Clustering of Environmental Parameters and the Risk of Acute Myocardial Infarction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Setting
2.2. Study Population
2.3. Environmental Data
2.4. Statistical Analysis
3. Results
3.1. Environmental Clusters
3.2. Study Population
3.3. Association of Environmental Clusters with Incidence of AMI
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Cardiovascular Diseases (CVDs). 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 14 February 2022).
- Roth, G.A.; Johnson, C.; Abajobir, A.; Abd-Allah, F.; Abera, S.F.; Abyu, G.; Ahmed, M.; Aksut, B.; Alam, T.; Alam KAlla, F. Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. J. Am. Coll. Cardiol. 2017, 70, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Zanobetti, A.; Peters, A. Disentangling interactions between atmospheric pollution and weather. J. Epidemiol. Community Health. 2014, 69, 613–615. [Google Scholar] [CrossRef] [PubMed]
- Hamanaka, R.B.; Mutlu, G.M. Particulate Matter Air Pollution: Effects on the Cardiovascular System. Front. Endocrinol. 2018, 9, 680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoek, G.; Krishnan, R.M.; Beelen, R.; Peters, A.; Ostro, B.; Brunekreef, B.; Kaufman, J.D. Long-term air pollution exposure and cardio- respiratory mortality: A review. Environ. Health 2013, 12, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- GBD 2017 Risk Factor Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1923–1994. [Google Scholar]
- Gasparrini, A.; Guo, Y.; Hashizume, M.; Lavigne, E.; Zanobetti, A.; Schwartz, J.; Tobias, A.; Tong, S.; Rocklöv, J.; Forsberg, B.; et al. Mortality risk attributable to high and low ambient temperature: A multicountry observational study. Lancet 2015, 386, 369–375. [Google Scholar] [CrossRef]
- Bhaskaran, K.; Hajat, S.; Haines, A.; Herrett, E.; Wilkinson, P.; Smeeth, L. Effects of ambient temperature on the incidence of myocardial infarction. Heart 2009, 95, 1760–1769. [Google Scholar] [CrossRef]
- Sun, Z.; Chen, C.; Xu, D.; Li, T. Effects of ambient temperature on myocardial infarction: A systematic review and meta-analysis. Environ. Pollut. 2018, 241, 1106–1114. [Google Scholar] [CrossRef]
- Turner, L.R.; Barnett, A.G.; Connell, D.; Tonga, S. Ambient temperature and cardiorespiratory morbidity: A systematic review and meta-analysis. Epidemiology 2012, 23, 594–606. [Google Scholar] [CrossRef] [Green Version]
- Claeys, M.J.; Rajagopalan, S.; Nawrot, T.S.; Brook, R.D. Climate and environmental triggers of acute myocardial infarction. Eur. Heart J. 2017, 38, 955–960. [Google Scholar] [CrossRef] [Green Version]
- Cheong, K.H.; Ngiam, N.J.; Morgan, G.G.; Pek, P.P.; Tan, B.Y.-Q.; Lai, J.W.; Koh, J.M.; Ong, M.E.H.; Ho, A.F.W. Acute Health Impacts of the Southeast Asian Transboundary Haze Problem—A Review. Int. J. Environ. Res. Public Health 2019, 16, 3286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sastry, N. Forest fires, air pollution, and mortality in southeast Asia. Demography 2002, 39, 1–23. [Google Scholar] [CrossRef]
- Sunchindah, A. Transboundary Haze Pollution Problem in Southeast Asia: Reframing ASEAN’s Response; Economic Research Institute for ASEAN and East Asia (ERIA): Jakarta, Indonesia, 2015. [Google Scholar]
- Marlier, M.E.; DeFries, R.S.; Voulgarakis, A.; Kinney, P.L.; Randerson, J.T.; Shindell, D.; Chen, Y.; Faluvegi, G. El Niño and health risks from landscape fire emissions in southeast Asia. Nat. Clim. Chang. 2012, 3, 131–136. [Google Scholar] [CrossRef] [Green Version]
- Reddington, C.L.; Yoshioka, M.; Balasubramanian, R.; Ridley, D.; Toh, Y.Y.; Arnold, S.; Spracklen, D.V. Contribution of vegetation and peat fires to particulate air pollution in Southeast Asia. Environ. Res. Lett. 2014, 9, 094006. [Google Scholar] [CrossRef] [Green Version]
- Ramakreshnan, L.; Aghamohammadi, N.; Fong, C.S.; Bulgiba, A.; Zaki, R.A.; Wong, L.P.; Sulaiman, N.M. Haze and health impacts in ASEAN countries: A systematic review. Environ. Sci. Pollut. Res. 2017, 25, 2096–2111. [Google Scholar] [CrossRef] [PubMed]
- Department of Statistics Singapore DSS. Population and Households Singapore: Government of Singapore. 2022. Available online: https://www.singstat.gov.sg/publications/reference/singapore-in-figures/population-and-households (accessed on 14 February 2022).
- Meteorological Service Singapore MSS. Climate of Singapore Singapore Singapore Government. Available online: http://www.weather.gov.sg/climate-climate-of-singapore/ (accessed on 13 February 2022).
- Health Promotion Board HPB. Singapore Myocardial Infarction Registry Annual Report 2019; Office NRoD, Ed.; Health Promotion Board: Singapore, 2021. [Google Scholar]
- Tunstall-Pedoe, H.; Kuulasmaa, K.; Amouyel, P.; Arveiler, D.; Rajakangas, A.M.; Pajak, A. Myocardial infarction and coronary deaths in the World Health Organization MONICA Project. Registration procedures, event rates, and case-fatality rates in 38 populations from 21 countries in four continents. Circulation 1994, 90, 583–612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Environmental Agency NEA. Frequently Asked Questions Singpore. 2020. Available online: https://www.nea.gov.sg/our-services/pollution-control/air-pollution/faqs (accessed on 13 February 2022).
- Armstrong, B.G.; Gasparrini, A.; Tobias, A. Conditional Poisson models: A flexible alternative to conditional logistic case cross-over analysis. BMC Med. Res. Methodol. 2014, 14, 122. [Google Scholar] [CrossRef] [Green Version]
- Phung, D.; Thai, P.K.; Guo, Y.; Morawska, L.; Rutherford, S.; Chu, C. Ambient temperature and risk of cardiovascular hospitalization: An updated systematic review and meta-analysis. Sci. Total Environ. 2016, 550, 1084–1102. [Google Scholar] [CrossRef]
- Barnett, A.G.; Dobson, A.J.; McElduff, P.; Salomaa, V.; Kuulasmaa, K.; Sans, S. Cold periods and coronary events: An analysis of populations worldwide. J. Epidemiol. Commun. Health 2005, 59, 551–557. [Google Scholar] [CrossRef] [Green Version]
- Michelozzi, P.; Accetta, G.; De Sario, M.; D’Ippoliti, D.; Marino, C.; Baccini, M.; Biggeri, A.; Anderson, H.R.; Katsouyanni, K.; Ballester, F.; et al. High Temperature and Hospitalizations for Cardiovascular and Respiratory Causes in 12 European Cities. Am. J. Respir. Crit. Care Med. 2009, 179, 383–389. [Google Scholar] [CrossRef] [Green Version]
- Goggins, W.B.; Chan, E.Y.; Yang, C.-Y. Weather, pollution, and acute myocardial infarction in Hong Kong and Taiwan. Int. J. Cardiol. 2013, 168, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Wu, P.J.; Chang, C.J.; Lee, C.H.; Chung, W.J.; Chen, T.Y.; Tseng, C.H.; Wu, C.C.; Cheng, C.I. Weather Impact on Acute Myocardial Infarction Hospital Admissions with a New Model for Prediction: A Nationwide Study. Front. Cardiovasc. Med. 2021, 8, 725419. [Google Scholar] [CrossRef] [PubMed]
- Dang, T.A.T.; Wraith, D.; Bambrick, H.; Dung, N.; Truc, T.T.; Tong, S.; Naish, S.; Dunne, M.P. Short-term effects of temperature on hospital admissions for acute myocardial infarction: A comparison between two neighboring climate zones in Vietnam. Environ. Res. 2019, 175, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Phung, D.; Guo, Y.; Thai, P.; Rutherford, S.; Wang, X.; Nguyen, M.; Do, C.M.; Nguyen, N.H.; Alam, N.; Chu, C. The effects of high temperature on cardiovascular admissions in the most populous tropical city in Vietnam. Environ. Pollut. 2016, 208, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Giang, P.N.; Van Dung, D.; Giang, K.B.; Van Vinhc, H.; Rocklöv, J. The effect of temperature on cardiovascular disease hospital admissions among elderly people in Thai Nguyen Province, Vietnam. Glob. Health Action 2014, 7, 23649. [Google Scholar] [CrossRef] [Green Version]
- Bourdrel, T.; Bind, M.-A.; Béjot, Y.; Morel, O.; Argacha, J.-F. Cardiovascular effects of air pollution. Arch. Cardiovasc. Dis. 2017, 110, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Bhaskaran, K.; Hajat, S.; Haines, A.; Herrett, E.; Wilkinson, P.; Smeeth, L. Effects of air pollution on the incidence of myocardial infarction. Heart 2009, 95, 1746–1759. [Google Scholar] [CrossRef] [Green Version]
- Mustafic, H.; Jabre, P.; Caussin, C.; Murad, M.H.; Escolano, S.; Tafflet, M.; Périer, M.; Marijon, E.; Vernerey, D.; Empana, J.; et al. Main air pollutants and myocardial infarction: A systematic review and meta-analysis. JAMA 2012, 307, 713–721. [Google Scholar] [CrossRef]
- Newell, K.; Kartsonaki, C.; Lam, K.B.H.; Kurmi, O.P. Cardiorespiratory health effects of particulate ambient air pollution exposure in low-income and middle-income countries: A systematic review and meta-analysis. Lancet Planet. Health 2017, 1, e368–e380. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Woodward, A.; Hou, X.-Y.; Zhu, T.; Zhang, J.; Brown, H.; Yang, J.; Qin, R.; Gao, J.; Gu, S.; et al. Modification of the effects of air pollutants on mortality by temperature: A systematic review and meta-analysis. Sci. Total Environ. 2016, 575, 1556–1570. [Google Scholar] [CrossRef]
- Analitis, A.; Donato, F.D.; Scortichini, M.; Lanki, T.; Basagana, X.; Ballester, F.; Astrom, C.; Paldy, A.; Pascal, M.; Gasparrini, A.; et al. Synergistic Effects of Ambient Temperature and Air Pollution on Health in Europe: Results from the PHASE Project. Int. J. Environ. Res. Public Health 2018, 15, 1856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, K.; Wolf, K.; Breitner, S.; Gasparrini, A.; Stafoggia, M.; Samoli, E.; Andersen, Z.J.; Bero-Bedada, G.; Bellander, T.; Hennig, F.; et al. Two-way effect modifications of air pollution and air temperature on total natural and cardiovascular mortality in eight European urban areas. Environ. Int. 2018, 116, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Parodi, S.; Vercelli, M.; Garrone, E.; Fontana, V.; Izzotti, A. Ozone air pollution and daily mortality in Genoa, Italy between 1993 and 1996. Public Health 2005, 119, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Stafoggia, M.; Schwartz, J.; Forastiere, F.; Perucci, C.A. Does Temperature Modify the Association between Air Pollution and Mortality? A Multicity Case-Crossover Analysis in Italy. Am. J. Epidemiol. 2008, 167, 1476–1485. [Google Scholar] [CrossRef] [Green Version]
- Roberts, S. Interactions between particulate air pollution and temperature in air pollution mortality time series studies. Environ. Res. 2004, 96, 328–337. [Google Scholar] [CrossRef]
- Jhun, I.; Fann, N.; Zanobetti, A.; Hubbell, B. Effect modification of ozone-related mortality risks by temperature in 97 US cities. Environ. Int. 2014, 73, 128–134. [Google Scholar] [CrossRef]
- Ren, C.; Williams, G.M.; Tong, S. Does particulate matter modify the association between temperature and cardiorespiratory diseases? Environ. Health Perspect. 2006, 114, 1690–1696. [Google Scholar] [CrossRef] [Green Version]
- Ren, C.; Tong, S. Temperature modifies the health effects of particulate matter in Brisbane, Australia. Int. J. Biometeorol. 2006, 51, 87–96. [Google Scholar] [CrossRef]
- Lee, W.; Choi, H.M.; Kim, D.; Honda, Y.; Guo, Y.-L.L.; Kim, H. Synergic effect between high temperature and air pollution on mortality in Northeast Asia. Environ. Res. 2019, 178, 108735. [Google Scholar] [CrossRef]
- Mokoena, K.K.; Ethan, C.J.; Yu, Y.; Quachie, A.T. Interaction Effects of Air Pollution and Climatic Factors on Circulatory and Respiratory Mortality in Xi’an, China between 2014 and 2016. Int. J. Environ. Res. Public Health 2020, 17, 9027. [Google Scholar] [CrossRef]
- Ji, S.; Zhou, Q.; Jiang, Y.; He, C.; Chen, Y.; Wu, C.; Liu, B. The Interactive Effects between Particulate Matter and Heat Waves on Circulatory Mortality in Fuzhou, China. Int. J. Environ. Res. Public Health 2020, 17, 5979. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.; He, Q.; Lin, H.M.; Kong, L.; Bentley, C.M.; Liu, W.; Zhou, D. High temperatures enhanced acute mortality effects of ambient particle pollution in the “oven” city of Wuhan, China. Environ. Health Perspect. 2008, 116, 1172–1178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Yang, J.; Guo, C.; Chen, P.-Y.; Ou, C.-Q.; Guo, Y. Particulate matter modifies the magnitude and time course of the non-linear temperature-mortality association. Environ. Pollut. 2015, 196, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Oh, J.; Kang, I.-S.; Ha, E.; Pyun, W.-B. Effect of Short-Term Exposure to Fine Particulate Matter and Temperature on Acute Myocardial Infarction in Korea. Int. J. Environ. Res. Public Health 2021, 18, 4822. [Google Scholar] [CrossRef]
- Qiu, H.; Yu, I.T.-S.; Wang, X.; Tian, L.; Tse, L.A.; Wong, T.W. Cool and dry weather enhances the effects of air pollution on emergency IHD hospital admissions. Int. J. Cardiol. 2013, 168, 500–505. [Google Scholar] [CrossRef]
- Li, N.; Ma, J.; Liu, F.; Zhang, Y.; Ma, P.; Jin, Y.; Zheng, Z.J. Associations of apparent temperature with acute cardiac events and subtypes of acute coronary syndromes in Beijing, China. Sci. Rep. 2021, 11, 15229. [Google Scholar] [CrossRef]
- Leibowitz, D.; Planer, D.; Weiss, T.; Rott, D. Seasonal Variation in Myocardial Infarction Is Limited to Patients with ST-Elevations on Admission. Chrono-International 2007, 24, 1241–1247. [Google Scholar] [CrossRef]
- Lee, S.; Lee, W.; Lee, E.; Jeong, M.H.; Rha, S.-W.; Kim, C.-J.; Chae, S.C.; Kim, H.-S.; Gwon, H.-C.; Kim, H. Effects of Asian dust-derived particulate matter on ST-elevation myocardial infarction: Retrospective, time series study. BMC Public Health 2021, 21, 68. [Google Scholar] [CrossRef]
- Gardner, B.; Ling, F.; Hopke, P.K.; Frampton, M.W.; Utell, M.J.; Zareba, W.; Cameron, S.J.; Chalupa, D.; Kane, C.; Kulandhaisamy, S.; et al. Ambient fine particulate air pollution triggers ST-elevation myocardial infarction, but not non-ST elevation myocardial infarction: A case-crossover study. Part. Fibre Toxicol. 2014, 11, 1. [Google Scholar] [CrossRef] [Green Version]
- Davoodabadi, Z.; Soleimani, A.; Pourmoghaddas, A.; Hosseini, S.M.; Jafari-Koshki, T.; Rahimi, M.; Shishehforoush, M.; Lahijanzadeh, A.; Sadeghian, B.; Moazam, E.; et al. Correlation between air pollution and hospitalization due to myocardial infarction. ARYA Atheroscler. 2019, 15, 161–167. [Google Scholar]
- Argacha, J.; Collart, P.; Wauters, A.; Kayaert, P.; Lochy, S.; Schoors, D.; Sonck, J.; de Vos, T.; Forton, M.; Brasseur, O.; et al. Air pollution and ST-elevation myocardial infarction: A case-crossover study of the Belgian STEMI registry 2009–2013. Int. J. Cardiol. 2016, 223, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Rich, D.Q.; Thevenet-Morrison, K.; Kipen, H.M.; Özkaynak, H.; Baxter, L.; Burke, J.; Crooks, J.; Lunden, M.; Kostis, J.; Zhang, J.; et al. Triggering of transmural infarctions, but not nontransmural infarctions, by ambient fine particles. Environ. Health Perspect. 2010, 118, 1229–1234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buszman, P.E.; Derbisz, K.; Kwasiborski, P.; Chrząszcz, P.; Mularska, M.; Baron, D.; Sobieszek, A.; Mendyk, A.; Skoczylas, P.; Cisowski, M.; et al. Impact of air pollution on hospital patients admitted with ST- and non-ST-segment elevation myocardial infarction in heavily polluted cities within the European Union. Cardiol. J. 2013, 27, 541–547. [Google Scholar] [CrossRef] [Green Version]
- Butland, B.K.; Atkinson, R.W.; Milojevic, A.; Heal, M.R.; Doherty, R.M.; Armstrong, B.G.; MacKenzie, A.I.; Vieno, M.; Lin, C.; Wilkinson, P. Myocardial infarction, ST-elevation and non-ST-elevation myocardial infarction and modelled daily pollution concentrations: A case-crossover analysis of MINAP data. Open Heart 2016, 3, e000429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rich, D.Q.; Kipen, H.M.; Huang, W.; Wang, G.; Wang, Y.; Zhu, P.; Ohman-Strickland, P.; Hu, M.; Philipp, C.; Diehl, S.R.; et al. Association between changes in air pollution levels during the Beijing Olympics and biomarkers of inflammation and thrombosis in healthy young adults. JAMA 2012, 307, 2068–2078. [Google Scholar] [CrossRef]
- Rückerl, R.; Ibald-Mulli, A.; Koenig, W.; Schneider, A.; Woelke, G.; Cyrys, J.; Heinrich, J.; Marder, V.; Frampton, M.; Wichmann, H.E.; et al. Air Pollution and Markers of Inflammation and Coagulation in Patients with Coronary Heart Disease. Am. J. Respir. Crit. Care Med. 2006, 173, 432–441. [Google Scholar] [CrossRef]
- Rückerl, R.; Phipps, R.P.; Schneider, A.; Frampton, M.; Cyrys, J.; Oberdörster, G.; Wichmann, H.E.; Peters, A. Ultrafine particles and platelet activation in patients with coronary heart disease—results from a prospective panel study. Part. Fibre Toxicol. 2007, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Rudež, G.; Janssen, N.A.; Kilinc, E.; Leebeek, F.W.; Gerlofs-Nijland, M.E.; Spronk, H.M.; Cate, H.T.; Cassee, F.R.; de Maat, M.P. Effects of Ambient Air Pollution on Hemostasis and Inflammation. Environ. Health Perspect. 2009, 117, 995–1001. [Google Scholar] [CrossRef] [Green Version]
- Samet, J.M.; Rappold, A.; Graff, D.; Cascio, W.E.; Berntsen, J.H.; Huang, Y.-C.T.; Herbst, M.; Bassett, M.; Montilla, T.; Hazucha, M.J.; et al. Concentrated Ambient Ultrafine Particle Exposure Induces Cardiac Changes in Young Healthy Volunteers. Am. J. Respir. Crit. Care Med. 2009, 179, 1034–1042. [Google Scholar] [CrossRef]
- Zhang, S.; Breitner, S.; Cascio, W.E.; Devlin, R.B.; Neas, L.M.; Ward-Caviness, C.; Diaz-Sanchez, D.; Kraus, W.E.; Hauser, E.R.; Schwartz, J.; et al. Association between short-term exposure to ambient fine particulate matter and myocardial injury in the CATHGEN cohort. Environ. Pollut. 2021, 275, 116663. [Google Scholar] [CrossRef]
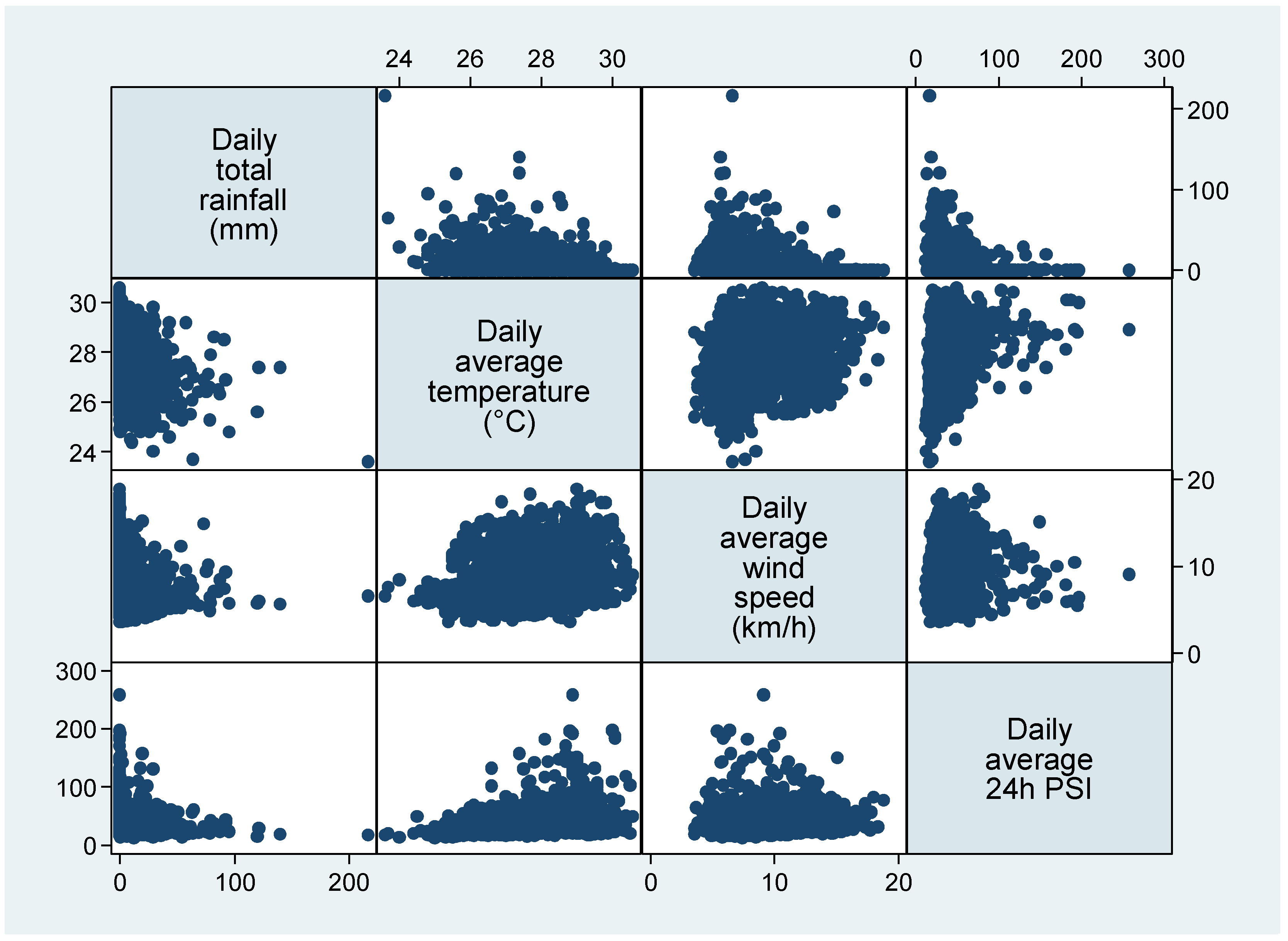
| Overall (n = 2191) | Cluster 1 (n = 686) | Cluster 2 (n = 1029) | Cluster 3 (n = 467) | p | |
|---|---|---|---|---|---|
| Daily total rainfall in mm, median (IQR) | 0.0 (0.0–4.0) | 0.0 (0.0–0.0) | 2.8 (0.4–13.6) | 0.0 (0.0–2.2) | <0.001 |
| Daily average temperature in deg C, median (IQR) | 27.9 (27.0–28.7) | 28.3 (27.6–28.9) | 27.3 (26.6–28.1) | 28.6 (27.8–29.0) | <0.001 |
| Daily average wind speed in km/h, median (IQR) | 7.8 (6.4–10.5) | 10.4 (8.4–12.2) | 6.6 (5.8–7.8) | 8.5 (6.5–11.1) | <0.001 |
| Daily 24 h average PSI, median (IQR) | 32.8 (25.7–47.0) | 30.5 (25.5–37.0) | 29.0 (23.5–35.7) | 59.2 (54.8–70.0) | <0.001 |
| AMI (n = 39,287) | Cluster 1 (n = 12,173) | Cluster 2 (n = 18,287) | Cluster 3 (n = 8681) | p | |
|---|---|---|---|---|---|
| Age, median (IQR) | 66.6 (56.6–78.2) | 66.4 (56.3–78.1) | 66.7 (56.6–78.1) | 66.8 (56.9–78.7) | 0.029 |
| Male, n (%) | 26,763 (68.1) | 8294 (68.1) | 12,417 (67.9) | 5955 (68.6) | 0.517 |
| Ethnicity, n (%) | |||||
| Chinese | 25,320 (64.5) | 7921 (65.1) | 11,717 (64.1) | 5598 (64.5) | 0.042 |
| Malay | 8131 (20.7) | 2520 (20.7) | 3784 (20.7) | 1792 (20.6) | |
| Indian | 5271 (13.4) | 1586 (13.0) | 2518 (13.8) | 1145 (13.2) | |
| Etiology, n (%) | |||||
| STEMI | 11,557 (29.4) | 3610 (29.7) | 5431 (29.7) | 2482 (28.6) | <0.001 |
| NSTEMI | 25,295 (64.4) | 7834 (64.4) | 11,585 (63.4) | 5777 (66.6) | |
| History of AMI/CABG/PCI, n (%) | 12,584 (32.0) | 3839 (31.5) | 5952 (32.6) | 2755 (31.7) | 0.136 |
| History of DM, n (%) | 17,544 (44.7) | 5379 (44.2) | 8266 (45.2) | 3836 (44.2) | 0.127 |
| History of HTN, n (%) | 27,961 (71.2) | 8630 (70.9) | 13,145 (71.9) | 6087 (70.1) | 0.008 |
| History of HLD, n (%) | 24,726 (63.0) | 7586 (62.3) | 11,564 (63.3) | 5492 (63.3) | 0.211 |
| Smoking, n (%) | 19,258 (49.0) | 6012 (50.3) | 8994 (50.0) | 4180 (49.0) | 0.152 |
| Cluster 1 | Cluster 2 | Cluster 3 | |
|---|---|---|---|
| Number of events | 12,173 | 18,287 | 8681 |
| Entire cohort | 1.00 | 1.00 (0.98–1.03) | 1.04 (1.01–1.07) a |
| Subgroups | |||
| Age | |||
| <65 years | 1.00 | 0.99 (0.96–1.02) | 1.02 (0.98–1.07) |
| ≥65 years | 1.00 | 1.01 (0.98–1.05) | 1.06 (1.02–1.11) a |
| Gender | |||
| Male | 1.00 | 1.00 (0.97–1.03) | 1.05 (1.01–1.09) a |
| Female | 1.00 | 1.01 (0.97–1.05) | 1.03 (0.98–1.09) |
| Ethnicity | |||
| Chinese | 1.00 | 0.99 (0.96–1.02) | 1.03 (0.99–1.07) |
| Malay | 1.00 | 1.00 (0.96–1.05) | 1.04 (0.98–1.10) |
| Indian | 1.00 | 1.04 (0.99–1.10) | 1.05 (0.98–1.12) |
| Etiology | |||
| STEMI | 1.00 | 1.00 (0.96–1.04) | 1.01 (0.96–1.06) |
| NSTEMI | 1.00 | 0.99 (0.96–1.02) | 1.07 (1.03–1.11) a |
| History of AMI/ CABG/ PCI | |||
| Yes | 1.00 | 1.04 (0.99–1.08) | 1.05 (0.99–1.11) |
| No | 1.00 | 0.99 (0.96–1.02) | 1.04 (1.00–1.08) a |
| History of diabetes | |||
| Yes | 1.00 | 1.03 (0.99–1.07) | 1.04 (1.00–1.09) |
| No | 1.00 | 0.98 (0.95–1.01) | 1.04 (1.00–1.08) a |
| History of hypertension | |||
| Yes | 1.00 | 1.02 (0.99–1.05) | 1.03 (0.99–1.07) |
| No | 1.00 | 0.97 (0.93–1.01) | 1.07 (1.02–1.13) a |
| History of hyperlipidemia | |||
| Yes | 1.00 | 1.02 (0.98–1.05) | 1.05 (1.01–1.09) a |
| No | 1.00 | 0.98 (0.94–1.02) | 1.02 (0.98–1.07) |
| Current/former smoker | |||
| Yes | 1.00 | 1.00 (0.97–1.03) | 1.02 (0.98–1.06) |
| No | 1.00 | 1.01 (0.97–1.04) | 1.07 (1.03–1.12) a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koo, G.P.Y.; Zheng, H.; Pek, P.P.; Hughes, F.; Lim, S.L.; Yeo, J.W.; Ong, M.E.H.; Ho, A.F.W. Clustering of Environmental Parameters and the Risk of Acute Myocardial Infarction. Int. J. Environ. Res. Public Health 2022, 19, 8476. https://doi.org/10.3390/ijerph19148476
Koo GPY, Zheng H, Pek PP, Hughes F, Lim SL, Yeo JW, Ong MEH, Ho AFW. Clustering of Environmental Parameters and the Risk of Acute Myocardial Infarction. International Journal of Environmental Research and Public Health. 2022; 19(14):8476. https://doi.org/10.3390/ijerph19148476
Chicago/Turabian StyleKoo, Geraldine P. Y., Huili Zheng, Pin Pin Pek, Fintan Hughes, Shir Lynn Lim, Jun Wei Yeo, Marcus E. H. Ong, and Andrew F. W. Ho. 2022. "Clustering of Environmental Parameters and the Risk of Acute Myocardial Infarction" International Journal of Environmental Research and Public Health 19, no. 14: 8476. https://doi.org/10.3390/ijerph19148476
APA StyleKoo, G. P. Y., Zheng, H., Pek, P. P., Hughes, F., Lim, S. L., Yeo, J. W., Ong, M. E. H., & Ho, A. F. W. (2022). Clustering of Environmental Parameters and the Risk of Acute Myocardial Infarction. International Journal of Environmental Research and Public Health, 19(14), 8476. https://doi.org/10.3390/ijerph19148476








