.65 to −8.19 | −28.30 to −8.02 | −29.90 to −26.96 | −9.38 to −6.48 | |
| RV apical (%) (d) | Parametric | −26.39 to −9.10 | −27.62 to −25.16 | −10.33 to −7.87 | −26.99 to −8.80 | −28.27 to −25.70 | −10.08 to −7.51 |
| Robust | −27.73 to −9.03 | −27.93 to −25.67 | −10.23 to −7.56 | −27.21 to −8.60 | −28.74 to −26.07 | −9.88 to −6.99 | |
| LV pGLS (%) (b) | Nonparametric | −24.65 to −14.62 | −25.21 to −24.10 | −15.14 to −12.24 | −24.90 to −13.18 | −25.62 to −19.50 | −17.26 to −10.48 |
| RVFWSL (%) (b) | Parametric | −28.11 to −10.45 | −29.38 to −26.85 | −11.72 to −9.18 | −29.06 to −9.69 | −30.43 to −27.70 | −11.06 to −8.32 |
| Robust | −28.69 to −10.68 | −30.09 to −27.64 | −11.99 to −9.85 | −29.57 to −8.60 | −31.23 to −28.37 | −11.21 to −8.17 | |
| RV4CSL (%) (e) | Parametric | −22.30 to −11.37 | −23.11 to −21.49 | −12.18 to −10.57 | −24.15 to −9.80 | −25.17 to −23.14 | −10.81 to −8.78 |
| Robust | −22.45 to −11.31 | −23.35 to −21.70 | −12.09 to −10.42 | −24.13 to −9.50 | −25.37 to −23.16 | −10.52 to −8.25 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toma, D.; Toganel, R.; Fagarasan, A.; Cucerea, M.; Gabor-Miklosi, D.; Cerghit-Paler, A.; Iurian, D.-R.; Gozar, H.; Moldovan, E.; Iancu, M.; et al. Interobserver Agreement and Reference Intervals for Biventricular Myocardial Deformation in Full-Term, Healthy Newborns: A 2D Speckle-Tracking Echocardiography-Based Strain Analysis. Int. J. Environ. Res. Public Health 2022, 19, 8620. https://doi.org/10.3390/ijerph19148620
Toma D, Toganel R, Fagarasan A, Cucerea M, Gabor-Miklosi D, Cerghit-Paler A, Iurian D-R, Gozar H, Moldovan E, Iancu M, et al. Interobserver Agreement and Reference Intervals for Biventricular Myocardial Deformation in Full-Term, Healthy Newborns: A 2D Speckle-Tracking Echocardiography-Based Strain Analysis. International Journal of Environmental Research and Public Health. 2022; 19(14):8620. https://doi.org/10.3390/ijerph19148620
Chicago/Turabian StyleToma, Daniela, Rodica Toganel, Amalia Fagarasan, Manuela Cucerea, Dorottya Gabor-Miklosi, Andreea Cerghit-Paler, Diana-Ramona Iurian, Horea Gozar, Elena Moldovan, Mihaela Iancu, and et al. 2022. "Interobserver Agreement and Reference Intervals for Biventricular Myocardial Deformation in Full-Term, Healthy Newborns: A 2D Speckle-Tracking Echocardiography-Based Strain Analysis" International Journal of Environmental Research and Public Health 19, no. 14: 8620. https://doi.org/10.3390/ijerph19148620
APA StyleToma, D., Toganel, R., Fagarasan, A., Cucerea, M., Gabor-Miklosi, D., Cerghit-Paler, A., Iurian, D.-R., Gozar, H., Moldovan, E., Iancu, M., & Gozar, L. (2022). Interobserver Agreement and Reference Intervals for Biventricular Myocardial Deformation in Full-Term, Healthy Newborns: A 2D Speckle-Tracking Echocardiography-Based Strain Analysis. International Journal of Environmental Research and Public Health, 19(14), 8620. https://doi.org/10.3390/ijerph19148620







