1. Introduction
Impaired insulin sensitivity in skeletal muscle is an early defect in the pathogenesis of type 2 diabetes since it can be detected several years before the development of overt diabetes [
1]. Insulin resistance in skeletal muscle is associated with an elevated adipose tissue mass [
2]. The interplay between adipose tissue and skeletal muscle is important as the body tries to defend itself against insulin resistance in the peripheral tissues; this interplay is regulated by several factors and through more than one pathway [
3]. Earlier studies revealed that adipose tissue secretes more than 50 different adipokines into circulation [
2], while recent proteomic studies identified even more adipokines being secreted into the circulation and exosomes [
4]. It is well-known that several adipokines and cytokines, including adiponectin, leptin, interleukin-6 (IL-6), monocyte chemoattractant protein-1 (MCP-1), INF-γ (interferon-γ) and tumor necrosis factor-α (TNF-α), can influence insulin signaling in skeletal muscle [
3]. The down-regulation of the insulin receptor substrate 1 (IRS-1) pathway by TNF-α and a reduced activation of 5′-AMP activated protein kinase and AMPK (AMP-activated protein kinase) due to leptin resistance have been associated with insulin resistance [
5,
6]. IL-6 induces the hepatic synthesis of C-reactive protein (CRP) and its levels have been linked with visceral adiposity [
7] but its role in obesity and insulin resistance is controversial. Monocyte chemoattractant protein-1 (MCP-1) also aggravates insulin resistance at doses similar to its physiological plasma concentrations (200 pg/mL) [
2]. In contrast to the other adipokines, adiponectin concentrations are low in obesity and at the stage of insulin resistance; therefore, adiponectin is considered to be an insulin-sensitizing hormone [
8]. Skeletal muscle cytokine expression can be increased via several pathways, including the p38MAPK and NF-kB pathways; this pattern of inflammatory expression seems to depend on the duration of obesity and the stage of type 2 diabetes. It has been reported that the INF-γ pathway is up-regulated in skeletal muscle cells isolated from insulin-resistant type 2 diabetic patients [
9]. On the other hand, rodent studies indicated that
INF-γ
−/− mice displayed better glucose tolerance compared to their wild type counterparts [
10].
There are both human and animal studies demonstrating that long-term physical activity increased the expression of the GLUT-4 gene in skeletal muscle in middle-aged active subjects as compared to their sedentary peers [
11]. Furthermore, in rats fed a high-fat diet, the expression of the GLUT-4 gene was decreased in skeletal muscle [
12]. Nevertheless, as far as we are aware, there is no information on the effects of a long-term exercise–diet intervention on GLUT-4 and IRS-1 gene expression.
Skeletal muscle is a major site of insulin-stimulated glucose disposal [
13]. In addition to insulin, physical exercise (i.e., muscle contraction and hypoxia) stimulates the translocation of the glucose transporter protein-4 (GLUT-4) to the plasma membrane. These two stimuli, i.e., insulin and physical exercise, act at least partially via independent pathways. These alternative pathways, which act in an additive manner to insulin-stimulated glucose uptake, could potentially improve the glycemic control of insulin-resistant subjects. AMPK is one of the key players in the pathway stimulated by muscle contraction [
14]. The increased ratio of AMP to ATP activates this energy sensor protein, which is heterotrimetric and consists of a catalytic α and regulatory β and γ subunits [
15]. The AMPK isoforms α1 and α2 are expressed in skeletal muscle [
16] and their activities can be increased by moderate-intensity exercise but not with low-intensity exercise equal to or below 40% of VO
2max [
17]. Thus, AMPK also plays a key role in the regulation of fatty acid oxidation in skeletal muscle and it can be activated by adiponectin and leptin, resulting in enhanced fatty acid oxidation and glucose uptake [
14]. In addition, the systemic effects of increased AMPK activity are regulated by many factors, i.e., adiponectin, insulin, IL-6, leptin and TNF-α. Understanding how these secreted signaling proteins regulate AMPK activity may yield clues to discovering novel therapeutic strategies for metabolic disorders, such as diabetes, insulin resistance and obesity [
15]. It has also been debated whether exercise can improve insulin sensitivity in overweight Individuals without accompanying weight loss [
18,
19]. Dube and co-workers reported that both diet-induced weight loss and exercise training improved insulin resistance in overweight-to-obese adults [
18]. Church et al. [
20] revealed that, in patients with type 2 diabetes, the combination of aerobic and resistance training improved glycemic control and maximum oxygen consumption when compared to the control group. The same results could not be achieved by either aerobic or resistance training alone [
21]. In this study, we explored the effects of a 2-year exercise–diet intervention on glucose metabolism in middle-aged obese subjects with impaired glucose tolerance (IGT) and separately in those who achieved or did not achieve a weight loss during this two year period. Here, we also report the responses of plasma levels of adipokines and cytokines to our long-term exercise and dietary intervention to provide evidence of new approaches that could be beneficial in the prevention and treatment of type 2 diabetes.
4. Discussion
In this study, at the two-year assessment of an intervention involving an increase in physical activity and a modification of diet, there was evidence of enhanced oxygen uptake (10%), increased oxidative capacity of skeletal muscle (37%) and decreased insulin resistance (60%) in the responders. Furthermore, the increased maximal oxygen uptake positively correlated with the improvements in insulin sensitivity. These improvements were associated with a complete prevention of type 2 diabetes as observed in the responders after a 5-year follow-up, while one out of every three of the non-responders (3/9) was diagnosed with type 2 diabetes.
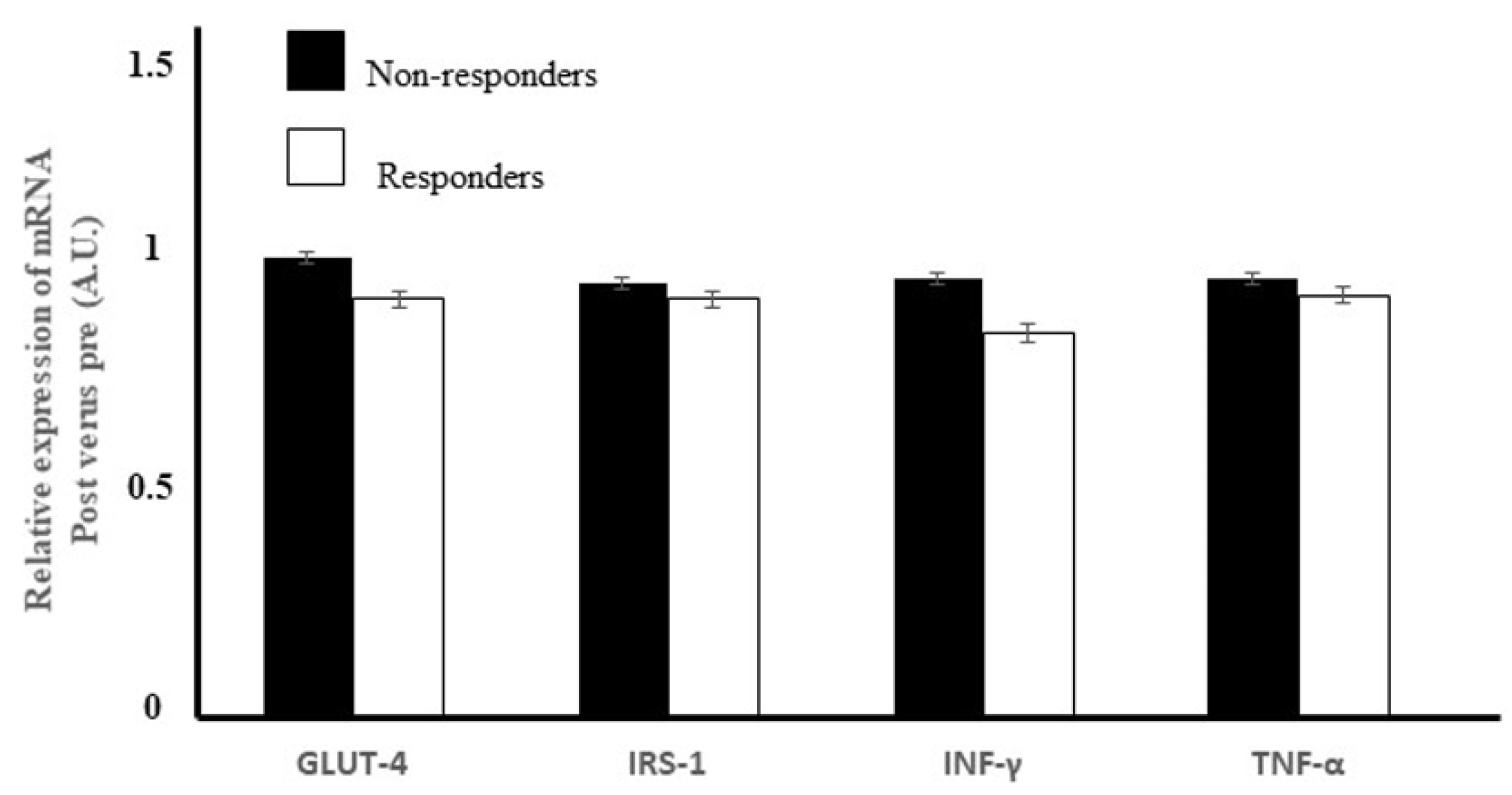
Obesity and physical inactivity are the main non-genetic risk factors for type 2 diabetes [
37]. Although increased physical activity and changes in daily dietary habits are the main lifestyle factors recommended for the prevention of type 2 diabetes [
38], after weight loss, only 10% of dieters manage to maintain weight loss in the long-term [
39]. On the other hand, the DPS (Diabetes Prevention Study) [
23] and several other trials have revealed that lifestyle changes can achieve both significant weight losses and improve glucose tolerance in middle-aged obese subjects with impaired glucose tolerance (IGT) [
40,
41,
42] and that these are accompanied with an increase in the concentration of adiponectin and decrease in those of serum IL-6 and TNF-α [
43,
44,
45]. In our study, we detected no changes in the serum levels of IL-6 or TNF-α or MCP-1 in either group, although serum adiponectin concentrations did increase in the responders. In contrast, the gene expressions of INF-γ or TNF-α in skeletal muscle did not change due to the intervention. The expression of pro-inflammatory genes was reported to be elevated in skeletal muscle cells of diabetic patients and interferon-γ-induced up-regulation leading to a decrease in insulin-stimulated glucose uptake [
46].
In the present study, in the individuals classified as responders to the exercise–diet intervention, serum leptin levels were reduced (27%), serum adiponectin concentrations increased (12%) and glucose control improved without any changes in the lipid metabolism. In a review, Klimcakova et al. [
47] concluded that as little as a 1% weight reduction would be enough to achieve a significant decrease in the plasma leptin concentration. Consistent with other reports [
48,
49,
50], in the present study, the exercise–diet intervention-induced a reduction in serum leptin levels, and this decline was positively correlated with weight loss in the responders. In the other studies, serum leptin decreased by 37% after weight stabilization due to the consumption of a hypocaloric diet for 16 weeks [
51], by 21% after a 3-month dynamic strength training program [
52] and by 14% after a 6-month program that combined a hypocaloric diet and moderate physical activity [
48]. In the study of Auerbach et al., a strong correlation was evident between changes in fat percentage and plasma leptin levels [
53].
In the literature, there is still controversy regarding the effects of regular exercise on adiponectin concentrations. An increase in adiponectin concentration was demonstrated in response to weight loss and glitazone therapy, but not after chronic exercise training [
54]. According to Madsen et al. [
55], a loss of at least 10% body weight is needed to increase systemic levels of total adiponectin. Nonetheless, many investigators reported that adiponectin levels increased in interventions that induced a major weight loss or a significant reduction of visceral fat; in these trials, it seems that weight loss was more efficient than an exercise-based intervention [
56]. However, Fatouros et al. [
57] reported that, while the leptin concentration was decreased by low-, moderate- and high-intensity resistance training in inactive obese men, in contrast, the adiponectin concentration rose only in the high-intensity training group. These changes seem to be strongly associated with changes in the ratio of muscle and adipose tissue mass. According to Mujumdar et al. [
58], a progressive long-term aerobic training (marathon training) increased the adiponectin concentration in middle-aged, overweight, untrained males and females, without affecting the insulin resistance in either group [
58]. The exercise intensity in our trial was not as high as in the studies of Fatouros et al. [
57] or Mujumdar et al. [
58]. Therefore, it is plausible to conclude that the increase in the adiponectin concentration observed in the present study was due to the combined effects of exercise and diet.
Both adiponectin and leptin are hormonal regulators of AMPK signaling and are generally perceived to exert positive effects on insulin sensitivity [
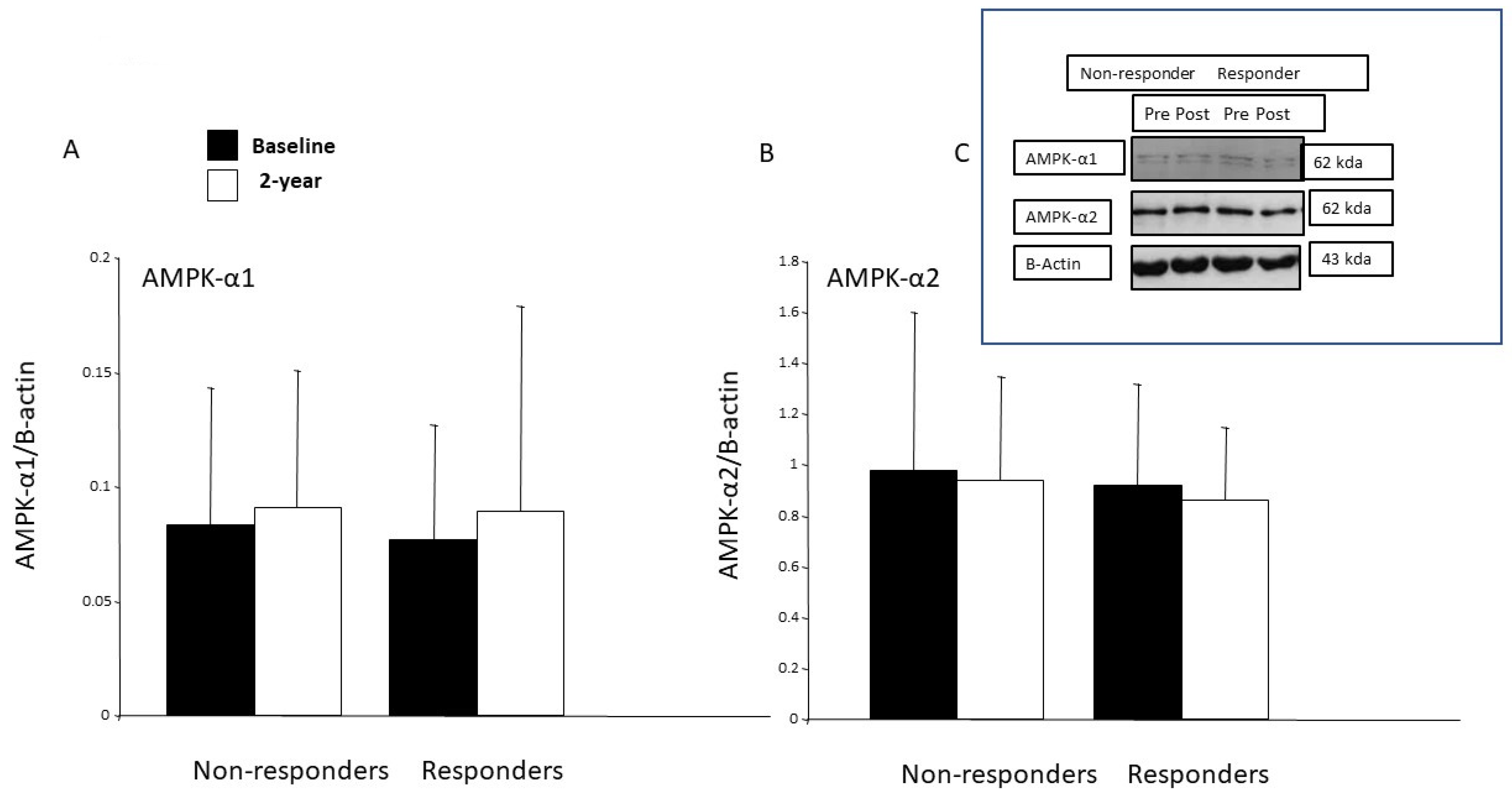
15]. The protein expression of AMPK- α1 was increased after one-month’s endurance training in healthy young males and in type 2 diabetes patients after 6 weeks of strength training [
59,
60]. However, in our study, the protein expressions of the AMPK-α1 and AMPK-α2 in the skeletal muscle remained unchanged in both groups after the 2-year intervention, and there was no correlation between the changes in the amounts of the AMPKs and CS. In work conducted in rodents, when AMPK is chronically activated, a similar adaptive pattern of an increase in mitochondrial oxidative enzymes was observed. Nevertheless, our study design did not allow to us to investigate either AMPK activity or signaling by this protein in skeletal muscle. However, in skeletal muscle, adiponectin activates both AMPK-α1 and AMPK-α2, while leptin activates only AMPK-α2, resulting in enhanced fatty acid oxidation [
61,
62,
63]. In addition, AMPK also regulates glucose homeostasis. The acute regulation of skeletal muscle glucose transport by exercise is multifactorial and has been attributed to several signaling pathways, including Ca
2+-calmodulin-dependent protein kinase (CaMK)II, AMPK and nitric oxide [
64]. Here, the diet and exercise intervention did not increase the expression of the
GLUT-4 gene in the skeletal muscle of either the non-responders or the responders. During exercise, AMPK activation occurs as a response to the contraction of fast-twitching muscles, triggering an up-regulation of hexokinase II expression, which subsequently increases
GLUT-4 gene expression and GLUT-4 translocation [
65,
66,
67]. In addition, when considering the response to exercise, AMPK and calcium/calmodulin-dependent protein kinases (CaMK)II contribute to the regulation of GLUT-4 expression via histone hyperacetylation of the GLUT4 promoter and increase GLUT4 transcriptional activity [
68]. In summary, the multifaceted regulation of GLUT-4 may explain the failure to detect any difference in GLUT-4 gene expression between responders and non-responders.
It has been shown that physical exercise increases the GLUT-4 content in both healthy controls and subjects with impaired insulin-stimulated glucose uptake [
69,
70,
71,
72]. In addition, in subjects with type 2 diabetes, GLUT-4 is normally expressed in skeletal muscle [
73,
74]. Moreover, an inhibition of protein synthesis did not counteract the exercise-mediated increase in muscle insulin sensitivity [
75]. Therefore, increased GLUT4 translocation, rather than its expression, seems to lie behind the improvements in insulin’s action post-exercise [
76]. Consistent with this hypothesis, we did not find any increase in the expression of the GLUT4 gene in the responders, despite their improved glycemic control and insulin sensitivity. While physical exercise may be able increase mRNA of GLUT4 in healthy and type 2 patients, it seems that the increase in GLUT mRNA is transient, returning to a pre-exercise level within 24 h [
77]. Our results can be interpreted as follows—our exercise–diet intervention had not increased the gene expression of GLUT4 by the end of the 2-year intervention, although it did seem that the exercise intensity could have been high enough to transiently induce the expression of GLUT4.
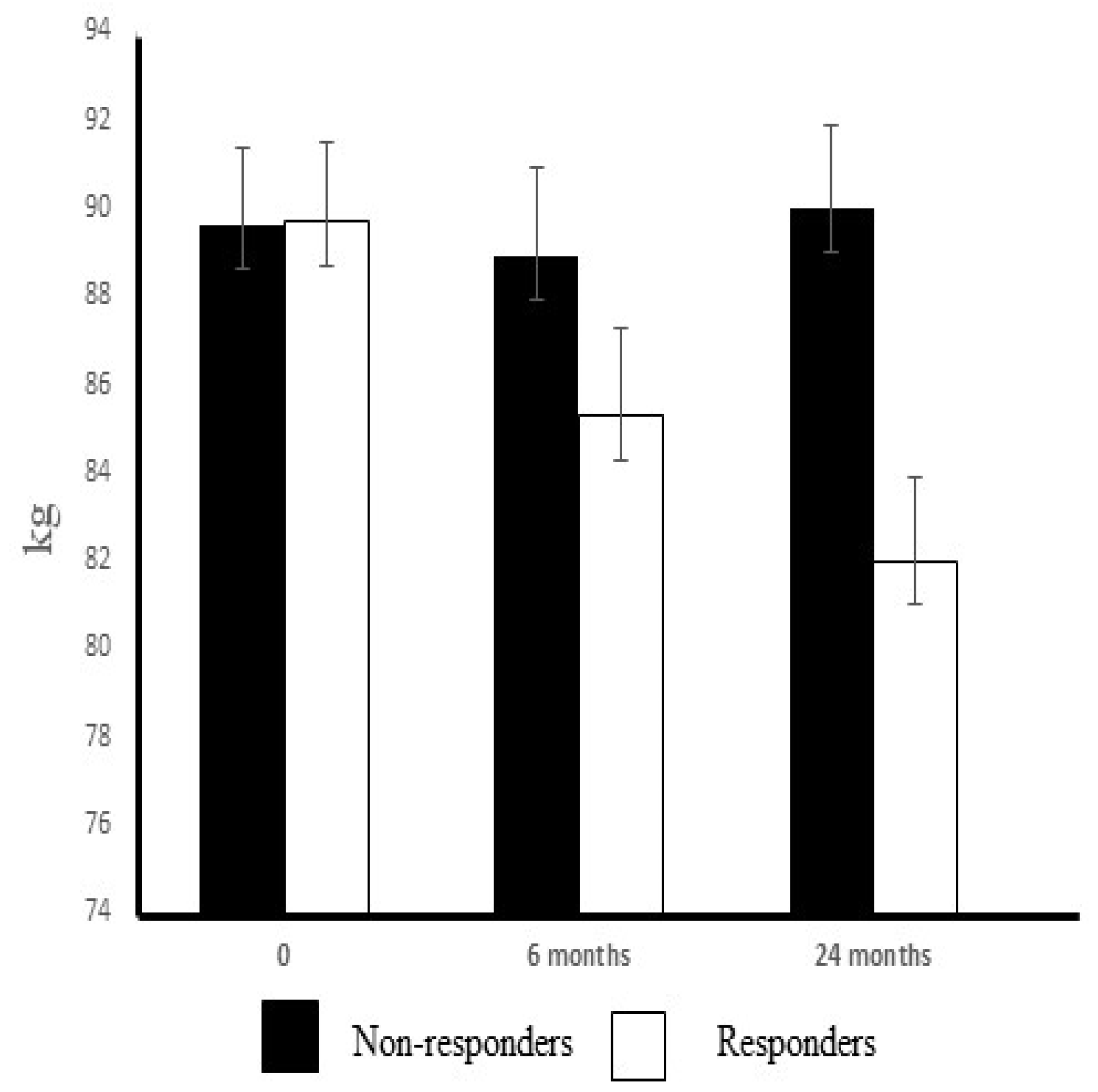
The failure of non-responders to achieve a weight loss and their lower training intensity may be responsible for their unchanged glucose control or serum adipokine concentrations. Interestingly, total cholesterol and LDL-cholesterol levels only decreased in the non-responders, despite the fact that marginal improvements in the dietary intake of fat and fiber occurred only in responders. On the other hand, serum levels of triglycerides and free fatty acid values were decreased in the responders but not in the non-responders. The chronic adaptation to exercise reduced the synthesis of triglycerides, which may have been a response to the enhanced lipid oxidation in trained skeletal muscle. Aerobic exercise was reported to reduce triglycerides, but this effect was only evident with an accompanying energy deficit [
78]. In addition, the exercise–diet intervention may have evoked reductions in free fatty acid levels, and this can improve the insulin sensitivity in both liver and skeletal muscle. In these tissues, the intracellular accumulation of lipids (diacylglycerol) impaired insulin signaling [
79]. As a limitation, our study design, did not allow us to reliably evaluate the individual impact of the exercise or changes in the effect of dietary habits on weight loss or improved glucose control. When considering glucose metabolism in skeletal muscle, the greatest changes due to dietary counselling were assumed to have occurred by 6 months. While this gives a good view of the changes in the regulation of the skeletal muscle metabolism induced by exercise, it does not allow us to separately distinguish the individual effects of either exercise or dietary counselling. In addition, in both groups, rather few participants were women, which needs to be considered while interpreting the results. Although we did not observe any difference between female and male subjects in the changes occurring in any of the parameters, because of the small sample size, we could not perform a more detailed statistical analysis. Dietary intake and the total amount of exercise did not significantly differ between responders and non-responders, but the intensity of the exercise might have been higher in responders (based on their increased citrate synthase activity), and this could contribute, at least partly, to these favorable effects, together with dietary modifications such as increased fiber intake and decreased fat intake. There was no difference in the total duration of physical activity, but the amount of exercise training in responders that consisted of two hours aerobic exercise and one hour strength and power exercise per week may be an optimal strategy to improve metabolism and achieve greater caloric expenditure. It seems that the sustained weight loss achieved by dietary modifications with a combination of aerobic and power-type strength exercises is likely to be the major contributor to the improved glucose control evident in the responders. There can be at least three possible explanations to account for the improved glucose control in the responders. The exercise–diet intervention: (1) reduced body weight with decreased adipose tissue mass resulting in a change to a more favorable adipokine profile; (2) the increased oxidative capacity of skeletal muscle coupled with increased fatty acid oxidation in the skeletal muscle, which reduces leptin resistance and increases adiponectin concentrations; and (3) the reduced level of the hepatic lipid content and weight-loss-related improvements of HOMA-IR, triggering decreased glucose production in liver and a loss of intra-hepatic lipids.
While this study had a strong focus on adipokines and cytokines in middle-aged obese subjects with impaired glucose tolerance, it is important to consider the clinical significance of the findings since dietary counselling and physical activity were the main approaches applied in the prevention and treatment of type 2 diabetes. This two-year exercise–diet intervention exerted a significant effect on fasting glucose levels and insulin sensitivity in responders. Insulin sensitivity is not a basic or standard clinical measurement, but it is an important health outcome that can be easily calculated using routine laboratory values. The extent of weight loss was also clinically significant in the responders (>5%), and together with the improvements of maximal oxygen uptake (large effect size), these changes can be considered to underpin the positive alterations in glucose metabolism evident in the responders. It has been claimed that even a minor weight loss should be enough to significantly decrease plasma leptin levels [
47], and in our study, the observed effect size of the changes of leptin was large. The weight loss is an important factor accounting for the decrease in the leptin levels, i.e., it correlated strongly with changes in the serum leptin concentration in responders leading to decreased leptin resistance. The observed effect size shows that successful weight loss and improved aerobic capacity can achieve clinically significant changes in glucose control.