Obstructive Sleep Apnea and Nonalcoholic Fatty Liver Disease in the General Population: A Cross-Sectional Study Using Nationally Representative Data
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. The Definition of NAFLD and Evaluation of Advanced Liver Fibrosis
2.3. The STOP-Bang Scoring System
2.4. Socioeconomic Status, Anthropometric Indices, and Laboratory Results
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Senaratna, C.V.; Perret, J.L.; Lodge, C.J.; Lowe, A.J.; Campbell, B.E.; Matheson, M.C.; Hamilton, G.S.; Dharmage, S.C. Prevalence of obstructive sleep apnea in the general population: A systematic review. Sleep Med. Rev. 2017, 34, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Mirrakhimov, A.E.; Sooronbaev, T.; Mirrakhimov, E.M. Prevalence of obstructive sleep apnea in Asian adults: A systematic review of the literature. BMC Pulm. Med. 2013, 13, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peppard, P.E.; Young, T.; Barnet, J.H.; Palta, M.; Hagen, E.W.; Hla, K.M. Increased prevalence of sleep-disordered breathing in adults. Am. J. Epidemiol. 2013, 177, 1006–1014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Framnes, S.N.; Arble, D.M. The Bidirectional Relationship Between Obstructive Sleep Apnea and Metabolic Disease. Front. Endocrinol. 2018, 9, 440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Türkay, C.; Ozol, D.; Kasapoğlu, B.; Kirbas, I.; Yıldırım, Z.; Yiğitoğlu, R. Influence of obstructive sleep apnea on fatty liver disease: Role of chronic intermittent hypoxia. Respir. Care 2012, 57, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Labarca, G.; Gower, J.; Lamperti, L.; Dreyse, J.; Jorquera, J. Chronic intermittent hypoxia in obstructive sleep apnea: A narrative review from pathophysiological pathways to a precision clinical approach. Sleep Breath 2020, 24, 751–760. [Google Scholar] [CrossRef]
- Trzepizur, W.; Boursier, J.; Le Vaillant, M.; Ducluzeau, P.H.; Dubois, S.; Henni, S.; Abraham, P.; Aubé, C.; Calès, P.; Gagnadoux, F. Increased liver stiffness in patients with severe sleep apnoea and metabolic comorbidities. Eur. Respir. J. 2018, 51, 1800601. [Google Scholar] [CrossRef] [Green Version]
- Drager, L.F.; Li, J.; Reinke, C.; Bevans-Fonti, S.; Jun, J.C.; Polotsky, V.Y. Intermittent hypoxia exacerbates metabolic effects of diet-induced obesity. Obesity 2011, 19, 2167–2174. [Google Scholar] [CrossRef]
- Chung, G.E.; Cho, E.J.; Yoo, J.J.; Chang, Y.; Cho, Y.; Park, S.H.; Shin, D.W.; Han, K.; Yu, S.J. Nonalcoholic fatty liver disease is associated with the development of obstructive sleep apnea. Sci. Rep. 2021, 11, 13473. [Google Scholar] [CrossRef]
- Shpirer, I.; Copel, L.; Broide, E.; Elizur, A. Continuous Positive Airway Pressure Improves Sleep Apnea Associated Fatty Liver. Lung 2010, 188, 301–307. [Google Scholar] [CrossRef]
- Alonso-Álvarez, M.L.; Terán-Santos, J.; Ordax Carbajo, E.; Cordero-Guevara, J.A.; Navazo-Egüia, A.I.; Kheirandish-Gozal, L.; Gozal, D. Reliability of home respiratory polygraphy for the diagnosis of sleep apnea in children. Chest 2015, 147, 1020–1028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, F.; Abdullah, H.R.; Liao, P. STOP-Bang Questionnaire: A Practical Approach to Screen for Obstructive Sleep Apnea. Chest 2016, 149, 631–638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pivetta, B.; Chen, L.; Nagappa, M.; Saripella, A.; Waseem, R.; Englesakis, M.; Chung, F. Use and Performance of the STOP-Bang Questionnaire for Obstructive Sleep Apnea Screening Across Geographic Regions: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2021, 4, e211009. [Google Scholar] [CrossRef] [PubMed]
- Petta, S.; Marrone, O.; Torres, D.; Buttacavoli, M.; Cammà, C.; Di Marco, V.; Licata, A.; Lo Bue, A.; Parrinello, G.; Pinto, A.; et al. Obstructive Sleep Apnea Is Associated with Liver Damage and Atherosclerosis in Patients with Non-Alcoholic Fatty Liver Disease. PLoS ONE 2015, 10, e0142210. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, D.; Kim, H.J.; Lee, C.H.; Yang, J.I.; Kim, W.; Kim, Y.J.; Yoon, J.H.; Cho, S.H.; Sung, M.W.; et al. Hepatic steatosis index: A simple screening tool reflecting nonalcoholic fatty liver disease. Dig. Liver Dis. 2010, 42, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Peleg, N.; Issachar, A.; Sneh-Arbib, O.; Shlomai, A. AST to Platelet Ratio Index and fibrosis 4 calculator scores for non-invasive assessment of hepatic fibrosis in patients with non-alcoholic fatty liver disease. Dig. Liver Dis. 2017, 49, 1133–1138. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.H.; Lee, H.W.; Yoo, J.-J.; Cho, Y.; Kim, S.U.; Lee, T.H.; Jang, B.K.; Kim, S.G.; Ahn, S.B.; Kim, H.; et al. KASL clinical practice guidelines: Management of nonalcoholic fatty liver disease. Clin. Mol. Hepatol. 2021, 27, 363–401. [Google Scholar] [CrossRef]
- Farney, R.J.; Walker, B.S.; Farney, R.M.; Snow, G.L.; Walker, J.M. The STOP-Bang equivalent model and prediction of severity of obstructive sleep apnea: Relation to polysomnographic measurements of the apnea/hypopnea index. J. Clin. Sleep Med. 2011, 7, 459–465. [Google Scholar] [CrossRef] [Green Version]
- Jamal, A.; Phillips, E.; Gentzke, A.S.; Homa, D.M.; Babb, S.D.; King, B.A.; Neff, L.J. Current Cigarette Smoking Among Adults—United States, 2016. MMWR Morb. Mortal Wkly. Rep. 2018, 67, 53–59. [Google Scholar] [CrossRef] [Green Version]
- Kweon, S.; Kim, Y.; Jang, M.-j.; Kim, Y.; Kim, K.; Choi, S.; Chun, C.; Khang, Y.-H.; Oh, K. Data resource profile: The Korea National Health and Nutrition Examination Survey (KNHANES). Int. J. Epidemiol. 2014, 43, 69–77. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.-J.G.; Lee, Y.J.; Jeong, D.-U. Differential Effects of Obesity on Obstructive Sleep Apnea Syndrome according to Age. Psychiatry Investig. 2017, 14, 656–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leong, W.B.; Arora, T.; Jenkinson, D.; Thomas, A.; Punamiya, V.; Banerjee, D.; Taheri, S. The prevalence and severity of obstructive sleep apnea in severe obesity: The impact of ethnicity. J. Clin. Sleep Med. 2013, 9, 853–858. [Google Scholar] [CrossRef]
- Johnson, R.F.; Hansen, A.; Narayanan, A.; Yogesh, A.; Shah, G.B.; Mitchell, R.B. Weight gain velocity as a predictor of severe obstructive sleep apnea among obese adolescents. Laryngoscope 2020, 130, 1339–1342. [Google Scholar] [CrossRef] [PubMed]
- Kuna, S.T.; Reboussin, D.M.; Strotmeyer, E.S.; Millman, R.P.; Zammit, G.; Walkup, M.P.; Wadden, T.A.; Wing, R.R.; Pi-Sunyer, F.X.; Spira, A.P.; et al. Effects of Weight Loss on Obstructive Sleep Apnea Severity. Ten-Year Results of the Sleep AHEAD Study. Am. J. Respir. Crit. Care Med. 2021, 203, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Dongiovanni, P.; Petta, S.; Mannisto, V.; Mancina, R.M.; Pipitone, R.; Karja, V.; Maggioni, M.; Kakela, P.; Wiklund, O.; Mozzi, E.; et al. Statin use and non-alcoholic steatohepatitis in at risk individuals. J. Hepatol. 2015, 63, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Shnaimer, J.A.; Dahlan, H.M.; Hanbashi, F.M.; Bahammam, A.S.; Gosadi, I.M. Assessment of the risk of obstructive sleep apnoea among patients with type 2 diabetes and its associated factors using the STOP-BANG questionnaire: A cross-sectional study. J. Taibah Univ. Med. Sci. 2022; in press. [Google Scholar]
- Li, J.; Grigoryev, D.N.; Ye, S.Q.; Thorne, L.; Schwartz, A.R.; Smith, P.L.; O’Donnell, C.P.; Polotsky, V.Y. Chronic intermittent hypoxia upregulates genes of lipid biosynthesis in obese mice. J. Appl. Physiol. 2005, 99, 1643–1648. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Xu, C.; Shi, J.; Ding, J.; Wan, X.; Chen, D.; Gao, J.; Li, C.; Zhang, J.; Lin, Y.; et al. Fatty acids promote fatty liver disease via the dysregulation of 3-mercaptopyruvate sulfurtransferase/hydrogen sulfide pathway. Gut 2018, 67, 2169–2180. [Google Scholar] [CrossRef]
- Chin, K.; Nakamura, T.; Takahashi, K.; Sumi, K.; Ogawa, Y.; Masuzaki, H.; Muro, S.; Hattori, N.; Matsumoto, H.; Niimi, A.; et al. Effects of obstructive sleep apnea syndrome on serum aminotransferase levels in obese patients. Am. J. Med. 2003, 114, 370–376. [Google Scholar] [CrossRef]
- Aron-Wisnewsky, J.; Minville, C.; Tordjman, J.; Lévy, P.; Bouillot, J.L.; Basdevant, A.; Bedossa, P.; Clément, K.; Pépin, J.L. Chronic intermittent hypoxia is a major trigger for non-alcoholic fatty liver disease in morbid obese. J. Hepatol. 2012, 56, 225–233. [Google Scholar] [CrossRef]
- Jullian-Desayes, I.; Joyeux-Faure, M.; Tamisier, R.; Launois, S.; Borel, A.-L.; Levy, P.; Pepin, J.-L. Impact of obstructive sleep apnea treatment by continuous positive airway pressure on cardiometabolic biomarkers: A systematic review from sham CPAP randomized controlled trials. Sleep Med. Rev. 2015, 21, 23–38. [Google Scholar] [CrossRef]
- Carreras, A.; Zhang, S.X.; Peris, E.; Qiao, Z.; Gileles-Hillel, A.; Li, R.C.; Wang, Y.; Gozal, D. Chronic sleep fragmentation induces endothelial dysfunction and structural vascular changes in mice. Sleep 2014, 37, 1817–1824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minville, C.; Hilleret, M.-N.; Tamisier, R.; Aron-Wisnewsky, J.; Clement, K.; Trocme, C.; Borel, J.-C.; Lévy, P.; Zarski, J.-P.; Pépin, J.-L. Nonalcoholic Fatty Liver Disease, Nocturnal Hypoxia, and Endothelial Function in Patients with Sleep Apnea. Chest 2014, 145, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Meszaros, M.; Tarnoki, A.D.; Tarnoki, D.L.; Kovacs, D.T.; Forgo, B.; Lee, J.; Sung, J.; Vestbo, J.; Müller, V.; Kunos, L.; et al. Obstructive sleep apnea and hypertriglyceridaemia share common genetic background: Results of a twin study. J. Sleep Res. 2020, 29, e12979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| NAFLD (-) (n = 3254) | NAFLD (+) (n = 1021) | p | |
|---|---|---|---|
| Age (years) | 55.6 (0.3) | 53.9 (0.4) | <0.001 |
| Men | 49.4 (0.9) | 56.7 (1.8) | 0.001 |
| Residence | 0.287 | ||
| Urban | 84.9 (1.9) | 83.5 (2.2) | |
| Rural | 15.1 (1.9) | 16.5 (2.2) | |
| Education | 0.454 | ||
| Middle school or lower | 21.0 (1.1) | 19.1 (1.4) | |
| High school | 35.2 (1.2) | 37.0 (1.9) | |
| College or more | 43.9 (1.6) | 43.9 (2.2) | |
| Household income | 0.984 | ||
| Lowest | 12.7 (0.8) | 12.5 (1.2) | |
| Middle low | 21.6 (1.1) | 21.1 (1.6) | |
| Middle high | 29.7 (1.2) | 30.2 (1.8) | |
| Highest | 35.9 (1.5) | 36.2 (2.0) | |
| Smoking | <0.001 | ||
| Never | 57.2 (1.0) | 50.1 (1.9) | |
| Former | 26.4 (1.0) | 27.2 (1.7) | |
| Current | 16.4 (0.8) | 22.7 (1.7) | |
| Adequate Physical activity ¶ | 0.073 | ||
| No | 56.6 (1.0) | 60.5 (1.9) | |
| Yes | 43.4 (1.0) | 39.5 (1.9) | |
| BMI (kg/m2) | <0.001 | ||
| <18.5 | 3.0 (0.4) | - | |
| 18.5–22.9 | 44.1 (1.1) | 2.4 (0.6) | |
| 23–24.9 | 30.7 (0.9) | 11.3 (1.2) | |
| ≥25 | 22.2 (0.9) | 86.3 (1.2) | |
| AST (IU/L) | 23.4 (0.2) | 27.3 (0.4) | <0.001 |
| ALT (IU/L) | 19.1 (0.2) | 36.5 (0.8) | <0.001 |
| SBP (mmHg) | 118.9 (0.4) | 123.9 (0.5) | <0.001 |
| DBP (mmHg) | 76.4 (0.2) | 80.7 (0.4) | <0.001 |
| Neck circumference (cm) | 34.9 (0.1) | 37.8 (0.1) | <0.001 |
| Waist circumference (cm) | 82.3 (0.2) | 94.4 (0.3) | <0.001 |
| Fasting glucose (mg/dL) | 99.6 (0.4) | 113.4 (1.1) | <0.001 |
| HbA1c (%) | 5.74 (0.02) | 6.26 (0.04) | <0.001 |
| HDL cholesterol (mg/dL) | 53.4 (0.3) | 46.5 (0.3) | <0.001 |
| LDL cholesterol (mg/dL) | 116.2 (2.2) | 119.1 (2.5) | 0.399 |
| Triglyceride (mg/dL) | 127.7 (2.1) | 178.3 (4.8) | <0.001 |
| Crude | Age and (Sex) Adjusted | Multivariable-Adjusted | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
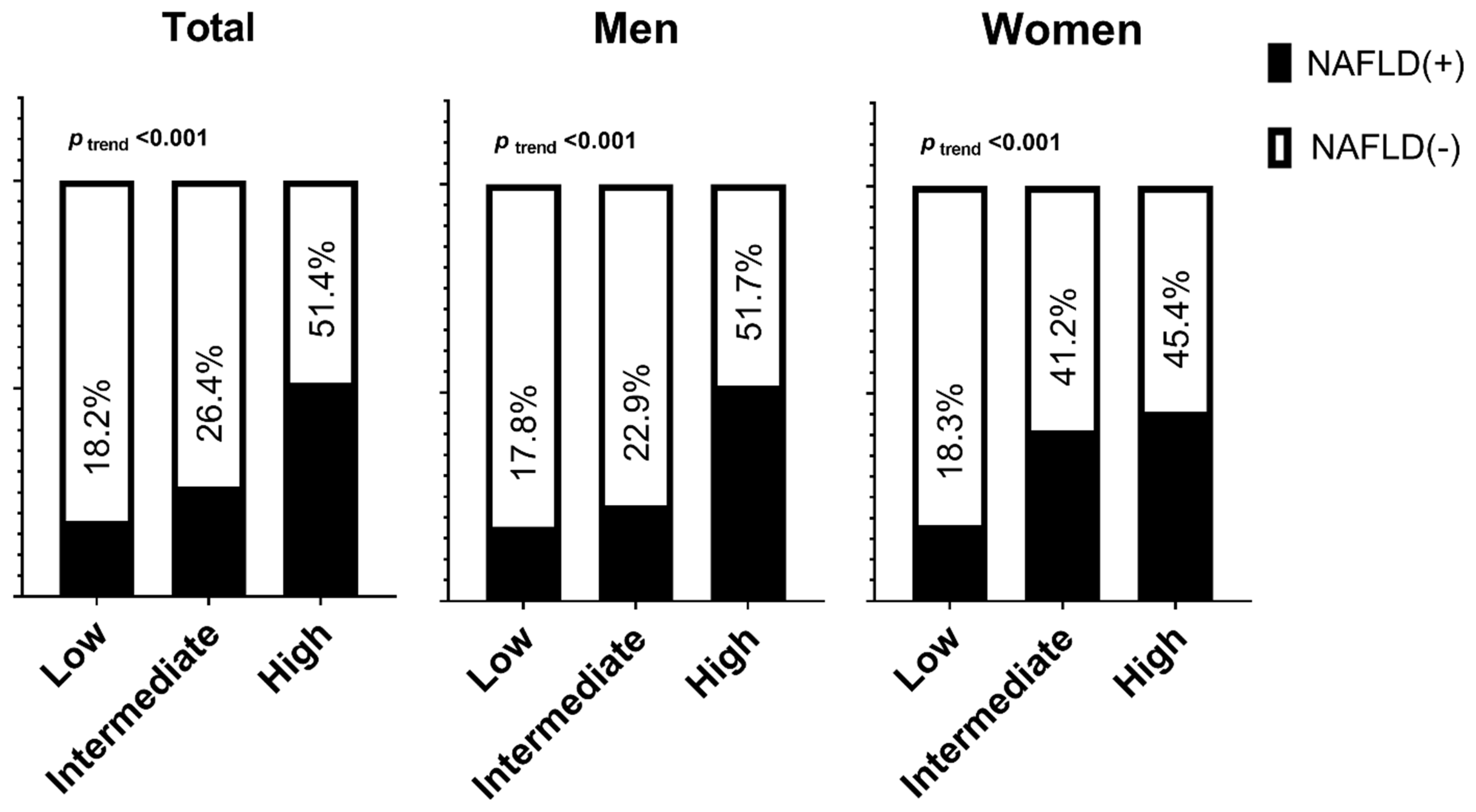
| All | <0.001 | <0.001 | <0.001 | |||
| Low | 1 | 1 | 1 | |||
| Intermediate | 1.76 (1.48–2.09) | 3.93 (2.97–5.21) | 2.59 (1.89–3.56) | |||
| High | 5.12 (3.64–7.21) | 12.79 (8.15–20.09) | 5.44 (3.16–9.36) | |||
| Men | <0.001 | <0.001 | <0.001 | |||
| Low | 1 | 1 | 1 | |||
| Intermediate | 1.63 (1.03–2.58) | 3.40 (1.78–6.49) | 3.44 (1.79–6.61) | |||
| High | 5.48 (3.18–9.46) | 8.63 (4.01–18.57) | 8.49 (3.92–18.37) | |||
| Women | <0.001 | <0.001 | <0.001 | |||
| Low | 1 | 1 | 1 | |||
| Intermediate | 3.25 (2.46–4.29) | 2.19 (1.53–3.16) | 2.13 (1.49–3.05) | |||
| High | 4.57 (1.10–18.99) | 2.69 (0.42–17.32) | 2.37 (0.36–15.48) | |||
| Crude | Age-Adjusted | Multivariable-Adjusted | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
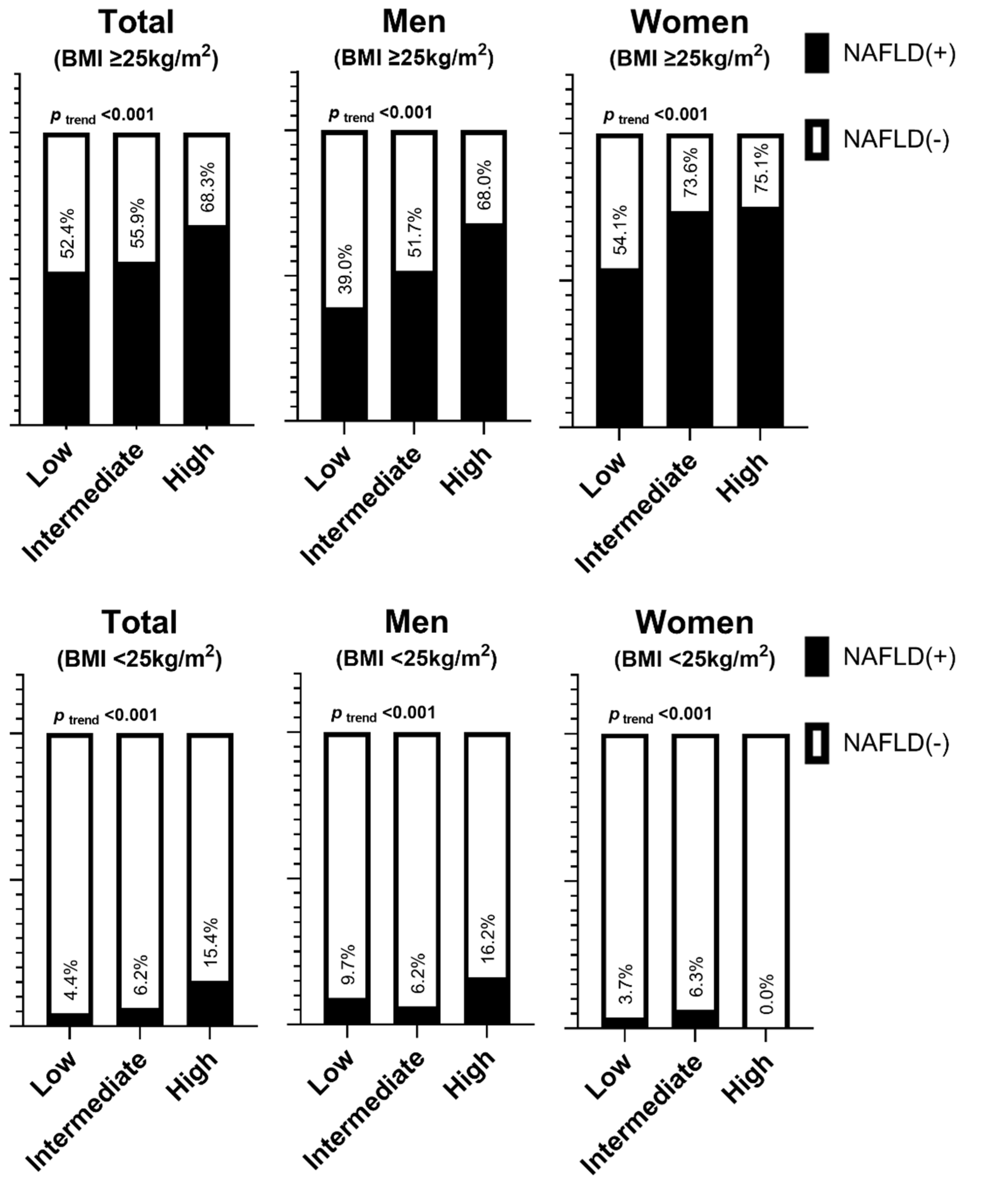
| Men | ||||||
| BMI ≥ 25 kg/m2 | <0.001 | <0.001 | <0.001 | |||
| Low | 1 | 1 | 1 | |||
| Intermediate | 1.67 (0.88–3.19) | 4.10 (2.06–8.16) | 4.29 (2.12–8.71) | |||
| High | 3.33 (0.58–7.04) | 9.76 (4.33–22.04) | 9.96 (4.32–22.94) | |||
| BMI < 25 kg/m2 | 0.687 | 0.003 | 0.008 | |||
| Low | 1 | 1 | 1 | |||
| Intermediate | 0.66 (0.29–1.50) | 2.96 (0.86–10.23) | 2.76 (0.82–9.36) | |||
| High | 1.93 (0.59–6.34) | 10.44 (2.23–48.95) | 8.53 (1.79–40.56) | |||
| Women | ||||||
| BMI ≥ 25 kg/m2 | <0.001 | <0.001 | <0.001 | |||
| Low | 1 | 1 | 1 | |||
| Intermediate | 2.36 (1.56–3.58) | 2.83 (1.81–4.43) | 2.71 (1.74–4.23) | |||
| High | 2.56 (0.28–23.39) | 3.23 (0.44–23.74) | 2.60 (0.34–19.70) | |||
| BMI < 25 kg/m2 | 0.161 | 0.853 | 0.837 | |||
| Low | 1 | 1 | 1 | |||
| Intermediate | 1.74 (0.86–3.51) | 1.13 (1.13–1.13) | 0.927 (NA–NA) | |||
| High | NA (NA–NA) | NA (NA–NA) | NA (NA–NA) | |||
| FIB-4 ≤ 1.3 vs. >1.3 | ||||||
|---|---|---|---|---|---|---|
| Crude | Age-Adjusted | Multivariable-Adjusted | ||||
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| Men | ||||||
| All | 0.005 | 0.157 | 0.135 | |||
| Low | 1 | 1 | 1 | |||
| Intermediate | 2.42 (0.54–10.79) | 0.42 (0.05–3.91) | 0.39 (0.04–3.48) | |||
| High | 4.71 (0.99–22.47) | 0.78 (0.08–7.57) | 0.75 (0.08–6.90) | |||
| BMI ≥ 25 kg/m2 | 0.004 | 0.191 | 0.189 | |||
| Low | 1 | 1 | 1 | |||
| Intermediate | 2.68 (0.34–20.84) | 0.36 (0.04–3.31) | 0.33 (0.04–3.16) | |||
| High | 5.46 (0.68–44.08) | 0.68 (0.07–6.78) | 0.65 (0.07–6.43) | |||
| BMI < 25 kg/m2 | 0.602 | 0.961 | 0.191 | |||
| Low | 1 | 1 | 1 | |||
| Intermediate | 1.99 (0.23–17.14) | 1.01 (0.46–2.18) | 10.34 (0.04–2698.53) | |||
| High | 1.97 (0.11–35.43) | 0.96 (0.12–7.86) | 1.48 (0.01–298.27) | |||
| Women | ||||||
| All | 0.015 | 0.458 | 0.831 | |||
| Low | 1 | 1 | 1 | |||
| Intermediate | 2.07 (1.30–3.28) | 0.89 (0.51–1.58) | 1.01 (0.56–1.81) | |||
| High | 0.43 (0.04–4.23) | 0.28 (0.02–4.01) | 0.46 (0.04–4.86) | |||
| BMI ≥ 25 kg/m2 | 0.02 | 0.487 | 0.837 | |||
| Low | 1 | 1 | 1 | |||
| Intermediate | 2.08 (1.29–3.33) | 0.91 (0.49–1.68) | 1.02 (0.54–1.93) | |||
| High | 0.43 (0.04–4.23) | 0.26 (0.02–3.96) | 0.44 (0.04–4.85) | |||
| BMI < 25 kg/m2 | 0.468 | 0.489 | 0.844 | |||
| Low | 1 | 1 | 1 | |||
| Intermediate | 1.78 (0.36–8.77) | 0.58 (0.12–2.80) | 0.80 (0.08–7.78) | |||
| High | NA (NA–NA) | NA (NA–NA) | NA (NA–NA) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, T.; Choi, H.; Lee, J.; Kim, J. Obstructive Sleep Apnea and Nonalcoholic Fatty Liver Disease in the General Population: A Cross-Sectional Study Using Nationally Representative Data. Int. J. Environ. Res. Public Health 2022, 19, 8398. https://doi.org/10.3390/ijerph19148398
Kim T, Choi H, Lee J, Kim J. Obstructive Sleep Apnea and Nonalcoholic Fatty Liver Disease in the General Population: A Cross-Sectional Study Using Nationally Representative Data. International Journal of Environmental Research and Public Health. 2022; 19(14):8398. https://doi.org/10.3390/ijerph19148398
Chicago/Turabian StyleKim, Taeyun, Hyunji Choi, Jaejun Lee, and Jehun Kim. 2022. "Obstructive Sleep Apnea and Nonalcoholic Fatty Liver Disease in the General Population: A Cross-Sectional Study Using Nationally Representative Data" International Journal of Environmental Research and Public Health 19, no. 14: 8398. https://doi.org/10.3390/ijerph19148398
APA StyleKim, T., Choi, H., Lee, J., & Kim, J. (2022). Obstructive Sleep Apnea and Nonalcoholic Fatty Liver Disease in the General Population: A Cross-Sectional Study Using Nationally Representative Data. International Journal of Environmental Research and Public Health, 19(14), 8398. https://doi.org/10.3390/ijerph19148398






